Introduction
Tracheostomy is a commonly performed procedure worldwide, often for critically ill patients with long-term dependence on mechanical ventilation. Over 100 000 patients receive a tracheostomy each year in the United States, with the tracheostomy tube remaining in place for days to weeks,[
1] while some become tracheostomy dependent for life. The most common reasons for a tracheostomy are prolonged mechanical ventilation, trauma, and upper airway obstruction.[
2]
Speech language pathologists (SLPs) work to assess, diagnose, and treat multiple speech, communication, cognitive linguistic, and swallowing disorders.[
3] In addition to diagnostic and rehabilitative services, SLPs provide education, training, and counseling to patients, improving compliance, outcomes, and quality of life, while potentially reducing length of stay, costs to patients and health care systems, and readmissions.[
4,
5] For tracheostomy patients, SLPs are trained to diagnose and treat dysphagia and aspiration sequelae via a variety of swallow evaluations. They assist in determining upper airway patency, implementation of ventilator in-line placement or general speaking valves, and collaborate regarding appropriate tracheostomy device selection. Finally, they provide inspiratory/expiratory muscle strength training, improving pulmonary rehabilitation by increasing overall strength and ability to clear the airway.[
6,
7]
Speaking valves are important for both speech and swallow function, and SLPs provide and train patients on their use. Valves redirect airflow through the vocal cords and upper airway, allowing for improved vocalization and restoration of normal upper airway flow. Speaking valve use is validated by numerous studies to aid in weaning, decannulation, olfaction, infection control, restoring positive airway pressure, oxygenation, secretion management, swallowing, speech production, and improved overall quality of life.[
8,
9,
10] Thus, it is prudent that most tracheostomy patient should be evaluated for a speaking valve, as they are not suitable for everyone. Cuff status, secretions, and upper airway patency can complicate use.
Swallow evaluations are performed as a clinical bedside swallow evaluation, fiber optic endoscopic evaluation of swallowing, or videofluoroscopy, also known as modified barium swallow. Bedside clinical swallow evaluation is an elaborate screen that assesses feeding position, consumption amount, need for supervision or adaptive equipment, and overall pleasure from eating and drinking.[
11] Suspicion for aspiration and need for further investigation is determined through synthesis of case history and clinical assessment. By evaluating all of these parameters, SLPs can make a statistically significant judgment regarding aspiration. Thus, a recommendation for a videofluoroscopy after a bedside clinical swallow evaluation should be heeded.[
11,
12] Swallow studies are not pass/fail, but instead provide information about ability to swallow as a dynamic process. A fiber optic swallow study can be performed bedside by SLPs to evaluate secretion management and swallow physiology. Videofluoroscopy is the gold standard of swallow studies[
13] and is performed in the radiology suite. The oral, pharyngeal, and esophageal phases of swallow are screened in real time to identify anatomical deficits, penetration and aspiration, and need for further management.[
14] One or all three studies may be performed, depending on the complexity of the patient.
The purpose of this study was to determine the rate of speech language pathology (SLP) utilization and devise a protocol to streamline the tracheostomy weaning process to make it safer and more efficient. The specific aims are to (1) measure the rate of SLP evaluation for patients after tracheostomy, (2) measure the rate of speaking valve and swallow study utilization, and (3) synthesize the results of our literature review and this study to develop a workflow for tracheostomy decannulation.
Methods
The authors designed and implemented a retrospective case series, which was approved by the University of Florida Jacksonville’s Institutional Review Board. Included subjects had undergone open or percutaneous tracheostomy at our institution, an urban level 1 trauma center, performed by either the Oral and Maxillofacial Surgery, Otolaryngology, or Acute Critical Care Surgery teams, from April 2016 to December 2018, with 6 months of documented follow-up. Subjects were excluded if they were converted to a laryngectomy, expired within the immediate postoperative period, were never weaned from the ventilator, or had missing/incomplete records.
Variables collected describing the sample included demographics, history and physical findings, tracheostomy history, and comorbidities as a continuous variable using the Charlson comorbidity index (CCI), without age, to standardize and emphasize preexisting conditions.[
15] Alcohol history was positive for moderate or greater drinking,[
16] and smoking if there was a self-reported history. Indication for tracheostomy was a categorical variable: upper airway obstruction (infection, trauma, tumor, foreign body, obstructive sleep apnea (OSA), and stenosis), prolonged mechanical ventilation (respiratory failure, management of secretions, or to promote weaning), or neurological or neuromuscular disease (diaphragm weakness, aspiration due to disease, coma, ineffective cough, or neurological injury).
The primary outcome was to measure the rate of SLP evaluation after tracheostomy. A general SLP evaluation addresses many components, however for this study we counted only those evaluating swallow, cognition, and speech production. In our center, the process of ordering a SLP evaluation requires final approval by the managing physician, but can be suggested to the surgical team by SLPs or nursing. SLP utilization was further evaluated by measuring speaking valve use, performance of any type of swallow study, and duration from surgery until the SLP evaluation was ordered by the surgical team. The goal was for every patient to have a general SLP evaluation at minimum, as evidence suggests that many tracheostomy patients can benefit from early evaluation by SLPs, as well as from using a speaking valve.[
17,
18,
19,
20] Three swallow studies were available: clinical bedside swallow evaluation, fiber optic endoscopic evaluation of swallowing, and videofluoroscopy. For the purpose of this study, we defined a “successful swallow study” as any subject recommended to initiate a diet because they were able to manage their secretions, masticate and move the bolus, and effectively and efficiently manage food consistencies with no signs or symptoms of aspiration. A failure was defined as a subject not being recommended a diet, or to stay nil per os/pleasure feeding.[
14] The primary predictor variable was etiology for the tracheostomy, and secondarily we evaluated subject demographics, comorbidities, and type of tracheostomy.
Secondarily we measured decannulation rates, mean duration the tracheostomies were present, and complications occurring within 30 days of decannulation. Complications included were respiratory failure, pneumonia, tracheostomy site infection, poor wound healing, granulation tissue needing intervention, and hemorrhage. Lastly, an interdisciplinary decannulation workflow was created, based on a review of the literature and our results to assist in decision-making and to optimize utilization of SLP rehabilitation services.
Study data were collected and managed using Research Electronic Data Capture tools hosted at the University of Florida.[
21] All data were gathered from the electronic medical record (EMR), based on available notes, and accessed through Epic (Epic Systems, Verona, WI). Data were analyzed as frequencies for categorical variables and as means with standard deviations for continuous variables. χ
2 test, ANOVA,
t-test, and binary logistic regression with odds ratio were used to further analyze data.
P values <0.05 were considered statistically significant. All analysis was completed utilizing MedCalc version 19 (MedCalc Software, Ostend, Belgium).
Results
A total of 255 subjects were included in this study, and demographics are detailed in
Table 1, as they relate to SLP evaluation. Only 197 (77.3%) had a SLP evaluation. The distribution of gender, age, BMI, and comorbidity score was consistent among those with and without SLP evaluation (
P > 0.05). Regarding indication for tracheostomy, subjects with upper airway obstruction were less likely to receive SLP evaluations (
P = 0.001). The majority this cohort received elective tracheostomy for head and neck cancer ablation and reconstruction, where decannulation usually occurs around 1 week postoperatively. Most tracheostomies were for upper airway obstruction (47%), followed by neuromuscular dysfunction (27%), and prolonged mechanical ventilation (25%). SLP evaluations were also generally ordered later into recovery, with a mean duration from surgery to SLP evaluation of 6 ± 8 days (median 4 days).
The minority of subjects in this study were evaluated and received a speaking valve (33.7%). Demographics regarding speaking valves are detailed in
Table 2. Those receiving percutaneous tracheostomies or requiring tracheostomies secondary to trauma were more likely to be cleared for a speaking valve. Results of speaking valve use related to tracheostomy outcomes are detailed in
Table 3 and
Table 4. Subjects cleared for a speaking valve, compared to those who were not, had a longer mean duration of tracheostomy, 63.0 versus 19.9 days, but overall had higher rates of being downsized and decannulated within the 6 months of follow-up. No subjects that were tracheostomy dependent for <7 days received a speaking valve, and conversely patients who had a tracheostomy for over 2 weeks had the highest incidence of valve utilization (31%).
Table 1.
Summary of subject demographic data stratified by SLP evaluation.
Table 1.
Summary of subject demographic data stratified by SLP evaluation.
| | All | General SLP evaluation | No SLP evaluation | P value |
|---|
| Sample size, n | 255 | 197 (77.3%) | 58 (22.7%) | |
| Gender | | | | 0.996 |
| | Male | 167 | 129 (77%) | 38 (23%) | |
| | Female | 88 | 68 (77%) | 20 (23%) | |
| Age (years) | Range | 15-88 | 15-88 | 17-86 | 0.08b |
| | Mean ± SD | 52.1 ± 17.6 | 50.9 ± 18.1 | 55.5 ± 15.5 | |
| CCI comorbidity score | Range | 0-18 | 0-18 | 0-10 | 0.383b |
| | Mean ± SD | 2.1 ± 2.3 | 2.0 ± 2.4 | 2.3 ± 1.9 | |
| BMI | Mean ± SD | 28.1 ± 9.4 | 28.0 ± 9.4 | 28.2 ± 9.4 | 0.887b |
| Tobacco, % | 141 | 108 (77%) | 33 (23%) | 0.781 |
| Alcohol, % | 46 | 35 (76%) | 11 (24%) | 0.835 |
| H&N cancer | 100 | 70 (70%) | 30 (30%) | <0.05 |
| OSA | 28 | 20 (71%) | 8 (29%) | 0.437 |
| History of trach | 22 | 14 (64%) | 8 (36%) | 0.112 |
| Emergent procedure | 19 | 18 (95%) | 1 (5%) | 0.06 |
| Percutaneous tracheostomy | 88 | 71 (81%) | 17 (19%) | 0.789 |
| Trauma induced | 98 | 87 (89%) | 11 (11%) | <0.0010.001c |
| Reason for trach | | | | |
| | Upper airway obstruction | 121 | 87 (72%) | 34 (28%) | |
| | Mechanical Ventilation | 64 | 52 (81%) | 12 (19%) | |
| | Neuromuscular Dysfunction | 70 | 58 (83%) | 12 (17%) | |
| Postoperative days until SLP evaluation (mean days) | | 5.9 ± 8.0 | N/A | |
Table 2.
Preoperative variables stratified by SLP specialty services.
Table 2.
Preoperative variables stratified by SLP specialty services.
Table 3.
SLP procedures stratified by duration of tracheostomy dependence.a
Table 3.
SLP procedures stratified by duration of tracheostomy dependence.a
| | | Tracheostomy dependent | Tracheostomy dependent | Tracheostomy dependent | Tracheostomy dependent | Not decannulated |
|---|
| All | <7 Days | 7-13 days | 14-30 Days | >30 Days | by 6 months |
|---|
| All subjects | 244 | 34 (14%) | 44 (18%) | 40 (16%) | 39 (16%) | 87 (36%) |
| With or without | | | | | | |
| SLP intervention | | | | | | |
| SLP service performed | | | | | | |
| General evaluation | 186 | 24 (13%) | 36 (19%) | 34 (18%) | 35 (19%) | 57 (31%) |
| Speaking valve | 81 | 0 | 6 (7%) | 25 (31%) | 25 (31%) | 25 (31%) |
| Swallow study | 126 | 11 (9%) | 26 (21%) | 25 (20%) | 29 (23%) | 35 (28%) |
Table 4.
SLP procedures stratified by tracheostomy outcomes.
Table 4.
SLP procedures stratified by tracheostomy outcomes.
| | All | Downsized | Decannulated | Mean duration of tracheostomy dependence (days) | Post decannulation complications |
|---|
| All subjects | 255 | 203 (80%) | 159 (62%) | 34.7 ± 84.2 | 13 (5%) |
| SLP evaluation | 197 | 172 (87%) | 134 (68%) | 38.5 ± 92.1 | 11 (6%) |
| No SLP evaluation | 58 | 31 (53%) | 25 (43%) | 17.2 ± 21.0 | 2 (3%) |
| P value | | <0.0001 | <0.001 | 0.082a | 0.726 |
| Speaking valve | 86 | 79 (92%) | 58 (67%) | 63.0 ± 131.4 | 7 (8%) |
| No speaking valve | 169 | 124 (73%) | 101 (60%) | 19.0 ± 29.5 | 6 (4%) |
| P value | | <0.001 | 0.276 | <0.0001a | 0.18 |
| Swallow study performed | 135 | 122 (90%) | 95 (70%) | 46.0 ± 107.0 | 9 (7%) |
| No swallow study | 120 | 81 (68%) | 64 (53%) | 19.2 ± 27.5 | 4 (3%) |
| P value | | <0.0001 | <0.01 | <0.01a | 0.149 |
| Successful swallow study | 79 | 71 (90%) | 59 (75%) | 50.7 ± 123.4 | 5 (6%) |
| Failed swallow study | 56 | 51 (91%) | 36 (64%) | 36.7 ± 65.4 | 4 (7%) |
| P value | | 0.846 | 0.169 | 0.44a | 0.816 |
Approximately half of subjects had a swallow study (52.9%), and most frequently via only a bedside evaluation (75%). Videofluoroscopy, either alone or combined with a bedside swallow study, were performed for a quarter of the subjects. Demographics for swallow study subjects are detailed in
Table 2. Those receiving a swallow study were more likely to have had an emergent tracheostomy or trauma etiology (
P < 0.05), while head and neck cancer subjects were less likely to undergo a swallow study (
P < 0.001). Similar to speaking valves, swallow studies were performed more often on subjects who were tracheostomy dependent longer, 63.0 days versus 19.2, and again were overall more likely to be downsized and decannulated (
Table 3 and
Table 4).
Results summarizing tracheostomy decannulation, downsize, mean duration of tracheostomy dependence, and complications are summarized in
Table 4. Overall, the majority of subjects were eventually decannulated (62%) during our follow-up period. SLP services were utilized more in subjects with a longer duration of tracheostomy dependence, and there was consistent improvement in downsize and decannulation rates in all cohorts utilizing SLP services. Head and neck cancer subjects overall had a shorter mean duration of tracheostomy dependence of 19.3 days, compared to 34.7 days overall. Acute complications after decannulation were evenly distributed among all SLP cohorts, with an overall complication rate of 5%, and no statistically significant difference between cohorts. A logistic regression with odds ratio (
Figure 1) was performed to analyze which variables conferred higher odds of eventually being decannulated. Tracheostomy etiology of head and neck cancer and trauma, those completing a successful swallow study all conferred increased odds of decannulation, while obesity and prior tracheostomy history all conferred lower odds of decannulation.
We secondarily evaluated what additional variables lead to successful decannulation. When looking at subjects who had an acute complication after being decannulated, it was found that having pneumonia and unmanageable secretions were both predictive for decannulating too early. As one third of those decannulated with pneumonia, and one quarter of those with unmanageable secretions had a postdecannulation complication. Positive predictive value of the following to predict a successful decannulation (without acute post decannulation complications), where all >93%: tracheostomy tube had been downsized, adequate cough to clear secretions, toleration of occlusive cap for 24 hours, and maintenance of SpO
2 >90% for 24 hours on room air. An alert and oriented subject, or a subject with a successful swallow study and receiving a speaking valve both had a positive predictive value of 87%. Additionally, increased body mass index was known in our population to negatively affect decannulation outcomes from a previous study and our regression analysis.[
22]
Figure 2 was created to highlight these findings, and can be utilized by nursing, SLPs, or a tracheostomy team simply as a guide when the surgical team can be alerted to patients’ readiness to be decannulated. After analyzing these results, with findings from our literature review[
3,
4,
18,
19,
20,
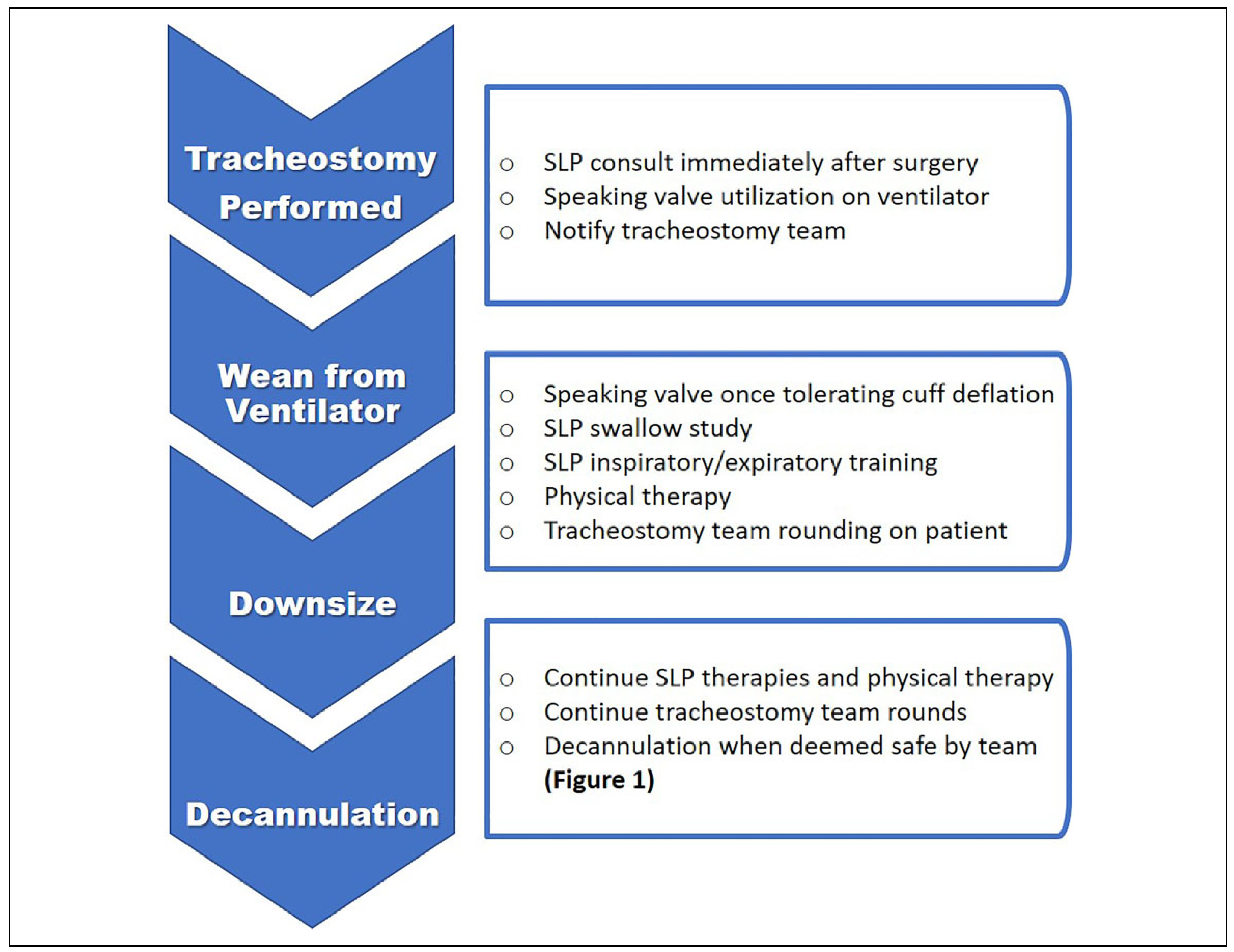
22] which was based on tracheostomy decannulation as well as commonly accepted criteria for endotracheal tube extubation a decannulation workflow process was created to assist in decision-making while progressing toward the goal of decannulation. We also used the same information to change our decannulation process to find which steps in the decannulation process could benefit from SLP intervention. We present this as a workflow pathway, which is presented in
Figure 3. It outlines a reasonable progression from surgery to decannulation in an ideal environment as well as how and when rehabilitation services can be utilized to optimize patient progression.
Discussion
Speech language pathology services were found to be underutilized and often requested later than recommended in this study. In our center, SLPs is trained to evaluate and begin rehabilitation of patients when they are still ventilator dependent, however most consultations did not occur until several days postoperatively, and for many patients after several weeks. The results show many missed opportunities for rehabilitation, as almost of a quarter of all tracheostomy subjects in this study were never evaluated by SLP. Although not every subject may need a SLP evaluation, the vast majority of our tracheostomy patients are quite complex secondary to traumatic brain injuries, morbid obesity, or undergoing extensive reconstruction of the oral cavity/pharynx due to cancer. Although the majority of the head and neck cancer subjects were evaluated by SLP (70%), they were one of the lowest utilizers of SLP. This is likely because the tracheostomy performed was elective to prevent temporary airway obstruction after surgery, and these subjects are often promptly decannulated on postoperative day 4 or 5 without SLP intervention. Still, not involving SLP in their care could be disadvantageous, as the majority underwent ablation and reconstruction of the oral cavity, causing significant alteration to their anatomy and speech/swallow. Furthermore, we did find that decannulation was met more often in patients under the care of SLP; however, it was difficult to evaluate a correlation between specialized SLP services (swallow evaluations and speaking valves) and other decannulation outcomes, as SLPs tended to treat subjects with more complex clinical courses and those with long-term tracheostomies unable to be weaned. While the benefits of these specialized services to result in decannulation may not be completely clear, the negative consequences of not utilizing speaking valves for rehabilitation is well documented to result in pulmonary deconditioning, decreased ability to communicate and swallow, and increased risk of aspiration which in turn can increased length of tracheostomy.[
8,
9,
10]
We found a lack of standardization and communication after tracheostomy which was the impetus for the creation of our hospital’s tracheostomy team. These frequent late, or often missed, consults to SLP were causing frequent delays in the rehabilitation and decannulation process. Additionally, there were regular urgent consults placed to SLP and the surgical airway teams on the day of discharge to give evaluation and guidance about decannulation, where rehabilitation potential is limited due to time constraints. Patients were frequently keeping their tracheostomies longer than needed from a lack of recognition and specified benchmarks indicating when to proceed with a safe downsize or decannulation. Poor follow-up after discharge also compounded the patient’s course. Having three surgical teams performing tracheostomies made it difficult for primary care services to coordinate proper follow-up after discharge, as a medicine team was often left managing the patients’ other hospital problems once the patient was surgically stable after tracheostomy. This caused many patients to remain tracheostomy dependent for unclear underlying medical or surgical conditions that were never followed up with the surgical team. SLP also functioned as the consistent provider throughout many of these patients’ care, thus it was recognized that an initial evaluation with SLP could lead to better continuity of care, as they remained on caseload while navigating through the decannulation process. SLP was consistent in providing multiple assessments, skilled intervention and treatment, and if appropriate, immediate referral back to the surgical team.
Tracheostomy teams are still quite rare in the United States, so our framework was based on guidance from literature review, as well as the requirement to meet our own unique needs following a preliminary meeting of the multidisciplinary team. Extensive evidence-based research supports the recruitment of tracheostomy teams, as they improve outcomes and reduce costs through reducing time of ventilator and tracheostomy dependence, deescalating need for intensive care, and reducing length of stay.[
4,
5,
23,
24,
25,
26] Goals were focused on ways to improve outcomes, safety, and overall standardization of care to reduce costs, length of patient stay, and unnecessary cannulation time. The team at large included surgical services performing tracheostomies, SLPs, respiratory therapy, nursing, case management, and information technology. A smaller team meets to round on all tracheostomy patients in the hospital once a week, where ongoing evaluation and recommendations are developed and then discussed with the responsible surgical teams.
Objectives were delineated for three phases of tracheostomy. First, preoperative decisions address selection of the proper tracheostomy tube, plans for future weaning, barriers to weaning, and potential adjunctive therapies that can be utilized to optimize the patient for decannulation. Proper tube selection is important, as many are incompatible with speaking valves and this can delay rehabilitation efforts. Second, the tracheostomy phase addresses issues of organization to wean the patient with use of speaking valves, swallow studies, when to downsize, occlusive capping, and ancillary rehabilitation services. We utilize
Figure 2 and
Figure 3 to track patients’ progression toward decannulation. Attention is focused on team troubleshooting for identification of upper airway problems such as stenosis or granulation tissue and quickly routing referrals to the surgical airway team for management. Finally, post-tracheostomy phase focuses on enriched training and education for staff, patients, caregivers, and coordination of care upon discharge. Potential innovations include home health care visits, specifically aimed clinics for post discharge care, referrals to outpatient SLP, and creation of a documentation flow sheet in the EMR to facilitate improved record keeping and communication between all team members for inpatients.
This study is inherently limited as it is a retrospective chart review, and inaccuracies in the EMR can cause overor underestimation of the variables. To decrease misinterpretation and bias, most variables were recorded as binary data regardless of the magnitude. Additionally, the majority of SLP evaluations were placed for patients deemed to be more medically complex, and for those who were not progressing as expected through the decannulation process. Consequently, the results will sometimes falsely reflect inferior results in the SLP cohorts. Ideally SLP would have been consulted on every tracheostomy case, so we could infer a better association between SLP utilization and decannulation outcomes.
The external validity is based on only a few studies investigating the role of SLP and tracheostomy outcomes. Those published find superior results when SLP is utilized supporting early intervention for communication with speaking valves,[
27] and that SLP services result in faster rates of decannulation, higher safety for subjects tolerating diets,[
28] and reduced length of hospital stay.[
29] The rate of SLP evaluation will likely vary hospital to hospital based on structure, patient population, and airway teams’ experiences. However, with the new trend of establishing tracheostomy teams, we believe there will be an increase of studies on this topic as well as more focus on how tracheostomy teams can utilize all the available resources to best optimize patients for decannulation.