Abstract
Objective: The aim of the systematic review was to analyze the current clinical evidence concerning the use of tissue engineering as a treatment strategy for reconstruction of segmental defects of the mandible and their clinical outcomes using individual patient data. Methods: A systematic review of the literature was conducted using PubMed and Cochrane Library on May 21, 2019. The eligibility criteria included patients in whom segmental mandibular reconstruction was carried out using tissue engineering as the primary treatment strategy. After screening and checking for eligibility, individual patient data were extracted to the extent it was available. Data extraction included the type of tissue engineering strategy, demographics, and indication for treatment, and outcomes included clinical and radiographic outcome measures, vitality of engineered bone, dental rehabilitation, and patient-reported outcome measures and complications. Results: Out of a total of 408 articles identified, 44 articles reporting on 285 patients were included, of which 179 patients fulfilled the inclusion criteria. The different tissue engineering treatment strategies could be broadly classified into 5 groups: “prefabrication,” “cell culture,” “bone morphogenetic protein (BMP) without autografts,” “BMP with autografts,” and “scaffolds containing autografts.” Most included studies were case reports or case series. A wide variety of components were used as scaffolds, cells, and biological substances. There was not a single outcome measure that was both objective and consistently reported, although most studies reported successful outcome. Discussion: A wide variety of tissue engineering strategies were used for segmental mandibular reconstruction that could be classified into 5 groups. Due to the low number of treated patients, lack of standardized and consistent reporting outcomes, lack of comparative studies, and low evidence of reported literature, there is insufficient evidence to recommend any particular tissue engineering strategy.
Introduction
Mandibular resection is the curative treatment of choice for many diseases ranging from benign to malignant tumors, chronic infections, necrotic conditions, and large avulsive trauma. Mandibular resections can result in functional impairment of mastication and speech. They can also lead to aesthetic deformities with a decrease in health-related quality of life. The treatment goals for patients requiring reconstruction of segmental defects of the mandible have evolved from purely closing the defect to providing better aesthetic and functional outcomes leading to a better quality of life. At the same time, efforts are being made to provide solutions that decrease patient morbidity associated with treatment.[1]
Currently, the most commonly used treatment method for large reconstruction of mandibular segmental defects is vascularized bone containing free flaps (fibular, scapular, or deep circumflex iliac artery–based composite flap, etc).[2] Smaller resection defects are reconstructed using nonvascularized free bone grafts, most commonly from the iliac crest. Other methods such as transport distraction osteogenesis have also been described for the reconstruction of segmental defects.[3]
Autogenous bone transfer has been the gold standard for reconstruction of segmental defects.[4] Autogenous tissues have the capability to provide all factors critical for bone defect reconstruction: viable bone cells for osteogenesis, growth factors, and proteins for osteoinduction and a scaffold for bone regeneration[5] without the risk of incompatibility. However, the use of autogenous bone often results in donor site morbidity. Many patients are unable to go through the long and irksome procedure of free flap transfer. In addition, the anatomical and functional complexity of the mandible cannot be easily replicated with the existing autogenous tissue transfer and mere reconstruction of continuity of the resulting mandibular defects will not solve functional impairments caused by the resection.
Due to these reasons, mandibular reconstruction is still a clinical challenge and tissue engineering has been envisaged as a strategy to overcome the problems of current treatment methods. Tissue engineering has been defined as “an interdisciplinary field that applies principles of engineering and life sciences toward the development of biological substitutes, which restore, maintain, or improve tissue function (p.920).”[6] It is based on the use of 3 principal components: scaffolds, bioactive substances, and cells.[6,7] Preclinical animal studies have shown promising results after reconstructing segmental defects of the mandible; however, there is a clear lack of evidence in clinical applications.[5] This systematic review was undertaken to analyze the current clinical evidence concerning the use of tissue engineering as a treatment strategy for reconstruction of segmental defects of the mandible. Our primary objective was to describe the current tissue engineering strategies that have been used for reconstruction of segmental defects of the mandible and their clinical outcomes.
Materials and Methods
A systematic review of the literature was conducted according to the PRISMA-IPD (Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Individual Patient Data) statement.[8,9,10] Electronic searches were conducted in PubMed and the Cochrane Library on May 21, 2019, to find the evidence related to the use of tissue engineering for mandibular reconstruction, defined as any combination of scaffolds, bioactive substances, and/or cells.
Search Strategy
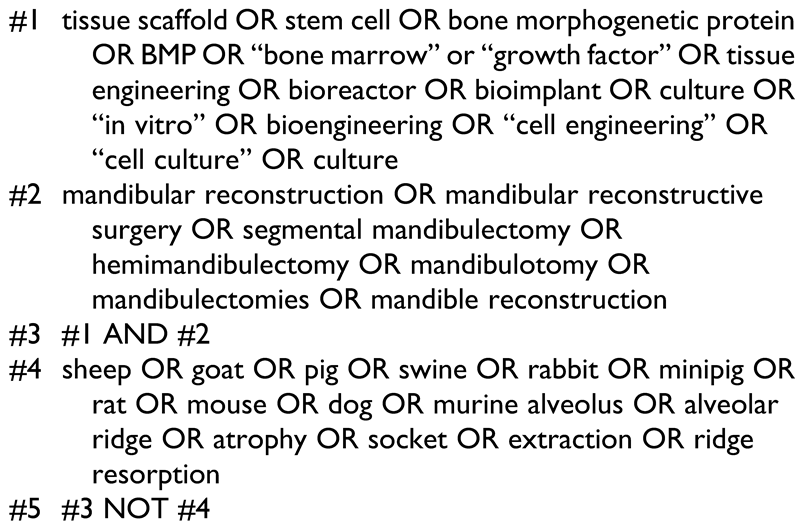
The search strategy is described in Table 1. Additional articles were identified by hand searching the reference lists of relevant articles (hand search). The available evidence consisted almost exclusively of case series and case reports, which, in most cases, presented information on an individual patient level. The eligibility criteria for this systematic review were as follows:

Table 1.
Search Criteria.
Inclusion criteria.
- Original clinical reports and studies with human subjects
- Any age
- Reconstruction of segmental defects of the mandible with tissue engineering as primary treatment
- Description of any outcome measure: radiological, clinical, functional, or bone vitality
- Minimum follow-up of 3 months
- Providing IPD on at least 1 parameter of interest
- Articles in English language
- Published within the last 20 years (1999 onward)
Exclusion criteria.
- Noncontinuity defects of the mandible
- Continuity defects secondary to fracture malunion or nonunion
- Gunshot injuries and resection defects without specification of defect size
- Isolated condylar or temporomandibular joint reconstruction
- Tissue engineering used as adjunct to vascularized bone free flaps
- Use of distraction osteogenesis alone.
Study Selection
Screening and eligibility assessment was performed by the first author (V.V.K.) who is a maxillofacial reconstructive surgeon. Individual Patient Data from eligible patients was extracted from each publication to the extent it was available. Whenever no IPD was available, the respective author was contacted per mail, requesting the missing information. Duplicate patient details were identified by comparing recruitment dates/treatment time/time frame during which patients were treated, comparing defect and reconstruction characteristics of the described patients and similarity in the author/institution details.
Data Extraction
Data extraction was done by the first author (V.V.K.), which included study design, direction of study (prospective/ retrospective), number of patients reported in the article, and number of patients eligible according to the inclusion/exclusion criteria of our review, demographics, indication for mandibular resection, use of radiotherapy, defect location, and length of defect. Indications for mandibular resection were categorized as benign pathologies (odontogenic cysts, tumors, developmental cysts, and tumors), malignant pathologies (carcinomas, osteosarcomas), infective (osteomyelitis), osteoradionecrosis, and trauma (road traffic accidents, gunshot injuries, ballistic injuries). The length of defect was collected in centimeters, whereas the defect location was described according to the affected structure(s) as high (H) if the condyle was affected, lateral (L) if the ascending ramus and/or body were affected, and central (C) if the symphysis was affected (modified from Jewer et al[11]). Data related to the mandibular defect treatment included the type of reconstruction (primary or secondary), use of maxillomandibular fixation (MMF), and additional grafts and surgical treatments.
Data Synthesis
Data were synthesized in a qualitative manner. For a better overview, outcomes were summarized in separate tables for each type of tissue engineering strategy, categorized as follows:
- Prefabrication: Entails the construction of a tissue-engineered mandible inside the body of the patient but that is away from the target site of segmental mandible reconstruction. It considers the patient as a “bioreactor.” Typically, a scaffold with or without bioactive substances and autogenous bone with or without bone substitutes is implanted in a vascularized region of the patient’s own body. This construct remains embedded until the fabricated tissue is viable. The construct is then transferred onto the mandibular defect and anastomosed by microvascular techniques or following flap delays.[12,13,14,15]
- Cell culture: Mesenchymal stem cells and/or progenitors harvested from the patient’s bone marrow or adipose tissue are isolated in the laboratory, cultured under preferential conditions over a few passages, concentrated and transferred into the defect.
- Bone morphogenetic protein without autografts: Bone morphogenetic protein (BMP) alone is placed in an absorbable carrier and into the defect to promote bone regeneration.
- Bone morphogenetic protein with autografts: In addition to BMP, varying amounts and types of autogenous tissues were used for reconstruction: bone marrow aspirates, platelet-rich plasma (PRP), and/or corticocancellous bone grafts.
- Scaffolds containing autografts: Scaffolds that have anatomic similarity to the segmental reconstruction defect, additionally filled with autogenous bone graft and placed in the defect.
Treatment Data
Treatment data were extracted based on the individual tissue engineering strategy and included the type of scaffold, bioactive substance (eg, BMP-2, BMP-7), cell type (mesenchymal, adipose, bone marrow, stem cells, autologous bone chips, PRP), region/flap of prefabrication, time taken for prefabrication, origin and type of cells used for cell culture, time in culture, and additional treatments (additional use of flaps, use of hyperbaric oxygen, etc).
Outcome Measures
Outcomes were recorded according to the following categories: (a) clinical and radiological (b) vitality of the engineered bone assessed by scintigraphy or histology, (c) dental rehabilitation with dental implants or dentures, (d) patient-reported outcome measures, and (e) complications and additional surgical procedures. Patient-reported outcome measures were only extracted if validated questionnaires were used. Reports merely stating “patients were doing well/patients were happy with the outcome” without using well-defined questionnaires were not included.
When parameters of interest were not provided as IPD, the summarized data given in the original article were reported in this review. Due to the study design and quality of the articles included in the systematic review, a meta-analysis of pooled data was not possible. Risk of bias assessment across studies, quantitative synthesis of results, summary measures, treatment effect, heterogeneity assessment, and publication bias could not be performed. A formal quality assessment was not considered to be necessary as all included studies were of a similarly low level of evidence.
Statistical Analysis
Individual patient data were aggregated according to treatment received and descriptive statistics were performed using Microsoft Excel (MS Excel 2016).
Results
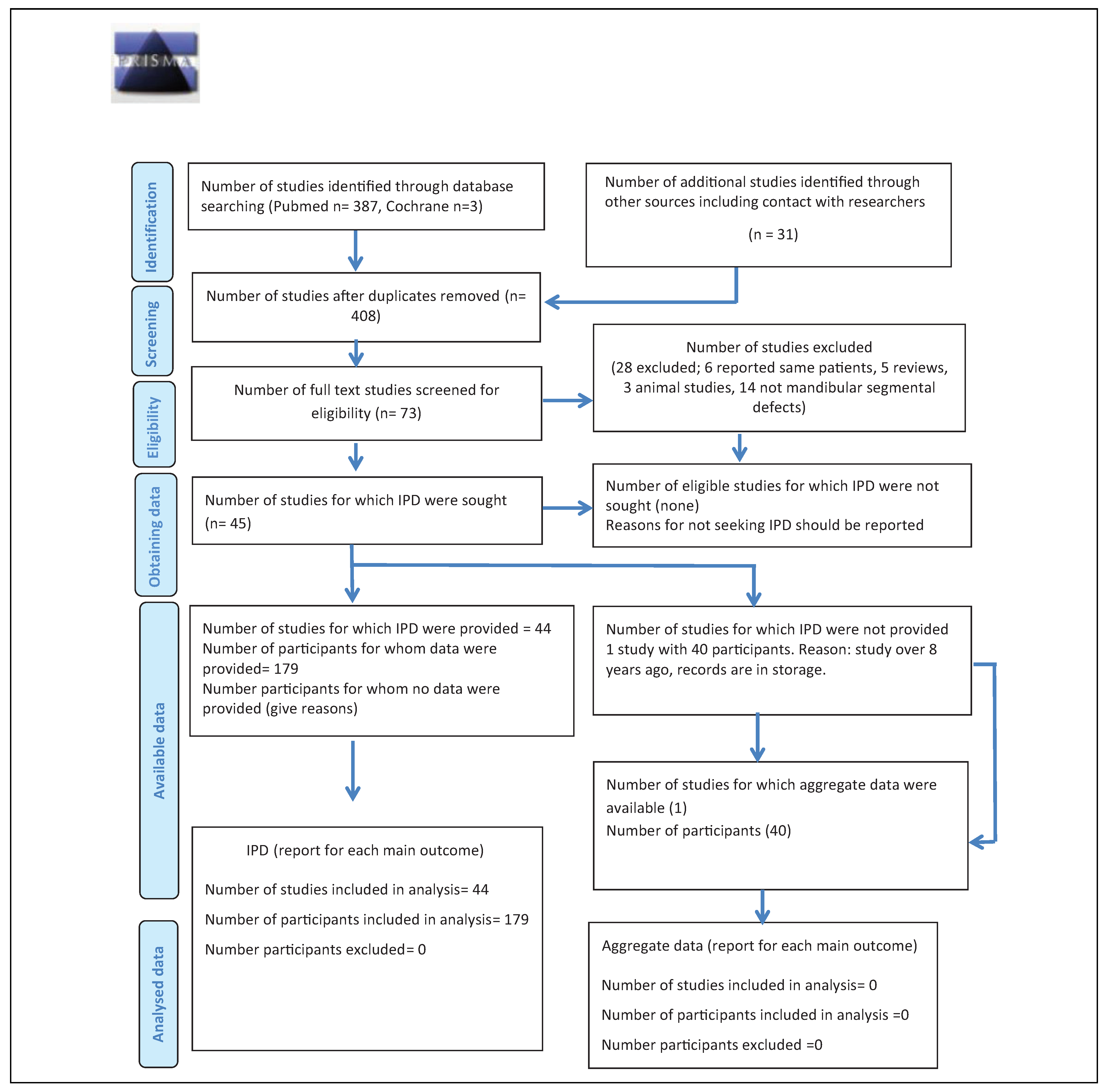
The search identified 387 articles in PubMed and 3 articles in Cochrane (Figure 1). Hand search of relevant articles identified 21 additional references. Once duplicates were removed, the abstracts of 408 articles were screened and 335 were considered ineligible. After reading the full text of 73 articles, 28 were excluded. Out of the remaining 45 articles, one study did not report on individual patient data of any parameter (neither baseline data nor outcome measures[16]) and the attempt to procure the respective IPD directly from the author remained fruitless. Therefore, 44 articles reporting on a total of 285 patients were included. Of these, 179 patients fulfilled the eligibility criteria.[13,15,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]
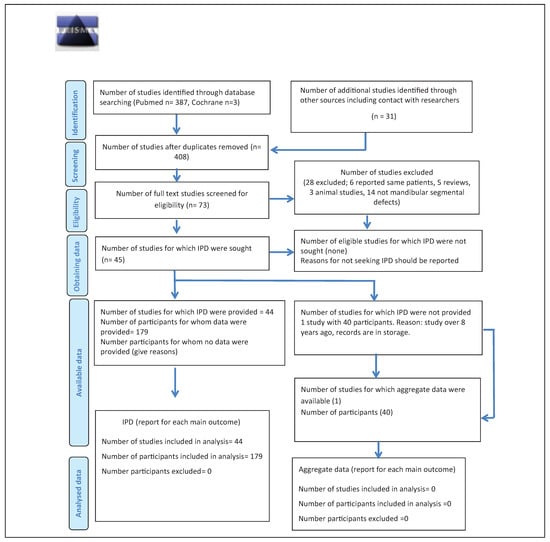
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Individual Patient Data flow diagram.
In instances where the same patient data had been reported in another publication,[12,14,22,59,60,61] the article that reported on longer term follow-up was included,[13,15,22,38] except for Sandor et al[59] and Wolff et al,[33] because the articles with shorter follow-up provided more detailed information. Several reports did not provide IPD on all parameters of interest,[18,24,32,39,56,59,62] While baseline characteristics were mostly given as IPD, the outcomes were often summarized for the whole study population included in the study.
Current Tissue Engineering Strategies
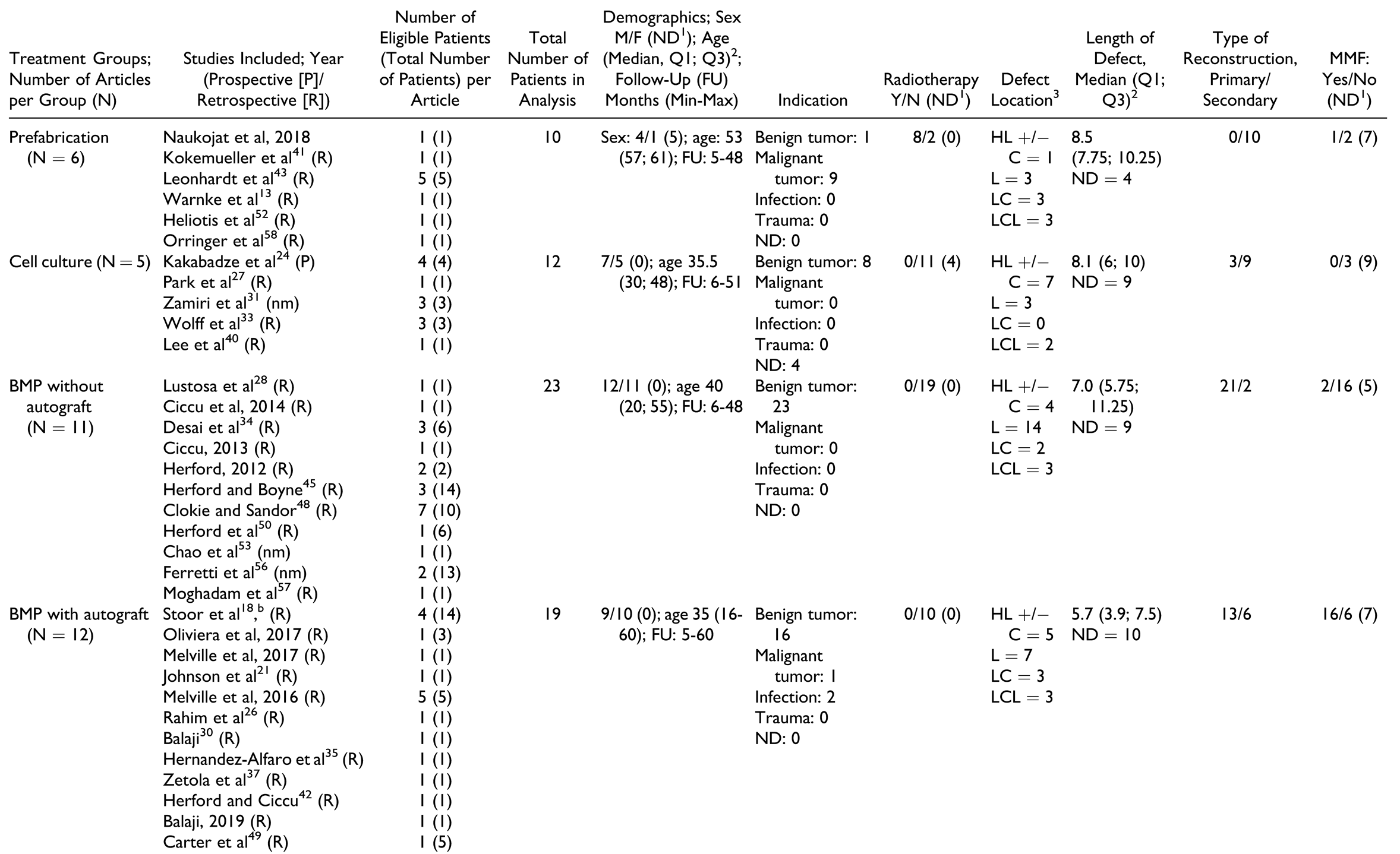
Due to the broad definition, a large number of treatment options qualified as “tissue engineering.” As described above, these treatment strategies were classified into 5 major treatment groups: prefabrication, cell culture, BMP without autografts, BMP with autografts, and scaffolds containing autografts. The number of studies and patients per treatment group are described in Table 2, whereas information on study design and treatment details (“individual treatment characteristics”) along with outcome parameters is given in Table 3, Table 4, Table 5, Table 6 and Table 7.

Table 2.
Publication Details and Baseline Characteristics According to Treatment Groups.a.

Table 3.
Treatment Characteristics and Outcomes of Prefabrication Group.

Table 4.
Treatment Characteristics and Outcome Measures for Patients Treated by Cell Cultures.

Table 5.
Treatment Characteristics and Outcome Measures of Patients Treated With BMP Without Autograft.

Table 6.
Treatment Characteristics and Outcome Measures of Patients Treated by BMP With Autograft.

Table 7.
Treatment Characteristics and Outcome Measures for Treatment Group “Scaffolds Containing Autograft.”
Included Study Characteristics
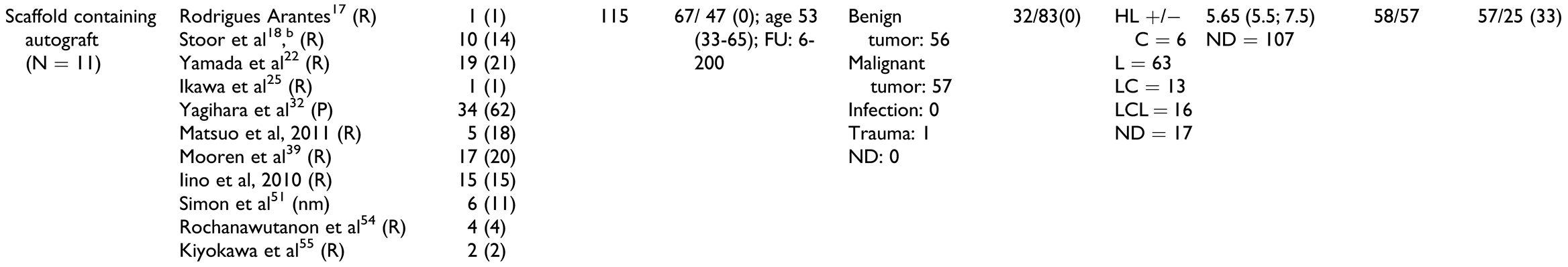
Overall and irrespective of treatment group, most studies included were case reports or case series (Table 2). Most reports were retrospective except for 2 prospective case series[24,32] and 4 studies in which the direction was either not mentioned or unclear.[31,51,53,56] The highest number of patients was in the group “scaffolds containing autografts” with 115 patients, and the smallest number of patients was in the group “prefabrication” with 10 patients. The “cell culture” group consisted of 12 patients, “BMP without autografts” had 23 patients, and “BMP with autografts” had 19 patients.
Thirteen out of the 44 articles reported on series of patients with heterogenous defect characteristics, some of them not fulfilling the eligibility criteria.[19,22,32,34,39,47,48,49,50,51,56,59,60] For these articles, individual patient data were examined and data from eligible patients were included in this review.
Baseline Patient and Defect Characteristics
Table 2 shows baseline characteristics of each treatment group. In general, patients in the “prefabrication” and the “scaffolds containing autografts” group had a higher median age compared to the other treatment modalities.
Treatment indications. The “prefabrication” group had the highest proportion of patients with malignant pathologies, followed by “scaffolds containing autografts” group, whereas the “cell culture,” “BMP with autografts,” and “BMP without autografts” groups contained mostly patients with benign tumors. Consequently, 8 of 10 patients in the “prefabrication” group and 32 of 115 patients in the “scaffolds containing autografts” group underwent therapeutic radiation.
Defect location and size. The most common defect location was the lateral aspect (body and ramus) of the mandible, but the distribution varied widely. The median defect size was similar in all treatment groups; however, IPD was provided only for 40 of 179 patients.
General Treatment Information
Timing of reconstruction. All patients in the prefabrication group and most patients in the cell culture group underwent secondary reconstruction, whereas the remaining patients underwent primary reconstruction. The use of MMF was only mentioned in a few reports with a higher proportion of use in patients treated with “BMP with autografts” and “scaffolds with autografts” compared with other treatment groups.[20,21,29,32,37,39,51,52,53]
Group-Specific Treatment Characteristics
There was a high degree of variability in the treatment strategies even within the same treatment group. The individual treatment characteristics as well as the outcome measures according to treatment group are summarized for each of the studies in Table 2, Table 3, Table 4, Table 5 and Table 6.
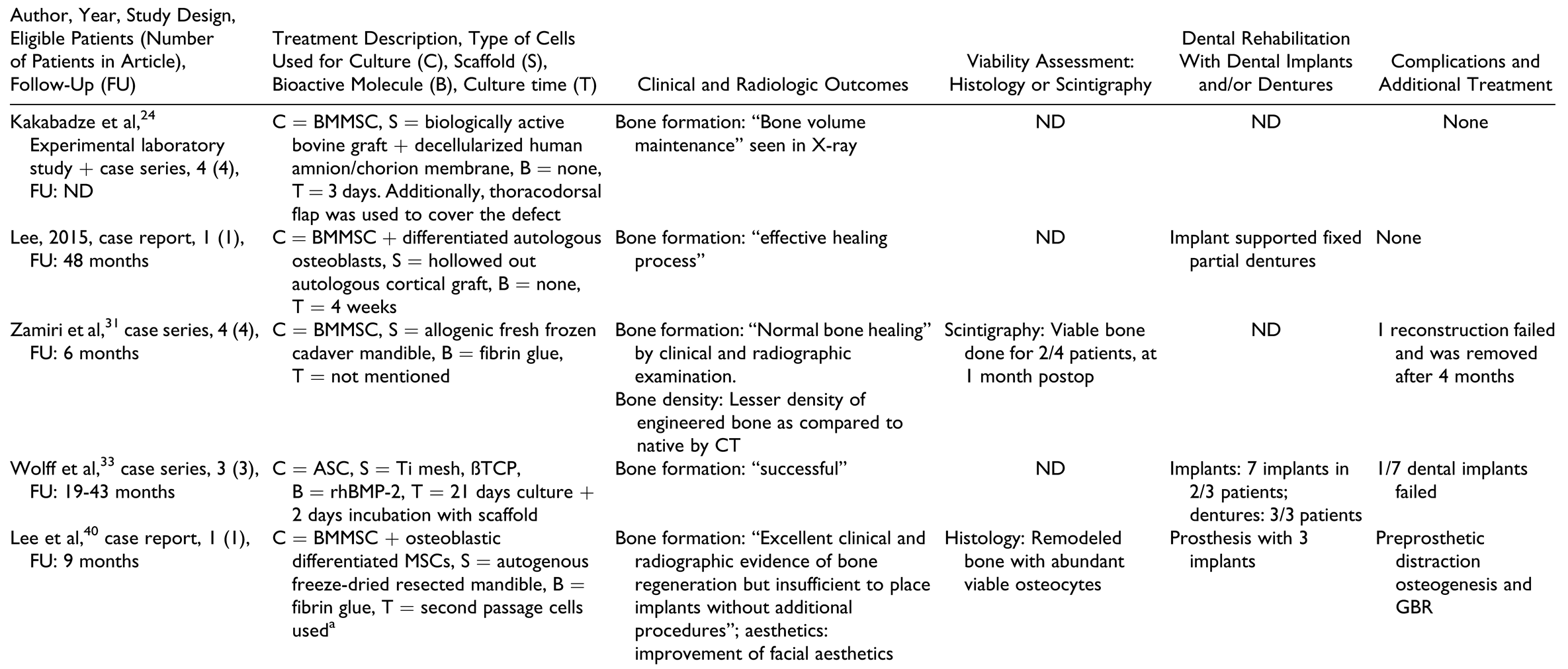
In the “prefabrication” group (Table 3), the donor area of prefabrication was gastrocolic omentum, latissimus dorsi, radial forearm, pectoralis major, and scapular fascia. Titanium mesh was the most commonly used scaffold, packed with bone substitute materials such as deproteinized bovine bone, beta tricalcium phosphate, hydroxyapatite, and/or autografts. Almost all cases treated by this method used BMP (either as BMP-2 or BMP-7). The time for prefabrication varied from 4 weeks to 6 months. All reports employed scintigraphy to check for viability of the construct before it was transplanted to the defect site; however, only 2 reports described scintigraphy as a measure to check viability of the reconstruction after flap transfer.[13,52]
In the “cell culture” group (Table 4), the most common type of cells used were mesenchymal stem cells from the bone marrow of the iliac crest and adipose tissue. The time of culture varied from 3 days to 4 weeks. The scaffolds used in this treatment group were biologically active bovine graft covered with decellularized human amnion/ chorion membrane, fresh frozen cadaver mandible, and autogenous freeze-dried resected mandible.
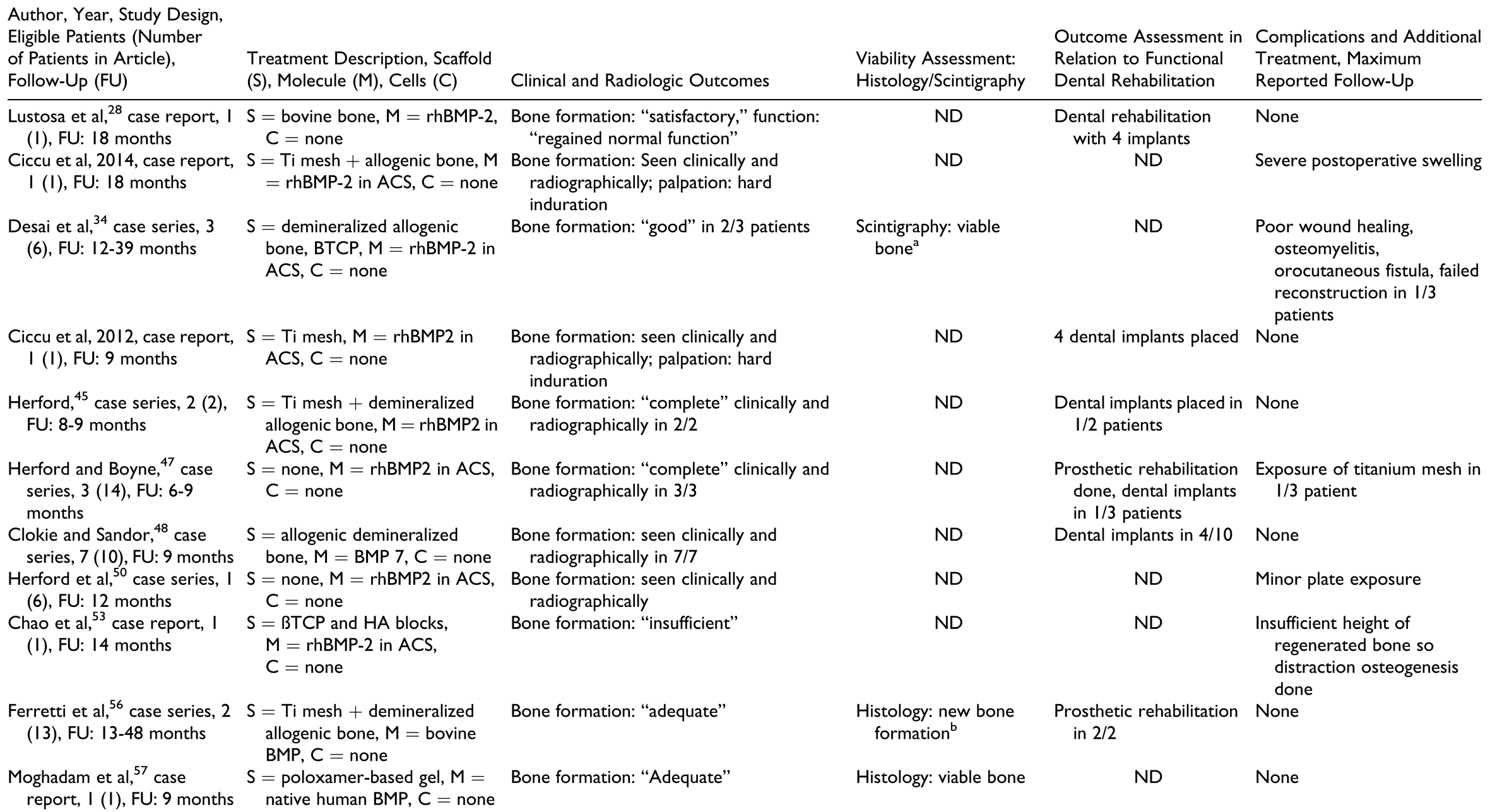
In the “BMP without autografts” group (Table 5), the scaffolds used were diverse, from bovine bone blocks, titanium mesh, and allogenic bone. The most commonly used BMP was recombinant human bone morphogenetic protein 2 (rhBMP-2). The amount of BMP used varied from 2 to 16 mg and seemed to depend on the size of the defect in most cases, although many reports did not mention the criteria for choosing a particular amount.
In the “BMP with autografts” group (Table 6), the scaffolds used were bovine bone blocks, titanium mesh, allogenic bone, resorbable polylactide mesh, and fresh frozen cadaver mandible cribs. Recombinant human bone morphogenetic protein 2 was the most commonly used BMP and the amount used seemed to vary depending upon the size of the defect, although many reports did not mention criteria for choosing a particular amount. Autografts were harvested from the costochondral region as well as from the iliac crest region and PRP was also used in some reports.
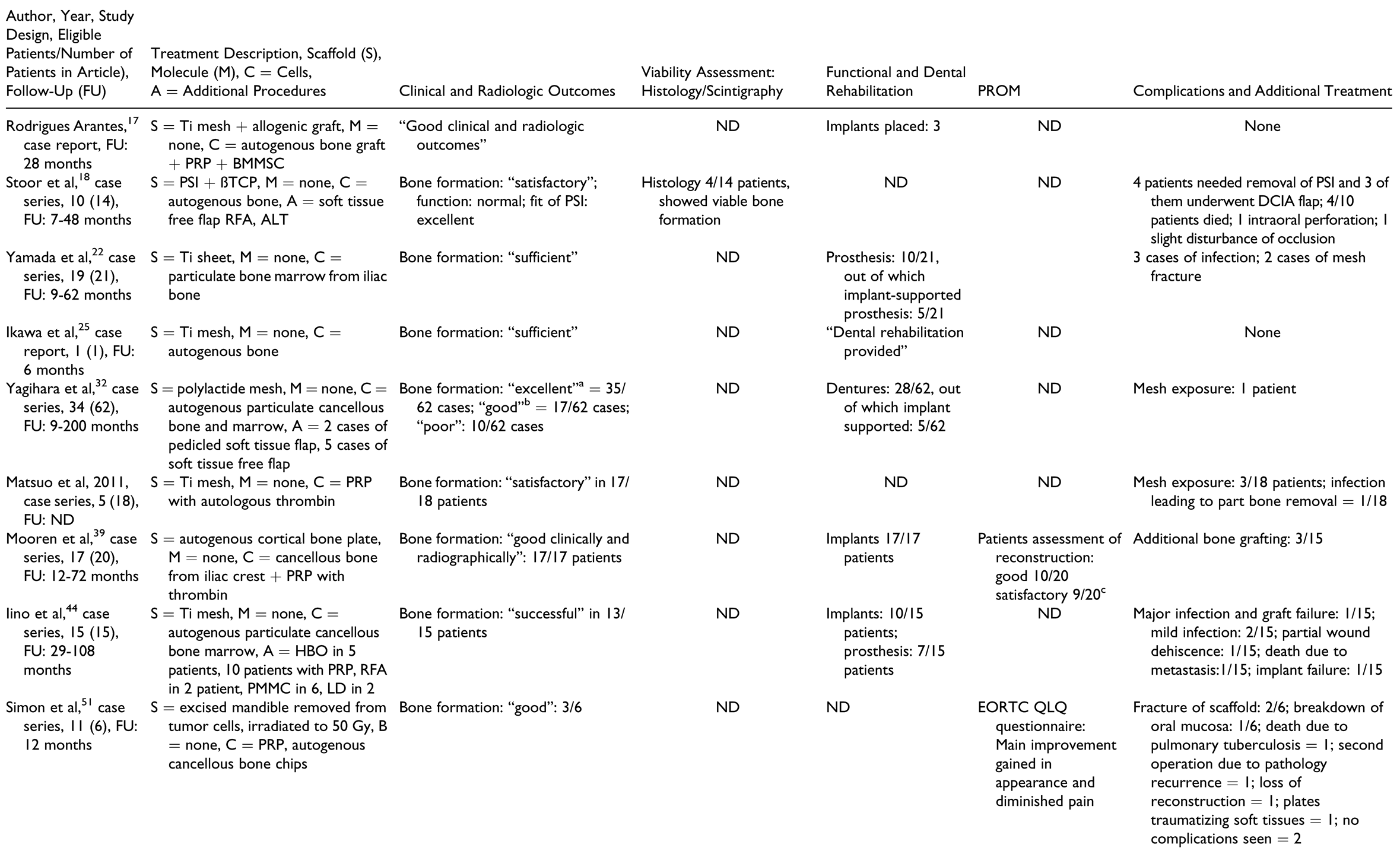
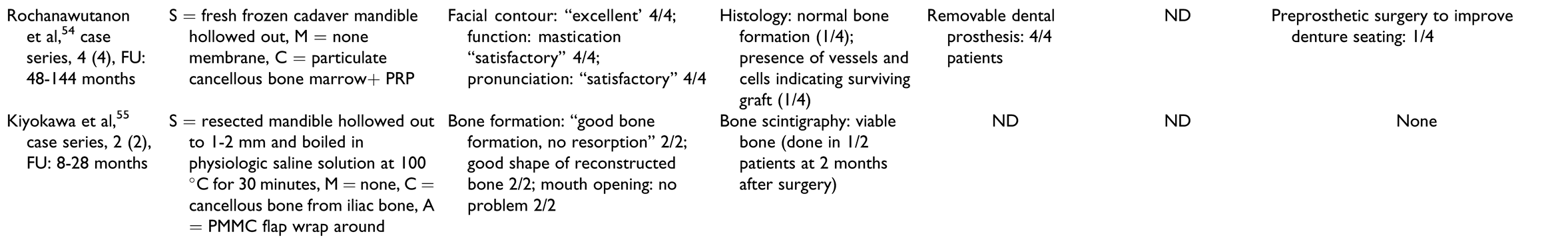
In the “scaffolds containing autografts” group (Table 7), the scaffolds used included titanium trays, either stock or patient-specific fabricated by electron beam melting technology, titanium meshes, polylactide meshes, autogenous cortical bone plates, and allogenic or autologous mandibles.
Outcomes
Outcome measures for individual patients are provided in Table 2, Table 3, Table 4, Table 5 and Table 6 for the respective treatment groups. There was not a single outcome measure that was both objective and consistently reported across all eligible studies.
Clinical and radiographic outcomes were not uniformly reported in ways that enabled comparison between groups or between patients within the same group. The most commonly reported radiologic outcome measures were “bone formation” and “bone healing,” but only one study performed a quantitative assessment.[32] Other radiologic outcome measures included “sufficient continuity of reconstructed bone” and amount of bone resorption. Clinical outcome measures included mandibular range of motion, functional outcomes such as eating, social interaction, ability to attend work, “sufficient wound healing,” bone resorption, and recurrence of pathology.
Histology and scintigraphy were additionally used to assess bone viability. Histology was reported for only 15 patients in total[15,18,19,33,35,56,57] and scintigraphy for 19 patients in total.[13,15,30,31,32,34,51,52,55] In contrast to other groups, all reports in the prefabrication group used scintigraphy to assess the vitality of the construct prior to flap harvest but not as an outcome measure of the reconstructed mandible. All reports on histology and/or scintigraphy as an outcome measure for reconstruction showed vital bone. Dental rehabilitation with dental implants and/or restoration with prostheses was reported for 2 of 10 patients in the “prefabrication” group, 4 of 12 in the “cell culture” group, 9 of 23 in the “BMP without autografts” group, 11 of 19 in the “BMP with autografts” group, and 71 of 115 in the “scaffolds containing autografts” group. There was only one report of implant failure in 1 patient.[44] Dental prosthetic rehabilitation (either with or without the use of dental implants) was successfully done in 1 of 10 patients in the “prefabrication” group, 5 of 12 patients in the “cell culture” group, 6 of 23 patients in the “BMP without autografts” group, 4 of 19 patients in the “BMP with autografts” group, and 50 of 115 patients in the “scaffold with autografts” group.
Patient-reported outcome measures with standardized questionnaires were reported in 2 of 44 studies only,[39,51] with only one of them reporting on individual patient data.[51] Complications were not reported in many studies and were not documented as objective outcomes in a structured manner. A higher number of complications, flap failures, and deaths were reported in patients from the “prefabrication” group, followed by the “scaffolds containing autografts” group. A common complication reported in the groups using BMPs was “increased swelling” during the first 2 postoperative weeks, with 1 patient requiring tracheostomy due to the severity of the swelling.[30]
Discussion
This systematic review provides a comprehensive description and categorization of the use of tissue engineering strategies for the reconstruction of segmental mandibular defects and a synthesis of the currently available evidence. Our findings show that in this field, tissue engineering strategies are extremely diverse. They ranged from the relatively simple treatment strategy of rhBMP-2 in a collagen carrier to the extremely complex use of the patient as a bioreactor to prefabricate a segment of mandible at a distant site that was then harvested and transplanted into the defect. It also included the use of autogenous iliac crest cortical bone plates filled with cancellous bone, mixed with autogenous PRP and subsequently fixed onto a titanium plate, or the use of bone grafts with titanium carriers/scaffolds. Of note, this strategy is similar to autogenous bone grafting alone, which is not regarded as tissue engineering strategy. However, the data were included in this systematic review to provide comprehensive data. This decision may be debatable and actually relies on the generally accepted definition of tissue engineering. At the moment, this definition is rather broad and makes it difficult to distinguish tissue engineering from other reconstruction techniques.
Although we were able to fit all treatment strategies into one of the 5 categories, there was little homogeneity within these groups. A wide variety of scaffolds, cell types, and bioactive substances were used. Scaffolds showed the maximum diversity: diseased mandibles,[40,51,55] cadaveric mandibles,[31,54] allogenic bone, heterogenic bone, and poly-lactide and titanium meshes/plates (either stock or patient-specific).[63] The most commonly documented bioactive substances were BMPs, whereas the most commonly used cells were autologous bone chips, bone marrow–derived mesenchymal stem cells, and PRP (which also contains bioactive substances).
Heterogeneity was also present concerning the patient populations in several studies, which reported a single technique used in patients with different indications. In combination with the aforementioned scarcity of homogenous treatment modalities, interpreting the role or the usefulness of each of the individual treatment characteristics for segmental mandibular reconstruction is difficult.
Likewise, there was heterogeneity in the reported outcome measures in spite of their importance for evaluating the effectiveness of a specific treatment strategy. The most commonly reported outcome measures were related to bone formation. Nearly all authors reported “successful outcomes,” irrespective of the treatment strategy, with “sufficient bone formation” or “positive radiographic and clinical outcomes.” However, these outcomes were hardly ever specified objectively. The lack of objective standardized outcomes precludes the valid comparison of results. Hence, we suggest caution in interpreting treatment outcomes based purely on subjective or arbitrary methods as successful.
At the same time, objective methods to evaluate segmental mandibular reconstruction are indeed scarce. Histology and scintigraphy are objective methods to assess respectively bone neogenesis and viability, but only few articles used this as an outcome measure. Successful reconstruction was reported in all instances and not a single publication mentioned cases of nonviable or unsuccessful bone regeneration. Since these results either come from case reports or from case series in which the outcome was reported for selected patients, it strongly suggests reporting bias.
The ability of reconstructed mandibles to support a dental prosthesis is an objective measure that shows that the reconstruction provides a sufficient foundation to support function. Although 97 out of the 197 eligible patients received dental implants, prosthodontic rehabilitation was reported for only 66 patients. There were no reports objectively specifying that reconstructed bone was of insufficient quality to support dental prostheses, so it is unclear what happened in the rest of the cases. Very few studies reported failure of dental implants. In interpreting these findings, one must keep in mind that dental implant placement is not required for all cases of segmental mandibular reconstruction. The use of dental implants in segmental reconstruction is well justified for adult patients but controversial prior to skeletal maturity. Additional scenarios where dental implants are not needed are patients who have defects posterior to the second molar, patients with sufficient remaining functional dentition, and patients who do not desire or cannot afford dental implant rehabilitation. These limitations should be considered when choosing dental prostheses as an outcome measure.
Patient-reported outcome measures using standardized questionnaires were reported in only 2 studies.[39,51] Even though these studies reported most patients having satisfactory treatment outcomes, the information is too sparse to generalize it.
In contrast to the rest of the outcomes, almost all studies reported specifically on the presence or absence of complications—if none were observed. There was a seemingly higher rate of complications, flap loss, and mortality in the “prefabrication” and “scaffolds with autografts” groups. However, the reason for this is likely related to the underlying disease rather than the reconstruction method. The “prefabrication” and “scaffolds with autografts” groups contained more patients with advanced recurrent diseases than the other groups and were often treated with adjuvant therapeutic radiation. Additionally, the “prefabrication” group primarily comprised patients who had recurrent malignant disease, and many of these patients died due to disease-related factors (not related to reconstruction) in the follow-up period. This is in stark contrast to most patients treated with BMP alone, who were young patients with benign tumors and in whom the resective and reconstructive therapy could be performed with preservation of the periosteum in most cases.
Strengths and Limitations
This is the first time an attempt to collate all available evidence on tissue engineering for reconstruction of segmental mandibular defect has been made. Here, we provide an overview of the current strategies with a functional categorization and a summary of the reported outcomes.
However, our study has several limitations. The level of evidence in the available publications was very low. Most of the included studies were retrospective case reports and case series; no comparative studies could be included. Moreover, several studies consisted of mixed patient populations of whom only a part was eligible for inclusion into our review. In many of these reports, the outcomes were not provided as IPD but as summary information, so it was not possible to draw any conclusions on our population of interest (segmental mandible reconstruction).[13,15,18,19,22,30,31,32,33,34,35,39,47,48,49,50,51,52,55,56,57,59] Objective or quantifiable outcome criteria were hardly ever used.[13,15,18,19,30,31,32,33,34,35,51,52,55,56,57] The low level of evidence, the variability in the patient population and in the treatments (even within the same treatment group), and the lack of objective outcome measures did not allow to perform a quantitative synthesis of results or a comparison among patients or treatment groups. The combination of selective reporting and the lack of standardized outcome reporting is an important methodological weakness seen in all studies and appears likely to have introduced bias. Finally, prognostic factors such as defect or patient characteristics could not be identified due to the scarcity of data.
Conclusion
A wide variety of tissue engineering strategies have been used for the reconstruction of segmental mandibular defects. After categorizing the most commonly used tissue engineering strategies into 5 treatment groups, a high variability of individual treatment strategies was seen. Currently, there is insufficient evidence to recommend any particular tissue engineering strategy for segmental mandibular reconstruction, given the low number of patients treated with any particular strategy and the low levels of evidence in the available literature. Future studies should report outcomes for segmental mandibular reconstruction in a more systematic, consistent, and objective manner.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by AOCMF through a Clinical Research Fellowship at AOCID granted to the first author, V.V.K.
Conflicts of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Kumar, V.V.; Jacob, P.C.; Ebenezer, S.; et al. Implant supported dental rehabilitation following segmental mandibular reconstruction—quality of life outcomes of a prospective randomized trial. J Craniomaxillofac Surg. 2016, 44, 800–810. [Google Scholar] [PubMed]
- Brown, J.S.; Lowe, D.; Kanatas, A.; Schache, A. Mandibular reconstruction with vascularised bone flaps: a systematic review over 25 years. Br J Oral Maxillofac Surg. 2017, 55, 113–126. [Google Scholar] [PubMed]
- Sacco, A.G.; Chepeha, D.B. Current status of transport-discdistraction osteogenesis for mandibular reconstruction. Lancet Oncol. 2007, 8, 323–330. [Google Scholar]
- Kontio, R. Update on mandibular reconstruction: computer-aided design, imaging, stem cells and future applications. Curr Opin Otolaryngol Head Neck Surg. 2014, 22, 307–315. [Google Scholar]
- Chanchareonsook, N.; Junker, R.; Jongpaiboonkit, L.; Jansen, J.A. Tissue-engineered mandibular bone reconstruction for continuity defects: a systematic approach to the literature. Tissue Eng Part B Rev. 2014, 20, 147–162. [Google Scholar] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science (New York, NY). 1993, 260, 920–926. [Google Scholar]
- National Institute of Biomedical Imaging and Bioengineering. Tissue engineering and regenerative medicine. 2018. Available online: https://www.nibib.nih.gov/science-education/science-topics/tissue-engineering-and-regenerative-medicine (accessed on 16 October 2019).
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009, 62, e1–e34. [Google Scholar] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009, 339, b2535. [Google Scholar] [CrossRef]
- Stewart, L.A.; Clarke, M.; Rovers, M.; et al. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015, 313, 1657–1665. [Google Scholar]
- Jewer, D.D.; Boyd, J.B.; Manktelow, R.T.; et al. Orofacial and mandibular reconstruction with the iliac crest free flap: a review of 60 cases and a new method of classification. Plast Reconstr Surg. 1989, 84, 391–403. [Google Scholar]
- Warnke, P.H.; Springer, I.N.; Wiltfang, J.; et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet (London, England). 2004, 364, 766–770. [Google Scholar] [PubMed]
- Warnke, P.H.; Wiltfang, J.; Springer, I.; et al. Man as living bioreactor: fate of an exogenously prepared customized tissue-engineered mandible. Biomaterials. 2006, 27, 3163–3167. [Google Scholar]
- Wiltfang, J.; Rohnen, M.; Egberts, J.H.; et al. Man as a living bioreactor: prefabrication of a custom vascularized bone graft in the gastrocolic omentum. Tissue Eng Part C Methods. 2016, 22, 740–746. [Google Scholar] [PubMed]
- Naujokat, H.; Acil, Y.; Gulses, A.; Birkenfeld, F.; Wiltfang, J. Man as a living bioreactor: long-term histological aspects of a mandibular replacement engineered in the patient’s own body. Int J Oral Maxillofac Surg. 2018, 47, 1481–1487. [Google Scholar]
- Marx, R.E.; Harrell, D.B. Translational research: the CD34+cell is crucial for large-volume bone regeneration from the milieu of bone marrow progenitor cells in craniomandibular reconstruction. Int J Oral Maxillofac Implants. 2014, 29, e201–e209. [Google Scholar]
- Rodrigues Arantes, E.B.; Cortezzi, W.; de Aguiar, A.B.; Merly, F.; Junior, V.M.; Netto, R. Reconstruction and mandibular rehabilitation after resection of juvenile aggressive ossifying fibroma using undifferentiated mesenchymal cells and osseointegrated implants: a case report. Implant dent. 2019, 28, 400–404. [Google Scholar] [PubMed]
- Stoor, P.; Suomalainen, A.; Mesimaki, K.; Kontio, R. Rapid prototyped patient specific guiding implants in critical mandibular reconstruction. J Craniomaxillofac Surg. 2017, 45, 63–70. [Google Scholar]
- Oliveira, M.R.; Gorla, L.F.; Gabrielli, M.A.; Pereira-Filho, V.A. Offlabel use of bone morphogenetic protein 2 in the reconstructions of mandibular continuity defects. J Craniofac Surg. 2017, 28, 227–230. [Google Scholar]
- Melville, J.C.; Nassari, N.N.; Hanna, I.A.; Shum, J.W.; Wong, M.E.; Young, S. Immediate transoral allogeneic bone grafting for large mandibular defects. Less morbidity, more bone. A paradigm in benign tumor mandibular reconstruction? J Oral Maxillofac Surg. 2017, 75, 828–838. [Google Scholar]
- Johnson, J.; Jundt, J.; Hanna, I.; Shum, J.W.; Badger, G.; Melville, J.C. Resection of an ameloblastoma in a pediatric patient and immediate reconstruction using a combination of tissue engineering and costochondral rib graft: a case report. J Am Dent Assoc. 2017, 148, 40–43. [Google Scholar]
- Yamada, H.; Nakaoka, K.; Sonoyama, T.; et al. Clinical usefulness of mandibular reconstruction using custom-made titanium mesh tray and autogenous particulate cancellous bone and marrow harvested from tibia and/or ilia. J Craniofac Surg. 2016, 27, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Melville, J.C.; Couey, M.A.; Tong, M.S.; Marx, R.E. Regeneration of a tooth in a tissue-engineered mandible after resection of a central giant cell tumor. Demonstrating evidence of functional matrix theory and ectodermal origin of teeth in a human model-a case report. J Oral Maxillofac Surg. 2017, 75, 850–857. [Google Scholar] [CrossRef]
- Kakabadze, A.; Mardaleishvili, K.; Loladze, G.; et al. Reconstruction of mandibular defects with autogenous bone and decellularized bovine bone grafts with freeze-dried bone marrow stem cell paracrine factors. Oncol Lett. 2017, 13, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, T.; Shigeta, Y.; Hirabayashi, R.; et al. Computer assisted mandibular reconstruction using a custom-made titan mesh tray and removable denture based on the top-down treatment technique. J Prosthodont Res. 2016, 60, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Rahim, I.; Salt, S.; Heliotis, M. Successful long-term mandibular reconstruction and rehabilitation using non-vascularised autologous bone graft and recombinant human BMP-7 with subsequent endosseous implant in a patient with bisphosphonate-related osteonecrosis of the jaw. Br J Oral Maxillofac Surg. 2015, 53, 870–874. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, B.C.; Kim, B.H.; Lee, J.I.; Lee, J. Up-and-coming mandibular reconstruction technique with autologous human bone marrow stem cells and iliac bone graft in patients with large bony defect. J Craniofac Surg. 2015, 26, e718–e720. [Google Scholar] [CrossRef]
- Lustosa, R.M.; Macedo Dde, V.; Iwaki, L.C.; et al. Continuity resection of the mandible after ameloblastoma—feasibility of oral rehabilitation with rhBMP-2 associated to bovine xenograft followed by implant installation. J Craniomaxillofac Surg. 2015, 43, 1553–1560. [Google Scholar] [CrossRef]
- Cicciu, M.; Herford, A.S.; Cicciu, D.; Tandon, R.; Maiorana, C. Recombinant human bone morphogenetic protein-2 promote and stabilize hard and soft tissue healing for large mandibular new bone reconstruction defects. J Craniofac Surg. 2014, 25, 860–862. [Google Scholar] [CrossRef]
- Balaji, S.M. Protein-signaled guided total jaw regeneration in infantile total mandibular resection. Ann Maxillofac Surg. 2014, 4, 198–200. [Google Scholar] [CrossRef]
- Zamiri, B.; Shahidi, S.; Eslaminejad, M.B.; et al. Reconstruction of human mandibular continuity defects with allogenic scaffold and autologous marrow mesenchymal stem cells. J Craniofac Surg. 2013, 24, 1292–1297. [Google Scholar] [CrossRef]
- Yagihara, K.; Okabe, S.; Ishii, J.; et al. Mandibular reconstruction using a poly(L-lactide) mesh combined with autogenous particulate cancellous bone and marrow: a prospective clinical study. Int J Oral Maxillofac Surg. 2013, 42, 962–969. [Google Scholar]
- Wolff, J.; Sandor, G.K.; Miettinen, A.; et al. GMP-level adipose stem cells combined with computer-aided manufacturing to reconstruct mandibular ameloblastoma resection defects: Experience with three cases. Ann Maxillofac Surg. 2013, 3, 114–125. [Google Scholar]
- Desai, S.C.; Sclaroff, A.; Nussenbaum, B. Use of recombinant human bone morphogenetic protein 2 for mandible reconstruction. JAMA Facial Plast Surg. 2013, 15, 204–209. [Google Scholar]
- Hernandez-Alfaro, F.; Ruiz-Magaz, V.; Chatakun, P.; Guijarro-Martinez, R. Mandibular reconstruction with tissue engineering in multiple recurrent ameloblastoma. Int J Periodontics Restorative Dent. 2012, 32, e82–e86. [Google Scholar] [PubMed]
- Cicciu, M.; Herford, A.S.; Stoffella, E.; Cervino, G.; Cicciu, D. Protein-signaled guided bone regeneration using titanium mesh and Rh-BMP2 in oral surgery: a case report involving left mandibular reconstruction after tumor resection. Open Dent J 2012, 6, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Zetola, A.; Ferreira, F.M.; Larson, R.; Shibli, J.A. Recombinant human bone morphogenetic protein-2 (rhBMP-2) in the treatment of mandibular sequelae after tumor resection. Oral Maxillofac Surg. 2011, 15, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, A.; Chiba, H.; Toyoda, J.; et al. Mandibular reconstruction using a tray with particulate cancellous bone and marrow and platelet-rich plasma by an intraoral approach. J Oral Maxillofac Surg. 2011, 69, 1807–1814. [Google Scholar]
- Mooren, R.E.; Merkx, M.A.; Kessler, P.A.; Jansen, J.A.; Stoelinga, P.J. Reconstruction of the mandible using preshaped 2.3-mm titanium plates, autogenous cortical bone plates, particulate cancellous bone, and platelet-rich plasma: a retrospective analysis of 20 patients. J Oral Maxillofac Surg. 2010, 68, 2459–2467. [Google Scholar]
- Lee, J.; Sung, H.M.; Jang, J.D.; Park, Y.W.; Min, S.K.; Kim, E.C. Successful reconstruction of 15-cm segmental defects by bone marrow stem cells and resected autogenous bone graft in central hemangioma. J Oral Maxillofac Surg. 2010, 68, 188–194. [Google Scholar]
- Kokemueller, H.; Spalthoff, S.; Nolff, M.; et al. Prefabrication of vascularized bioartificial bone grafts in vivo for segmental mandibular reconstruction: experimental pilot study in sheep and first clinical application. Int J Oral Maxillofac Surg. 2010, 39, 379–387. [Google Scholar]
- Herford, A.S.; Cicciu, M. Recombinant human bone morphogenetic protein type 2 jaw reconstruction in patients affected by giant cell tumor. J Craniofac Surg. 2010, 21, 1970–1975. [Google Scholar]
- Leonhardt, H.; Pradel, W.; Mai, R.; Markwardt, J.; Lauer, G. Prefabricated bony radial forearm flap for secondary mandible reconstruction after radiochemotherapy. Head Neck. 2009, 31, 1579–1587. [Google Scholar]
- Iino, M.; Fukuda, M.; Nagai, H.; et al. Evaluation of 15 mandibular reconstructions with Dumbach Titan Mesh-System and particulate cancellous bone and marrow harvested from bilateral posterior ilia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009, 107, e1–e8. [Google Scholar] [PubMed]
- Herford, A.S. rhBMP-2 as an option for reconstructing mandibular continuity defects. J Oral Maxillofac Surg. 2009, 67, 2679–2684. [Google Scholar]
- Balaji, S.M. Mandibular cystic defect: a composite approach with rhBMP-2 and rib graft. J Maxillofac Oral Surg. 2009, 8, 27–30. [Google Scholar] [PubMed]
- Herford, A.S.; Boyne, P.J. Reconstruction of mandibular continuity defects with bone morphogenetic protein-2 (rhBMP-2). J Oral Maxillofac Surg. 2008, 66, 616–624. [Google Scholar] [PubMed]
- Clokie, C.M.; Sandor, G.K. Reconstruction of 10 major mandibular defects using bioimplants containing BMP-7. J Can Dent Assoc. 2008, 74, 67–72. [Google Scholar]
- Carter, T.G.; Brar, P.S.; Tolas, A.; Beirne, O.R. Off-label use of recombinant human bone morphogenetic protein-2 (rhBMP-2) for reconstruction of mandibular bone defects in humans. J Oral Maxillofac Surg. 2008, 66, 1417–1425. [Google Scholar]
- Herford, A.S.; Boyne, P.J.; Williams, R.P. Clinical applications of rhBMP-2 in maxillofacial surgery. J Calif Dent Assoc. 2007, 35, 335–341. [Google Scholar]
- Simon, E.N.; Merkx, M.A.; Shubi, F.M.; Kalyanyama, B.M.; Stoelinga, P.J. Reconstruction of the mandible after ablative surgery for the treatment of aggressive, benign odontogenic tumours in Tanzania: a preliminary study. Int J Oral Maxillofac Surg. 2006, 35, 421–426. [Google Scholar]
- Heliotis, M.; Lavery, K.M.; Ripamonti, U.; Tsiridis, E.; di Silvio, L. Transformation of a prefabricated hydroxyapatite/osteogenic protein-1 implant into a vascularised pedicled bone flap in the human chest. Int J Oral Maxillofac Surg. 2006, 35, 265–269. [Google Scholar]
- Chao, M.; Donovan, T.; Sotelo, C.; Carstens, M.H. In situ osteo-genesis of hemimandible with rhBMP-2 in a 9-year-old boy: osteoinduction via stem cell concentration. J Craniofac Surg. 2006, 17, 405–412. [Google Scholar] [CrossRef]
- Rochanawutanon, S.; Suddhasthira, T.; Pairuchvej, V.; Vajaradul, Y. Long term follow-up of reconstruction with allogeneic mandibular bone crib packed with autogenous particulate cancellous bone marrow. Cell Tissue Bank. 2002, 3, 183–197. [Google Scholar] [PubMed]
- Kiyokawa, K.; Tai, Y.; Tanaka, S.; Inoue, Y. A new regenerative approach to oromandibular reconstruction after the resection of head and neck malignant tumor. J Craniofac Surg. 2002, 13, 337–346. [Google Scholar] [PubMed]
- Ferretti, C.; Ripamonti, U. Human segmental mandibular defects treated with naturally derived bone morphogenetic proteins. J Craniofac Surg. 2002, 13, 434–444. [Google Scholar] [PubMed]
- Moghadam, H.G.; Urist, M.R.; Sandor, G.K.; Clokie, C.M. Successful mandibular reconstruction using a BMP bioimplant. J Craniofac Surg. 2001, 12, 119–127. [Google Scholar]
- Orringer, J.S.; Shaw, W.W.; Borud, L.J.; Freymiller, E.G.; Wang, S.A.; Markowitz, B.L. Total mandibular and lower lip reconstruction with a prefabricated osteocutaneous free flap. Plast Reconstr Surg. 1999, 104, 793–797. [Google Scholar]
- Sandor, G.K.; Numminen, J.; Wolff, J.; et al. Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Transl Med. 2014, 3, 530–540. [Google Scholar]
- Matsuo, A.; Chiba, H.; Takahashi, H.; Toyoda, J.; Hasegawa, O.; Hojo, S. Bone quality of mandibles reconstructed with particulate cellular bone and marrow, and platelet-rich plasma. J Craniomaxillofac Surg. 2011, 39, 628–632. [Google Scholar] [CrossRef]
- Sandor, G.K.; Tuovinen, V.J.; Wolff, J.; et al. Adipose stem cell tissue-engineered construct used to treat large anterior mandibular defect: a case report and review of the clinical application of good manufacturing practice-level adipose stem cells for bone regeneration. Int J Oral Maxillofac Surg. 2013, 71, 938–950. [Google Scholar]
- Yamada, H.; Nakaoka, K.; Horiuchi, T.; et al. Mandibular reconstruction using custom-made titanium mesh tray and particulate cancellous bone and marrow harvested from bilateral posterior ilia. J Plast Surg Hand Surg. 2014, 48, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Thor, A.; Palmquist, A.; Hirsch, J.M.; Rannar, L.E.; Derand, P. Omar Clinical, morphological, and molecular evaluations of bone regeneration with an additive manufactured osteosynthesis plate. J Craniofac Surg. 2016, 27, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. The Authors 2020.