Introduction
Correction of post-traumatic orbital defects remains a challenge for the maxillofacial surgeon. Functional and aesthetic outcome with respect to correct positioning and projection of the globe depends highly on the symmetry of the orbital shape and volume. Therefore, the goal of treatment is to restore a normal orbital volume and shape by anatomically reducing fracture fragments and placing a supporting structure on the defect orbital floor and/or wall [
1,
2,
3,
4,
5].
Indications for surgical treatment of a traumatic orbital deformity include (1) double vision caused by an incarceration of orbital muscle or the fine ligament system, documented by forced duction examination and suggested by multislice computed tomography (MSCT) or cone beam computed tomography (CBCT) scans; (2) radiographic evidence of an extensive fracture, which indicates a high probability of enophthalmos; (3) enophthalmos or exophthalmos produced by an orbital volume change; and/or (4) a fractured area larger than 2 cm
2 or more than 50% of the entire orbital floor area [
2,
3,
4,
5].
Adequate functional and aesthetic orbital repair, however, often remains a challenge even to the experienced surgeon due to limited visualization. The narrow surgical field makes proper implant position and placement difficult. This difficulty is increased by individual variability of the complex conical orbital structure. All these factors contribute to an often unpredictable result of orbital reconstruction. Failure to adequately restore orbital volume may lead to residual ex- or enophthalmos and/or diplopia. An increase of 1 cm
3 in orbital volume can result in a 1-mm enophthalmos on average, while an increment of 2 cm
3 or more can give rise to significant functional and aesthetic consequences. In a later stage, enophthalmos can be caused by periorbital fat atrophy, fibrosis, or loss of periorbital support [
1,
3,
4,
5,
6,
7,
8].
In the past years, developments in surgical planning, techniques, and technology have greatly improved surgical outcomes today. Nevertheless, implant positioning can still be subject to human error. Intraoperative (IO) navigation has known a rapid evolution as a viable tool in assisting the surgeon in the planning and reconstruction of the orbit.[
1,
9] More recently, IO-CBCT offers additional evaluation of the accuracy of bony orbital reconstruction during surgery [
10].
The purpose of this study was to retrospectively evaluate whether the combination of IO navigation-guided orbital reconstruction and IO-CBCT has the potential to re-establish orbital volume, and associated function and aesthetics, in a more predictable manner in patients with post-traumatic orbital defects. Moreover, we aimed to suggest an optimal workflow for use in orbital trauma reconstruction.
Materials and Methods
Study Design and Sample
A retrospective cohort study was performed after approval from the Hospital Institutional Review Board (B049201731057).
The records of all consecutive patients who presented at the Division of Maxillofacial Surgery for orbital surgery between January 2012 and December 2018 were reviewed. Only data from patients who underwent primary reconstruction for unilateral orbital deformities larger than 2 cm2 secondary to traumatic injury were included for analysis. Besides the pure blowout fractures, fractures of the zygoma maxillary complex were also included when there was no displacement of the zygoma and only an orbital deformity was diagnosed. Patients were excluded from the study when treated conservatively, when presenting with more extensive defects involving larger areas of the maxillofacial skeleton, when no supporting plate was placed on the orbital floor, and when there was no post-operative MSCT or CBCT available.
The included patients were divided into 3 groups, namely (1) patients for whom IO navigation with Brainlab Iplan software (Brainlab, Munich, Germany) was used, (2) patients where only IO-CBCT (Siemens, Berlin, Germany) was used, and (3) patients where both IO navigation and IO-CBCT were combined.
Outcome Variables
The absolute difference in volume between the operated and the contralateral orbit (cm3) was defined as the primary end point. The contralateral orbit served as a control for the operated orbit. This comparison was made rather than one of the postoperative and preoperative volumes of the affected orbit, since this would not take into account the normal orbital volume. Age and gender were examined as potential confounding variables.
Surgical Procedure
All of the orbital reconstructions were performed by a staff member surgeon, assisted by a resident surgeon. Five staff member surgeons were involved in the included reconstructions. After preoperative clinical examination and radiographic evaluation, a surgical correction was planned, if necessary. If prominent swelling hindered adequate examination, the patient was re-evaluated 5 days post-operatively when swelling had desisted.
If IO navigation was used, a CBCT scan (see below) was performed prior to surgery for presurgical planning and IO navigation [
11].
One single-shot intravenous antibiotics (2 g amoxicillin clavulanic acid or 600 mg clindamycin) prophylaxis was administered preoperatively. The orbital defect was visualized using a transconjunctival approach (inferior fornix transconjunctival using a retroseptal or preseptal route) with or without a transcaruncular medial extension, more rarely by a subtarsal approach or by the use of traumatic lacerations in the region which allowed for visualization of the orbital defect, depending on the preference of the operating surgeon [
12].
In all included cases, a commercially available preformed orbital matrix reconstruction plate was used (Synthes Maxillofacial, West Chester, Philadelphia). A large or small variant of the reconstruction plate was used depending on the size of the defect, as determined by preoperative imaging and IO evaluation.
If IO-CBCT was performed, the surgical field was first covered with a sterile drape. After correct positioning of the device, the scan procedure was performed. The scan was then evaluated using the Brainlab module in the theater as described subsequently.
Clinical evaluation was performed at 1, 2, and 6 weeks postoperatively, considering adequate functional and aesthetic recovery. More specifically, enophthalmos and exophthalmos were systematically registered in any case of suspected trauma to the orbital region. At first evaluation, this is a clinical appreciation of the orbital position. There was no quantification of any discrepancy in this position using Hertel exophthalmometers or a similar device. However, the clinical position of the eyebulb was always described in detail at preoperative and subsequent postoperative evaluations which allowed for registration of possible improvement or deterioration of this clinical factor. Furthermore, any limitation of eye movement and infraorbital hypoesthesia was registered.
Planning and IO Navigation with Brainlab Iplan Software
Surgical IO navigation was performed using the validated procedure described by Boeckx et al.[
11] using a modified wax-bite dental splint for registration. After adjustment of the grayscale window and bony segmentation, the healthy orbit was mirrored to the affected orbit. A stereolithography file of the commercially available preformed orbital matrix reconstruction plate (Synthes Maxillofacial) was then imported into the Brainlab planning module. This allowed for virtual placement of the plate with respect to the normal individual anatomy, provided by the mirrored healthy orbit. The planning was then transferred to the operating theater where the Brainlab navigation tool allowed for verification and comparison of the actual plate position to the planned position [
6,
8,
11,
13].
Intraoperative CBCT
Intraoperative-CBCT scanning to evaluate adequate plate positioning after orbital defect reconstruction was performed with a mobile CBCT apparatus (Siemens). After scanning, the Digital Imaging and Communications in Medicine files were immediately transferred to the fully integrated Brainlab module in the theater allowing for immediate 3-dimensional (3-D) evaluation of plate and position with consecutive surgical adaptation if necessary [
10,
14,
15].
Measurement of the Reconstructed Orbit
First, the fractured area was calculated on the preoperative CBCT by measuring the maximal extent of the fracture on sagittal and coronal slices. Only patients with affected areas larger than 2 cm2 were taken into account for further evaluation.
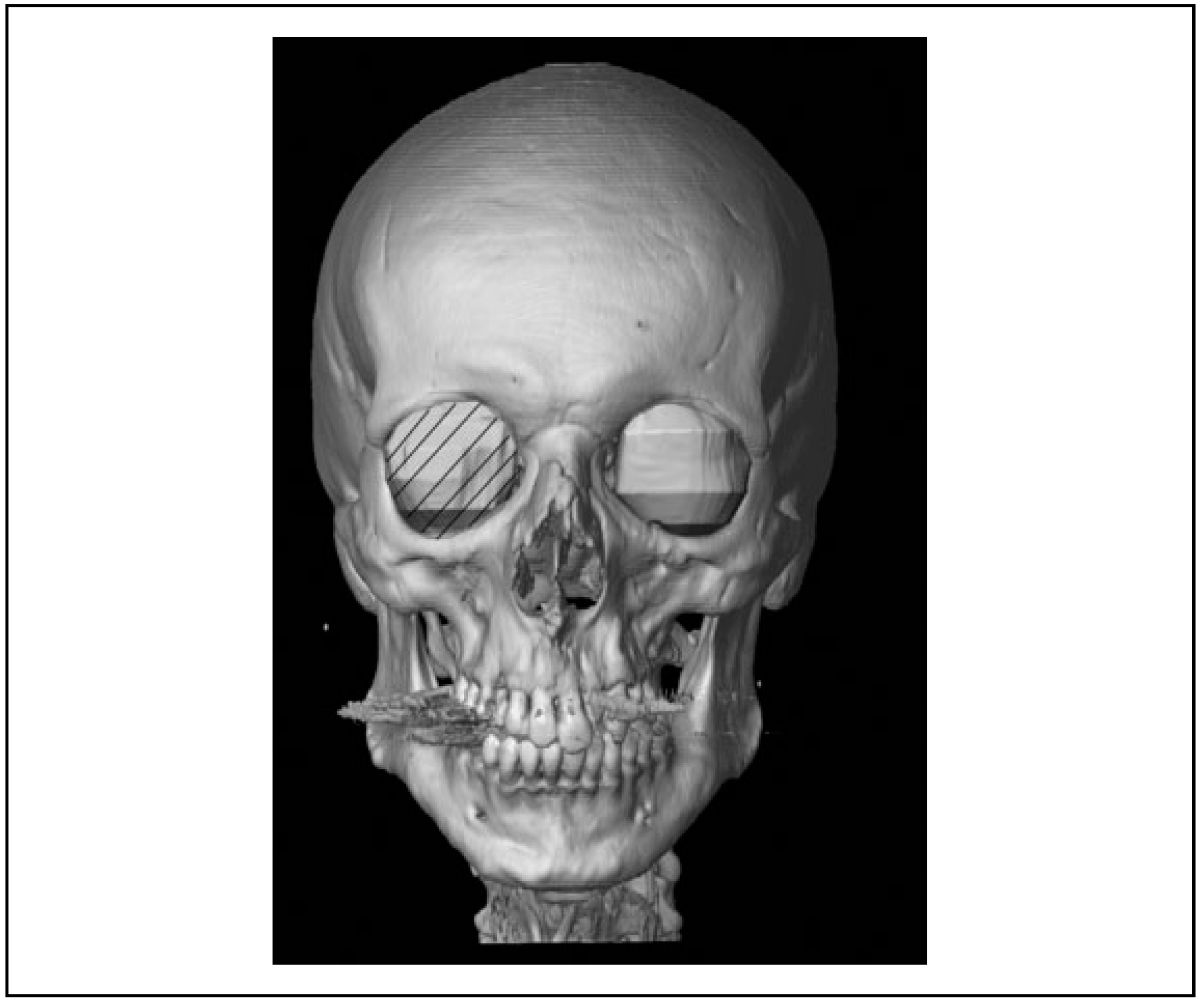
Volume measurement of the unaffected, the fractured, and the reconstructed orbit was obtained by segmentation of the orbital volume with Brainlab Iplan software (
Figure 1). The CT data sets were collected and imported, and an initial evaluation of its quality was performed, taking note of the patient’s anatomy [
15].
The segmentation procedure was based on the validated procedure described by Jansen et al.[
7] as the semiautomatic segmentation with manual adjustments. This procedure consists of an automatic method with subtraction of bone and air mask and a manual adjustment for errors using built-in functionalities in the software. First, the position of the scan was optimized so that the skull was in a true horizontal position. The sagittal plane was used to evaluate and mark the inferior orbital fissure by following the inferior rectus muscle and to define the apical limit. Finally, the axial slices were used to delineate the lateral and medial borders.
Data Collection, Management, and Statistical Analyses
All data were collected from the medical charts by the resident (B.D.C) and registered in an MS Office Excel file.
Descriptive statistics were computed for each group. A paired sample t test was applied to compare pre- and post- operative orbital volume within each group. A one-way analysis of variance test and post hoc Tukey test were performed for statistical comparison of the mean orbital volume difference between the 3 independent groups. Pearson correlation was used to evaluate the correlation between mean orbital volume difference and gender and age, respectively. Finally, operating times were evaluated using Kruskal-Wallis test.
Data management and statistical analysis were performed using IBM SPSS statistics for Windows, version 20.0 (IBM Corporation, Armonk, New York). Probabilities of less than 0.05 were accepted as significant.
Results
In the period from January 2012 to December 2018, 81 cases with a unilateral post-traumatic orbital defect were treated at the aforementioned department. Of the initial 81 cases, 20 were excluded after screening of the medical file since these cases confined more extensive trauma or the defect did not involve the orbital floor, 28 were excluded because neither IO navigation nor IO-CBCT was performed, and 11 were excluded since there was no suitable postoperative CBCT for adequate segmentation of the orbital volume. The remaining cases were then divided into 3 mentioned groups (ie, IO navigation only [NAV], IO-CBCT only [CBCT], and both IO navigation and IO-CBCT [NAV-CBCT]).
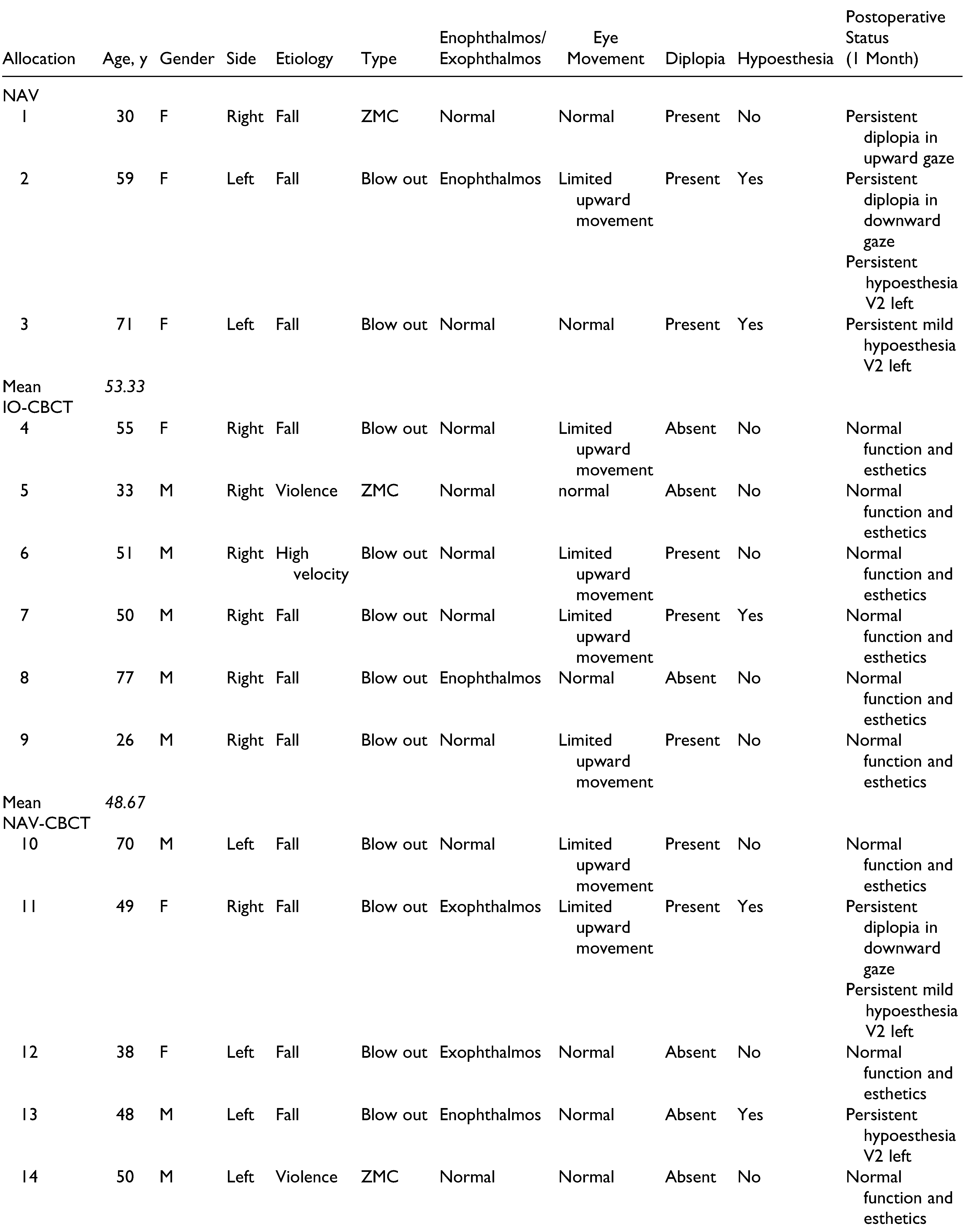
The mean age of all included patients was 51 (26–77) years. The predominant cause of trauma for all groups was a fall (72.7%), followed by violence (18.2%). Preoperative enophthalmos was seen in 5 patients, and exophthalmos was noted in 2 patients. Nine patients presented themselves with limited eye movement and 10 with diplopia (
Table 1). The mean fractured area for all cases was 4.55 cm
2 (2.09–7.29;
Table 2).
Intraobserver agreement of volumetric difference measurement was determined by calculating the intraclass correlation coefficient (ICC) based on a second segmentation of 5 randomly selected patients, 2 weeks after initial segmentation. An ICC value of 0.939 was calculated. Therefore, the segmentation procedure seems to be reliable, and risk of variation caused by measurement error is minimal.
As for the volumetric difference, no significant difference between the contralateral and the reconstructed orbit could be determined within the 3 groups (
p > 0.05). The mean difference between the contralateral and the operated orbit differed significantly between the 3 groups with a value of 3.05 (standard deviation [SD] 2.25) cm
3 for NAV, 3.72 (SD 2.41) cm
3 for CBCT, and 1.47 (SD 1.29) cm
3 for NAV-CBCT (
Table 3).
When comparing the 3 groups for volumetric difference between the contralateral and operated orbit, a significant difference between the CBCT group and the NAV-CBCT group could be determined (p = 0.046). The latter giving a significantly smaller volumetric difference between the 2 orbits. The difference between the CBCT group and the NAV group showed no statistical trend toward significant lower orbital volumetric difference favoring one of both groups. Finally, no statistical difference was observed when comparing the NAV group and the NAV-CBCT group. Gender or age did not correlate with difference in orbital volume (p > 0.05).
The operating time was retrieved for each procedure and did not differ significantly between the 3 groups with a value of 107 (SD 49.7) minutes for NAV, 120 (SD 62.5) minutes for CBCT, and 91 (26.7) minutes for NAV-CBCT (
Table 2).
Persistent diplopia was registered in 2 of the 3 included patients for the NAV group, 6 weeks postoperatively. Also, a mild hypoesthesia of the infraorbital nerve was seen in 2 patients within this group. In the CBCT group, all patients showed normal function and satisfying aesthetics, 6 weeks postoperatively. Finally, complete restitution of former aesthetics and function was seen in 6 of 13 cases in the NAV-CBCT group, 6 weeks postoperatively. In this group, persistent diplopia was registered in 3 cases, limited eye movement in 2 cases, and infraorbital hypoesthesia in 5 cases (
Table 1).
Discussion
Approximately 20 years ago, the CBCT technique was introduced. Since then smaller devices have been developed for 3-D image data acquisition. The development of mobile CBCT devices in the operating theater has offered the option of IO radiological control, giving rise to better surgical outcomes since secondary corrections could be performed instantaneously and surgical revisions could be avoided [
14,
16].
The advantages of IO navigation in predictably restoring orbital volume have been well described in the literature. Essig et al.,[
9] using data of 94 orbital reconstructions, demonstrated that even in complex orbital fractures, true-to-original reconstruction is feasible using an individually bent titanium mesh and IO navigation. Zimmerer et al.[
13] demonstrated, in 195 patients, that orbital reconstruction is significantly more precise when individualized implants are used. The same was seen for IO navigation. When individualized implants were used in combination with IO navigation, it was difficult to attribute the registered improved precision to one of both factors. Nevertheless, the authors conclude that individualization and IO navigation provide a clear clinical benefit in orbital reconstruction. In the methodology of this multicenter trial, IO-CBCT is mentioned as a mean of acquiring postoperative CBCT images for analyses of orbital volume. There is, however, no description of a specific workflow regarding the use of IO-CBCT, nor the comparison of reconstructions performed with and without IO-CBCT. The emphasis in this study was put on the use of IO navigation and individualized titanium reconstruction plates.
Based on 15 consecutive patients with complex primary or secondary unilateral post-traumatic and postablative orbital deformities, Bell and Markiewicz[
17] described preoperative computer modeling and IO navigation as a useful guide for a more accurate reconstruction of complex orbital injuries and postablative orbital defects. Markiewicz et al.[
1] showed in a study on 23 patients who underwent IO navigation-guided orbital reconstruction that the mean difference in orbital volume and globe projection between the contralateral orbit and the operated orbit was 1.3 cm
3 and 2.4 mm, respectively. Novelli et al.[
6] reported that in most of their cases, the volume of the reconstructed orbit was equal to that of the healthy orbit, with a variation of less than 1 cm
3, when surgical navigation was used. Furthermore, Shin et al.[
8] stated that improvements in both functional and aesthetic outcomes, with prevention of injury of important anatomical structures could be obtained by the use of navigation-assisted technology in orbital surgery [
9,
13].
The aim of this study was to determine whether additional imaging by IO-CBCT during surgery with IO navigation could assist in a more predictable restoration of the orbital volume in unilateral post-traumatic patients. The workflow for combination of IO navigation and a final control using IO-CBCT 3-D imaging has been described by Wilde and Schramm.[
10] Also, Bitterman et al.[
16] mentions IO imaging based on IO-CBCT for quality control when computer-assisted surgery is used. However, the advantage of this combination was not substantiated by data gathering and statistical analysis.
Herford et al. (2017) discusses a case report of secondary reconstruction of a complex orbital fracture, using IO navigation in combination with a polyetheretherketone patient-specific implant. They suggest that for complex reconstruction, the use of individualized implants may be warranted as a beneficial tool to approach the initial anatomical configuration. The IO navigation has proved to increase precision and accuracy of implant placement, rendering this a favorable combination for more complex cases, such as secondary reconstructions [
18].
The current institutional protocol entails the combination of both IO navigation and IO-CBCT as a final control of an accurate plate position. Based on our experience, it is believed that this combination gives a more predictable outcome and provides the surgeon with sufficient information concerning plate positioning, rendering secondary procedures for correction of this position redundant. This protocol consists of a 5-stage workflow, which is described in detail in
Figure 2.
The cost linked to these additional evaluation methods should also be taken into account when considering the application of IO navigation and/or IO-CBCT. The acquisition of the necessary hardware (IO-CBCT apparatus, IO navigation module) and software (IO navigation) demands a financial effort. Considering the fact that application of these techniques could avoid a second procedure for adjustment of an initial reconstruction, these costs might be deemed reasonable. There is, however, no additional cost toward the patient since the modified wax bite is created in house and requires little manufacturing time (approximately 10 min) and relatively inexpensive materials as described by Boeckx et al. [
11].
To our knowledge, this is the first study which not only considers the well-known advantages of IO navigation but also attempts an objectivation of the potential advantage of combining IO navigation with IO-CBCT.
The results of this study showed that the combination of both the latter techniques resulted in the smallest volumetric difference when comparing the contralateral to the operated orbit. However, statistical analyses did not show a significant difference that could support evidence of the potential clinical added value of IO-CBCT when IO navigation is used.
In the NAV CBCT group, there was a need for one revision after evaluation of the IO-CBCT on the Brainlab module. More specifically, there seemed to be a craniocaudal shift in the apical part of the reconstruction plate, which was not assessable clinically and did not give rise to major discrepancies when checking the position by means of IO navigation. This demonstrates nicely the added value a final check by means of IO-CBCT can have, even when using IO navigation.
The impact of IO navigation and/or CBCT on the operating time was evaluated and implied that combination of both techniques did not lead to a considerable extension of procedural time. On the contrary, when revising mean operating times, NAV-CBCT seems to be faster than NAV or CBCT alone. This could, however, not be confirmed in a statistical significant manner. The current protocol takes into account an additional 6 min for performance and evaluation of an IO-CBCT and 10 min for installing and registering the IO navigation module. It can be stated that once a systematic workflow for use of these techniques is in place, the possible benefits gained warrant the additional operating time. Furthermore, in difficult cases with a large fractured area and very little anatomical reference, these additional techniques can reduce total operating time since the surgeon is guided toward correct implant placement, rendering secondary adjustments scarce.
Moreover, the results of our study should be interpreted with caution due to some study limitations. First, although the segmentation procedure used for measurement of the orbital volume has been validated, it seems fair to state that the determination of the contour of the volume is prone to error given the human component in this workflow. However, intraobserver agreement was high, indicating that the performed segmentations and orbital volume measurements are reliable.
Second, the number of patients who could be included in our analyses is relatively limited. Patients were included in the respective groups, based on the workflow that was implemented, which was mostly dependent on the availability of the IO-CBCT apparatus and IO navigation module and the surgeon’s preference. Although the difference in patients per group in our current sample might give power issues for statistical analyses, our volumetric measurement results are indicative for favoring the combination of IO navigation and IO-CBCT, however, this was only proved in a statistical significant way in comparison to IO-CBCT alone, not IO navigation alone. However, considering the good postoperative functional and aesthetic outcome, IO-CBCT as an additional check-up might reduce postoperative complaints such as persistent diplopia or hypoesthesia, reducing necessity of reoperation.
Third, the retrospective design does not enable long-term clinical and radiologic data regarding late-appearing alterations such as late enophthalmos or visual defects. Moreover, a control group where neither IO-CBCT nor IO navigation is used would also provide more information regarding the added value of these technologies. The latter only being possible if standard postoperative CBCT is available.