Orbital floor fractures are a common sequela of blunt injury to the face and are considered an evolutionary safety mechanism to prevent injury to the globe.[
1,
2] The mechanism of this injury is thought to be either from buckling of the orbital floor itself or secondary to a hydraulic effect from an acute increase in intraorbital pressure.[
3]
In the adult population, orbital floor fractures result in volume expansion of the orbit, with muscle and fat prolapse into the maxillary sinus. Decision to repair is based on the presence of symptoms and findings such as diplopia, enophthalmos, hypoglobus, infraorbital dysthesia, or proptosis, as well as the size of the floor fracture.[
4] Although surgical criteria are variable, close observation is a generally accepted principle for asymptomatic patients presenting with defects less than 50% of the orbital floor surface. Orbital floor repair is recommended for patients presenting with either muscle entrapment or with fractures greater than 50% of the orbital floor surface area, as the majority of these patients will develop enophthalmos, hypoglobus, and diplopia as edema decreases.[
5]
Reconstruction of the orbit requires meticulous preoperative planning, careful dissection and surgical technique, as well as a detailed analysis of implant selection, sizing, and contour. However, there is controversy regarding appropriate selection of implanted materials.[
6] Once considered the gold standard, autogenic bone had the advantages of low cost and biocompatibility, but is less commonly used due to prolonged operating time, donor-site morbidity, and variable degrees of bone resorption.[
7,
8] A plethora of alloplastic materials have been described, including sheets of polytetrafluoroethylene, hydroxyapatite, porous polyethylene (PPE), silicone, and gelatin film, as well as metals such as titanium (Ti) and Vitallium alloy.[
9]
Ti, PPE, and PPE/Ti composites are commonly used to treat orbital floor fractures and carry advantages and disadvantages inherent in the material. Ti is easily malleable, highly biocompatible, and meshed to promote soft-tissue integration, thus preventing migration. However, there have been reports of orbital adherence, resulting in gaze restriction.[
10,
11] PPE has advantages of easy contouring and smooth edges but carries a higher risk of infection and migration, as do PPE/Ti composites.[
12,
13] Resorbable plating systems present an alternative to permanent options and have been successfully used to treat small-to-medium size orbital floor defects; however, much of the current literature is composed of small studies which report outcomes for one implant type without comparison to other existing options.[
14] Moreover, the majority of studies report absorbable plate outcomes for small-to-moderate size fractures only. In one retrospective comparison of absorbable and nonabsorbable plating systems, no type of implant was superior to the other in either immediate or long-term outcomes.[
15] In this study, we sought to compare outcomes of a resorbable plating system with those of Ti and PPE/Ti composites for all orbital floor fractures treated at our institution.
Methods
After obtaining institutional approval, a retrospective chart review was performed of all patients for whom orbital floor repair was performed at Dartmouth-Hitchcock Medical Center between 2010 and 2016, using the ICD-9 code 21390. Surgeries were performed by faculty in the departments of plastic surgery, otolaryngology, and ophthalmology with the assistance of resident physicians. Patients were excluded if follow-up was less than 1 year, age was less than 18 years, repair was after neoplasm resection, or trauma caused significant globe injury prior to the repair (
Figure 1). At our institution, the most common resorbable orbital floor implant used was the Delta resorbable plating system, which is composed of 85% poly L-lactide, 10% poly glycolide, and 5% poly D-lactide, 1.7 mm thickness (Stryker Corporation, Kalamazoo, MI). The two most commonly used nonabsorbable implants utilized at our institution were Ti mesh (0.4 mm thickness, Stryker Corporation) and a PPE/Ti composite (MEDPOR TITAN MTM, 0.85 mm thickness, Stryker Corporation). Two cases in which Gelfilm (Pfizer, New York, NY) and two cases in which calvarial bone were utilized were excluded to improve homogeneity of the absorbable and nonabsorbable implant cohorts, respectively. Implant selection was based on a combination of surgeon and patient preference.
Operative data retrieved included implant type, implant fixation, fracture size, surgical approach, intraoperative complications, and whether antibiotic and steroids were administered. All patient comorbidities were reviewed, as well as alcohol and tobacco use. Presence of diplopia, entrapment, enophthalmos, hypoglobus, and maxillary nerve hypo- or hyperesthesia was determined pre- and postoperatively. All documented postoperative complications were recorded in addition to follow-up time for each patient.
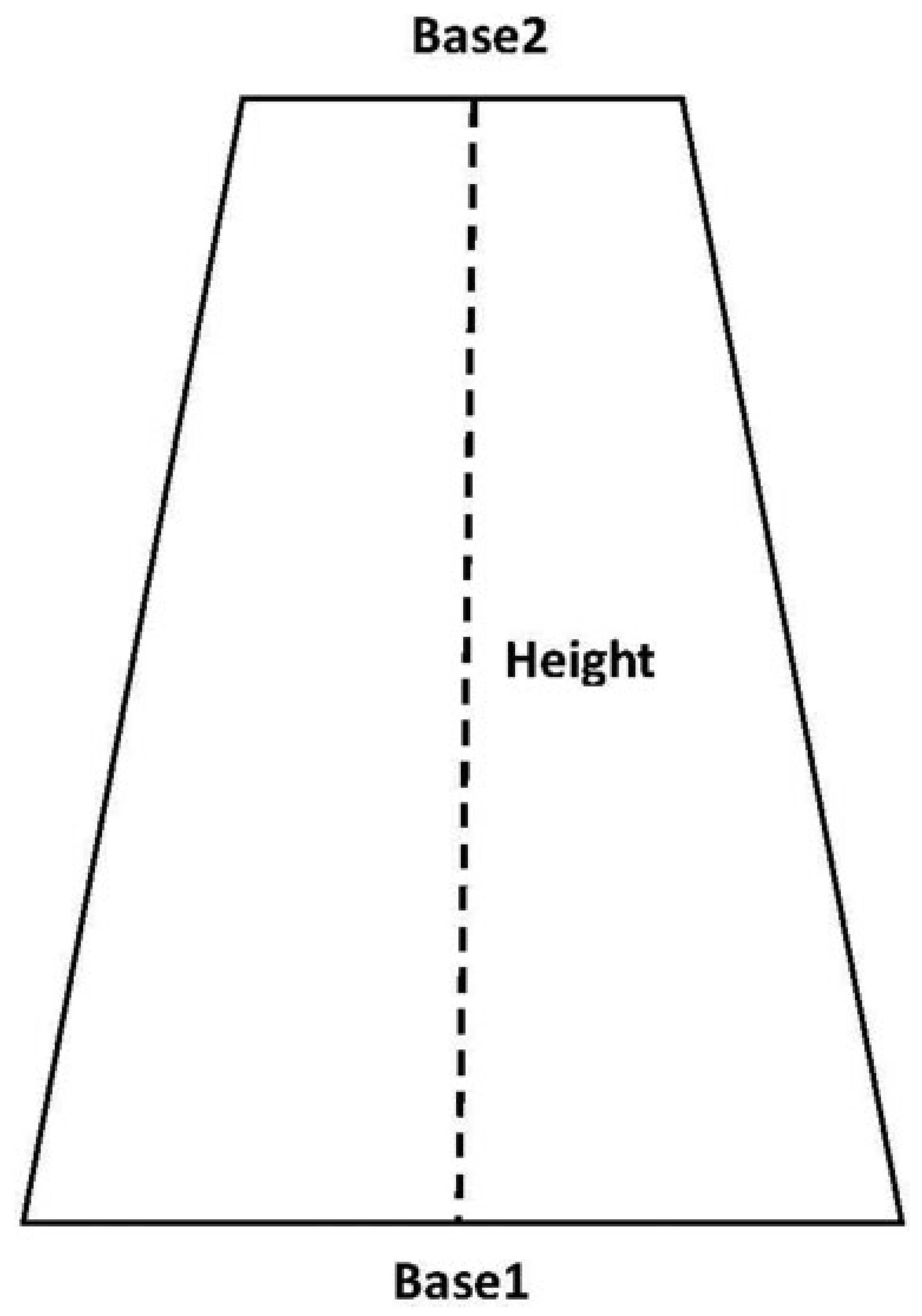
The surface area of 43% of orbital floor defects in the absorbable group and 36% in the nonabsorbable group was determined from operative note documentation. The remainder of defect surface areas was determined by measuring the length of the fracture on sagittal CT, as well as fracture width on coronal scans at the anterior-most and posterior-most aspects of the fracture. Defect surface area was estimated by calculating the surface area of a trapezoid (Surface area = [{Base 1 + Base 2}/2] × Height), with height equivalent to fracture length, and Base 1 and Base 2 equivalent to fracture width anteriorly and posteriorly (
Figure 2). This methodology was used to calculate surface area because the floor of the orbit narrows from anterior to posterior and thus bony defect shapes tend to approximate a trapezoid.
Statistical analyses were conducted using unpaired t-test for contiguous data and Fisher’s exact test for categorical data (Microsoft Excel 2013, Redmond, WA).
Results
Demographics
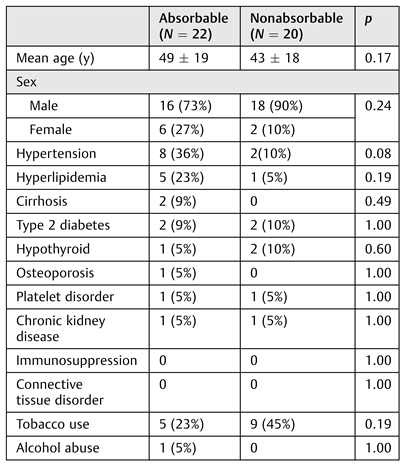
There was no significant difference in age, sex, or comorbidities between the absorbable and nonabsorbable plate groups (
Table 1). Tobacco use was more prevalent in the nonabsorbable group than in the absorbable plate group at 45 and 23%, respectively; however, this difference was not significant (
p = 0.19).
Orbital Floor Defect Surface Area and Additional Fractures
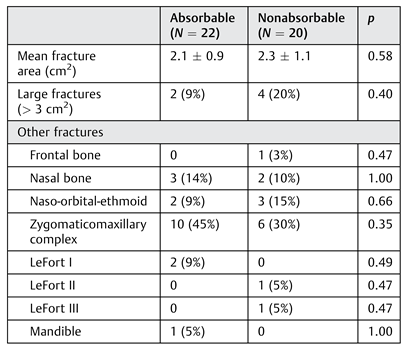
The mean orbital floor defect surface area was 2.1 (SD: ± 0.9 cm
2, range: 0.4–3.6 cm
2) for the absorbable plate group and 2.3 (SD: ± 1.1 cm
2, range: 0.6–4.4 cm
2) for the nonabsorbable plate group (
p = 0.58;
Table 2). There were a total of six large fractures (defined as fracture with a surface area >3 cm
2), two in the absorbable implant group and four in the nonabsorbable implant group (
p = 0.40). There were no significant differences in the number of additional fractures sustained between groups.
Operative Details
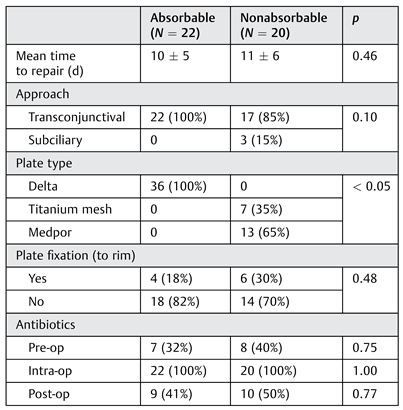
The mean time from injury to fracture repair was 10 ± 5 and 11 ± 6 days for the absorbable and nonabsorbable groups, respectively (
p = 0.46;
Table 3). Within the nonabsorbable plate group, 7 (35%) plates used were Ti mesh, and the other 13 (65%) were Ti/PPE composites. Implant fixation to the orbital rim with screws was more commonly performed in the nonabsorbable group than in the absorbable group; however, there was no significant difference (
p = 0.48). There was also no significant difference regarding antibiotic use during any phase of care between groups.
Outcomes
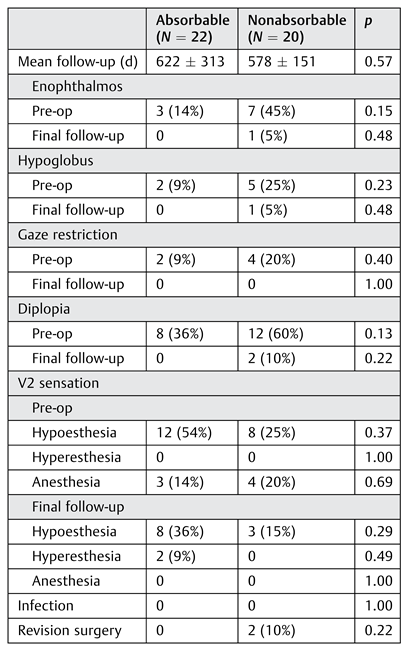
Mean follow-up time was 622 (SD ± 313) and 578 (SD ± 151) days for absorbable and nonabsorbable implant groups, respectively (
p = 0.57;
Table 4). There were no significant differences between groups regarding enophthalmos, hypoglobus, gaze restriction, diplopia, or infraorbital nerve sensation either preoperatively or postoperatively. For all cases in which there was enophthalmos, hypoglobus, or diplopia at the final follow-up appointment, all patients were asymptomatic in primary gaze, and diplopia was present only in upgaze or downgaze. These patients demonstrated marked improvement in diplopia at presentation and did not desire revision surgery. There were no cases of implant infection in either group, although there was one case of incisional cellulitis in the nonabsorbable implant group.
No revision surgeries were required for the absorbable implant cohort; however, there were two cases in the nonabsorbable implant cohort that required revision. The first patient had developed progressive diplopia in primary and upgaze, with significant limitation of elevation and left hypotropia at follow-up. ATi/PPE implant had beenplaced originally. Upon revision surgery, scarring and adhesions were found near the posterior edge of the implant andwere released. The second revision patient had initially been treated with a Ti/PPE implant. Over the course of 1 month, the patient developed persistent diplopia and unchanged vertical dystopia. The patient presented to the operating room for revision surgery and the implant was found to have migrated anteriorly, resting on the edge of the inferior orbital rim and causing 2 to 3 mm of excessive globe elevation. The implant was dissected from the orbital septum and floor of the orbit and removed without difficulty. A dense scar had formed on the orbital floor and the globe was in an ideal position without a plate; thus, a plate was not replaced. Diplopia had resolved completely at 3 months after revision surgery and the patient never required an implant to be replaced.
Discussion
The ideal material for orbital floor repair remains controversial, as there is no perfect implant available for all types of fractures. Autologous bone, often iliac or calvarial, was once the gold standard, but this is now performed less commonly due to prolonged operating time, donor-site morbidity, variable degrees of bone resorption, and readily available alloplastic materials.[
8] However, despite these shortcomings, excellent results may be obtained using autologous bone.[
16]
Alloplastic materials, both resorbable and nonresorbable, are more commonly used today in the repair of orbital floor defects. Ti is widely used for its mechanical strength, malleability, and visibility on imaging. Additionally, Ti stimulates osteoblasts, aiding in osseointegration.[
17] However, risks of using Ti include development of adhesions, and it has been postulated that attaching the plate to the orbital rim increases this risk.[
10,
11] PPE is another alloplastic material used in orbit reconstruction due to biocompatibility, ease of shaping, and a lower risk of adhesions; however, reported disadvantages include radiolucency, migration, and inflammation. Ti/PPE composites were developed to allow PPE visibility on imaging and the strength of Ti, with the advantages of a lower risk of adhesions associated with the PPE component. However, the major weakness native to all nonresorbable implants lies in the nature of the presence of a permanent foreign body. If the implant is not placed perfectly or if adhesions or infections develop, the entire implant must be removed. In our study, there were two cases in which nonabsorbable implants, both Ti/PPE composites, resulted in complications that required revision surgery. One patient had developed adhesions at the posterior aspect of the implant resulting in gaze restriction. Although PPE reduces the risk of adhesions compared with Ti, it is important to note that a degree of risk remains. The second revision was a patient who had a Ti/PPE implant that had migrated anteriorly, causing persistent diplopia. In this case, the implant had not been fixed and the complication was likely secondary to surgical technique.
Absorbable implants have been utilized since the 1990s and are characterized based on strength and degradation time. After placement, implants undergo hydrolysis, which is a time-variable process and based on the number of hydrolysable bonds present in each type of material used. For example, poly(lactide-co-glycolide) fully degrades in 12 months, whereas poly(co-lactide) absorbs in 24 months. The acidic byproducts of hydrolysis serve to create scar tissue, which suspends orbital contents. However, by the same token, resorbable plates have been shown to cause inflammatory reaction, scarring, and adherence, leading to gaze restriction.[
18] Similar to nonabsorbable implants, it appears that the risk of gaze restriction increases with close approximation to the infraorbital rim. Restriction was not a complication that we experienced using the PLL/PG/PDL resorbable plate, which is reported to maintain 78 and 50% of its initial strength at 2 and 6 months, respectively.[
19]
Some authors have advocatedusing nonresorbable plates for large orbital floor fractures due to ease of three-dimensional reconstruction, and using resorbable plates as an option for smaller floor fractures.[
20] However, others postulate that absorbable plates are a reasonable option for large orbital floor fractures, especially when combined with bone substitute, because orbital contents are not load-bearing.[
21] In our study, the mean fracture surface area was 2.1 (SD: ± 0.9 cm
2, range: 0.4–3.6 cm
2) and 2.3 cm
2 (SD: ± 1.01 cm
2, range: 0.6–4.4 cm
2) for the absorbable and nonabsorbable groups, respectively, a fracture size which we would describe as moderate. Given that there were no significant differences in either outcomes or complications between absorbable and nonabsorbable implants, this suggests that for fractures of a moderate size, absorbable implants are a viable option, a finding consistent with prior studies.[
15] There were only a total of six large (>3.0 cm
2) orbital floor fractures, two in the absorbable group and four in the nonabsorbable group (
p = 0.40). Although there is no statistically significant difference in the number of large fractures between groups or outcomes associated, meaningful conclusions cannot be made given the small sample size.
There was no significantdifferencebetween absorbable and nonabsorbable implant groups in regard to whether a transconjunctival or subciliary approach was used (p = 0.10). As this study was focused on the impact of the implants themselves, not the approach utilized, complications involving the eyelid secondary to approach such as entropion and ectropion were not reported. However, there were no reports of eyelid swelling secondary to hydrolysis of absorbable implants.
It should be noted that in this study, the surface area of fractures not measured directly in the operating room was estimated using the formula for the surface area of a trapezoid, compared with prior studies which estimated surface area using formulae for ellipses or squares.[
19,
22,
23] Using squares and ellipses to estimate fracture surface area may result in overestimation due to one width measurement used in calculation, whereas a trapezoid, while not perfect, accounts for the natural tapering of orbit width in an anterior-to-posterior fashion.
Cost is yet another factor that increasingly has come into play in medical decision making. Determining the cost of orbital implants is incredibly complex, as costs vary by a variety of factors such as production company, regional distribution, hospital contracts, implant size, and implant type. However, in general, absorbable implants are more costly than their nonabsorbable counterparts, especially Ti, and this may be a factor when deciding which implant type to use. Patientspecific implants have shown promising outcomes, particularly for treatment of complexorbital fractures; however, there may be an associated increase in cost as well as down time for construction of the implant which needs to be considered.[
24,
25] Given ease of placement, smooth edges, and accuracy of defect approximation, patient-specific implants will likely become increasingly utilized in the future.
Weaknesses of this study include the retrospective nature of the study, as well as the power which may not be large enough to account for smaller variances in outcomes or complications. Postoperative CT imaging is not routinely performed at our institution and is reserved for cases in which patients present with new or persistent ocular complaints such as diplopia, gaze restriction, or enophthalmos. Finally, a mean follow-up time of 622 days for the absorbable implant group may not be sufficiently long enough to account for late- occurring enophthalmos after complete implant absorption.