Orbital wall fractures account for 10 to 25% of all facial fractures,[
1,
2,
3] with the majority of these fractures involving the orbital floor.[
4,
5] Repair is commonly performed to alleviate intractable diplopia and correct potential enophthalmos.[
6,
7,
8] One of the feared complications of surgical repair of orbital fractures is postoperative orbital compartment syndrome from accumulation of blood within the orbit. The increased orbital pressure can limit blood flow to the eye and optic nerve, resulting in permanent vision loss.[
9]
As the orbital floor forms the roof of the maxillary sinus, in the setting of a floor fracture the sinus is a potential space into which orbital bleeding can drain. It has been postulated that non-meshed implant materials may prevent drainage of blood from the orbit into maxillary sinus.[
10] This has led some surgeons to drill holes into these implants or to prefer uncoated meshed titanium implants.[
10] Yet there remains little extant data addressing whether non-mesh implants actually reduce blood drainage from the orbit to the maxillary sinus. Similarly, some surgeons place drains intraoperatively to prevent orbital accumulation of blood and prevent associated complications [
9]; again, little data exist regarding the efficacy of this measure.
This study aimed to determine if non-mesh implant materials inhibit the drainage of blood into the maxillary sinus following surgical repair of orbital floor fractures relative to mesh implant materials and if drain placement impacted the accumulation of blood in the maxillary sinus.
Results
Chart review revealed 320 cases eligible for study inclusion. In accordance with prior power calculations, 92 cases were selected for measurement and analysis, with 23 coming from each of the four treatment categories. Within this group, 46 patients underwent placement of a manually bent Medpor Titan Barrier porous polyethylene-coated titanium sheet (Stryker, Kalamazoo, MI) and 46 patients underwent placement of a Matrix MIDFACE Titanium Preformed Orbital Plate (Depuy Synthes, West Chester, PA;
Figure 2). As these constituted the vast majority of the implants used, these were utilized as the representative plates for the non-mesh and mesh implant groups, respectively, and those receiving alternative implant materials were excluded from analysis. Drain placement, when performed, was based on surgeon preference and involved placement of an approximately 3 to 5 cm length of = inch rubber or silicone Penrose drain material superior to the orbital implant, which transited the unsutured retroseptal transconjunctival incision and was secured in place with a 6–0 nylon frost suture tarsorrhaphy. Each operation was performed by one of four board-certified oculoplastic surgeons, with no difference between surgeons in the distribution of either implant type or drain placement (
p = 0.4 vs. 0.3, respectively) on Pearson’s chi-squared test. All patients, regardless of implant type or drain placement, had a patch applied consisting of a single piece of ½ inch Xeroform gauze, two eye pads, and paper tape; unfortunately, it was infeasible to record the volume of blood absorbed by each participant’s eye pads. In all patients, the maxillary sinus was evacuated of any appreciable blood products intraoperatively. There was no use of local anesthetic or intraocular anticoagulants in any case, and packing of the sinus did not occur.
While all participants had an orbital floor fracture present, several others had concomitant medial wall or orbital roof fractures as shown in
Table 1. Medial wall fractures were significantly more prevalent than orbital roof fractures (
p < 0.001). Medial wall fractures were significantly more common among those receiving mesh implants than those receiving non-mesh implants (
p < 0.001) but did not differ significantly based on drain presence or absence (
p = 0.5). The prevalence of orbital roof fractures was nearly identical across all treatment groups (mesh vs. non-mesh implants,
p = 0.6; drain vs. no drain presence,
p = 1.0). The CT images that were used for blood and sinus volume measurements had to be taken within 36 h of surgery for inclusion in this study. The breakdown of whether these images were taken the same day as the operation or the day after the operation was fairly even, as shown in
Table 2. There was no significant difference in the day on which CT images were taken based on implant material or drain presence (
p = 0.7 and 0.4, respectively).
The mean patient age was 37 years at the time of surgery (range: 18–69 years), and the majority of patients (79.4%) were male. The most common approach was the preseptal transconjunctival approach (96.7%), with the remaining 3.3% using a transconjunctival approach with lateral canthotomy. None of the aforementioned variables (i.e., age, sex, surgical approach) demonstrated significant differences between treatment groups (
p = 0.7, 0.3, 0.3, respectively;
Table 3).
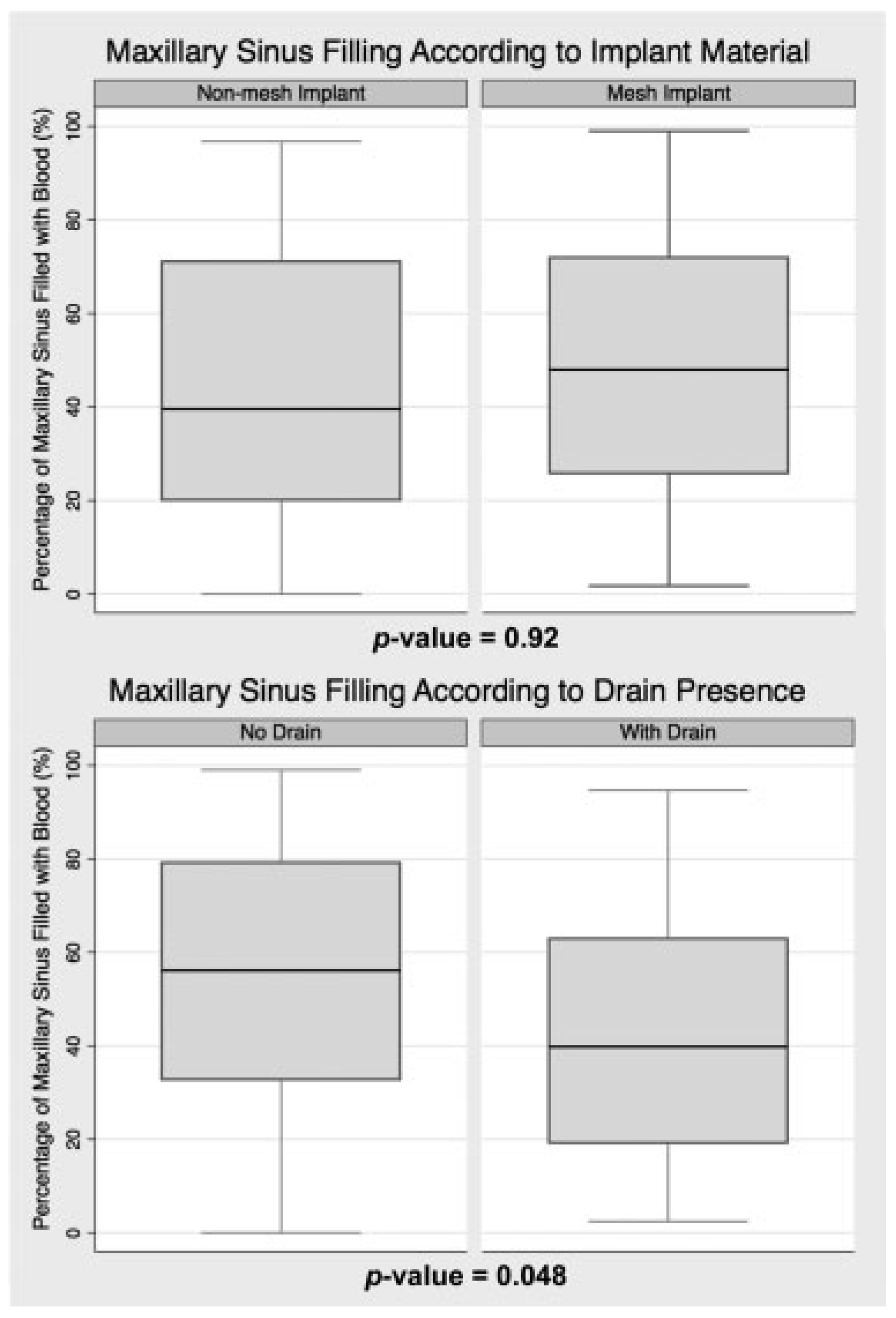
Postoperative maxillary sinus filling was 49% ± 29% on average. Given that the mean maxillary sinus size was 19 mL ± 6 mL, this left an average of 10 mL of unfilled sinus space. Eleven percent of cases (n = 10) demonstrated extreme sinus filling, which was defined as 90% or more of the maxillary sinus occupied with blood, and at least one such case was present within each of the four treatment groups (non-mesh, without drain: three cases; non-mesh, with drain: one case; mesh, without drain: five cases; mesh, with drain: one case). There was no significant difference in maxillary sinus size between those with and without extreme sinus filling (p = 0.4).
As shown in
Figure 3, sinus filling was similar regardless of the implant material used (
p = 0.9), with average sinus filling of 46% ± 29% for non-mesh and 51% ± 30% for mesh. Fewer non-mesh cases demonstrated extreme sinus filling (8.7%) than mesh cases (13.0%), but this was not statistically significant (
p = 0.5).
Figure 3 demonstrates the statistically significant association between intraoperative drain placement and a reduction in maxillary sinus filling (
p = 0.048). On average, the maxillary sinus filling was 43% ± 27% in cases involving a drain and 54% ± 30% in those without a drain. Drain placement was also associated with a statistically significant reduction in the percentage of cases with extreme sinus filling (
p = 0.044), as eight cases without a drain demonstrated greater than 90% sinus filling, but only two cases with a drain demonstrated such filling.
When assessed according to implant material, the impact of drain placement was similar for both non-mesh and mesh cases. Non-mesh cases averaged 52% ± 29% sinus filling without a drain, but 40% ± 27% with one (p = 0.8). Mesh cases average 57% ± 31% filling without a drain, but 45% ± 27% filling with one (p = 0.5).
Discussion
Internal orbital fracture repair is a common procedure performed to reduce functional and aesthetic sequelae of facial trauma. Severe vision loss is a rare but well-recognized complication of these procedures that can occur from an orbital hemorrhage-related compartment syndrome.[
9,
11,
12,
13] In postoperative orbital compartment syndrome, excessive bleeding is restricted to the confines of the orbit leading to an increase in pressure that can quickly compromise ocular perfusion.[
11,
12,
13] Thus, it is desirable that blood be able to pass from the orbit to a secondary space, be it the maxillary sinus or the outside world. Our first aim was to evaluate the maxillary sinus’ role as a reservoir for orbital blood; this was achieved by analyzing differences in sinus filling between those treated with and without an orbital drain. In our data, cases with a drain (mesh and non-mesh implant cases) had only 80% the level of sinus filling of their non-drain counterparts (mean sinus filling: 43% with drain, 54% without drain;
p = 0.048). It may be that the Penrose drain removes sufficient blood to prevent passage of any orbital blood into the maxillary sinus, in which case all sinus blood would be from another source, such as sinus mucosal bleeding; in this case, the difference in sinus blood filling between drain and non-drain cases would correspond directly with the total amount of orbital blood that passes into the sinus. Alternatively, drain placement may simply reduce the amount of blood entering the sinus, indicating that some or all of the sinus blood originated in the orbit in both drain and non-drain cases. In either of thesescenarios, the reduction in sinus filling in drain cases is presumed to be reduced passage of orbital blood into the maxillary sinus.
Given this apparent ability of the maxillary sinus to serve as a reservoir for orbital blood, it is desirable that blood be able to circumvent an implant placed during orbital floor fracture repair. Nevertheless, innovations in orbital implant design have largely focused on restoring the complex orbital shape and preventing orbital contents from adhering to porous materials, rather than allowing the passage of blood.[
14,
15,
16] It has been postulated that non-mesh implants serve as a barrier to blood flow from the orbit to the sinus. For instance, Nguyen et al. reported that “regarding implant choice, nonporous implants such as MEDPOR TITAN do not allow orbital blood to drain into the maxillary sinus. One possible solution to this is to drill holes in the implant to allow for drainage, which is now the senior author’s (N.R.M.) practice.”[
10] In an effort to provide evidence for such practices, we sought to determine differences in mesh and non-mesh implants’ abilities to allow passage of blood into the sinus. If meshed material did allow for greater blood passage, then mesh cases would presumably demonstrate greater sinus filling than non-mesh cases. However, this was not seen. In fact, mesh and non-mesh cases showed very similar sinus filling, whether a drain was present (mean: 45 vs. 40%, respectively) or not (mean: 57 vs. 52%, respectively). Both cohorts also demonstrated similar reductions in sinus filling (12% for both mesh and non-mesh groups). The similarity in findings suggests that blood can transit into the maxillary sinus by going around non-meshed implants.
Cases with a drain present left, on average, 57% of the maxillary sinus unfilled. This indicates that the maxillary sinus often does not fill to capacity with blood in the postoperative period and, thus, can serve as a reservoir for orbital blood to drain into to prevent excessive buildup within the orbit. It is worth noting, however, that 10.9% of cases did demonstrate greater than 90% sinus filling on imaging and thus essentially reached their maximum capacity. These cases are not likely a result of patients simply having particularly small maxillary sinuses, as the average sinus size did not differ significantly between these and other cases (p = 0.4). When considering this subset of cases, it is important to realize that while this filling may have been due to mucosal bleeding, it may also have been due to drainage of orbital blood, or a combination thereof. In any case, this accumulation of blood in the sinus could potentially be relieved via the semilunar canal into the middle meatus of the nasal cavity should pressure from orbital blood begin to build up. Thus, even in these instances, the orbit may still benefit from draining into the maxillary sinus. But given the serious clinical sequelae associated with orbital blood accumulation, this subset of cases may provide additional impetus for intraoperative drain placement as drains were associated with a significant reduction in the number of such cases with sinus filling of 90% or greater (p = 0.044).
As referenced earlier, average maxillary sinus filling was highly similar regardless of which implant material was used. As such, use of a mesh implant appears to have little or no effect on the amount of postoperative blood accumulation within the sinus. However, drain placement leads to a clear and statistically significant reduction in blood accumulation within the maxillary sinus. This suggests that drains are effective in removing orbital blood. This is helpful in providing an additional safety measure to prevent orbital blood accumulation and to prevent unnecessary accumulation of additional blood into the maxillary sinus, which might contribute to discomfort or lead to overflow into the nasal column. This also suggests that drains might be used to prevent orbital blood accumulation in orbital wall fractures that do not involve the orbital floor, as an intact orbital floor would not allow for blood drainage into the maxillary sinus; this, however, is speculative and further research is warranted. It is worth acknowledging, however, that there were two cases in which a drain was present, but sinus filling still exceeded 90%. This extreme sinus filling may have been due to mucosal bleeding or it may have been due to orbital blood draining; in the latter case, this would suggest that these drains can fail and should be monitored for effective passage of blood postoperatively. It is worth noting that, even in these cases, no loss of vision or other clinical complications were seen.
Limitations of this study include a retrospective review methodology. Due to practical limitations, we were unable to assess all available implants and instead opted to focus on the most commonly used mesh and non-mesh products in our practice. We sought to reduce any possibility of selection bias by randomizing case selection for analysis. Nevertheless, this was not a randomized control trial and, as such, a significant difference in the prevalence of medial wall fractures was seen based on which implant material was used. It is possible that there is a difference in the amount of bleeding in cases with medial wall fractures or even that some of the ethmoid sinus air cells can act as an additional reservoir for bleeding. Our study is based on individual postoperative CT scans, as it is not possible to directly visualize the passage of blood into the maxillary sinus and we did not directly measure drain output. Patients with excessive orbital hemorrhage with blood accumulation were not included in this study because these events are rare and such patients receive emergent hemorrhage evacuation before any CT imaging of the maxillary sinuses can be acquired. This study was not able to account for fracture size, but given that all patients met criteria for repair, all fractures were likely substantial. Postoperative fracture coverage is also difficult to assess. It is standard practice to extend the implant posteriorly to the palatine bone; however, a small portion of the posterolateral defect near the inferior orbital fissure or groove can sometimes remain uncovered (
Figure 4) and may be too small to visualize reliably on postoperative imaging. We did not compare sinus filling between pre and postoperative imaging because blood was evacuated from the maxillary sinus at the onset of surgery and because most preoperative images were taken days or weeks before the operation. Data from multiple surgeons were included in this study, and both drain placement and implant material were selected based on surgeon preference, which is another limitation of this study.
Drainage of orbital blood can be critical in the postoperative period following repair of orbital floor fractures, be it into the maxillary sinus or the outside world. Our findings suggest that there is no significant difference in the postoperative accumulation of blood in the maxillary sinus between non-mesh implants (i.e., Medpor Titan Barrier porous polyethylene-coated titanium sheet) and mesh implants (i.e., Matrix MIDFACE Titanium Preformed Orbital Plate). Consequently, some practices—such as drilling holes into non-mesh implants or avoiding them outright due to concerns about blood drainage—may be unnecessary. Orbital drains also demonstrate a significant reduction in postoperative blood accumulation within the maxillary sinus, indicating that they can effectively reduce buildup of blood within the orbit, perhaps even for nonfloor orbital fractures. Unfortunately, given its potential as a negative space for blood egress, we also demonstrate that the maxillary sinus can fill excessively. These findings serve to inform intraoperative decision making when treating orbital floor fractures and, as such, contribute to improved patient care.