Autologous Reconstruction of a Face Transplant Candidate
Abstract
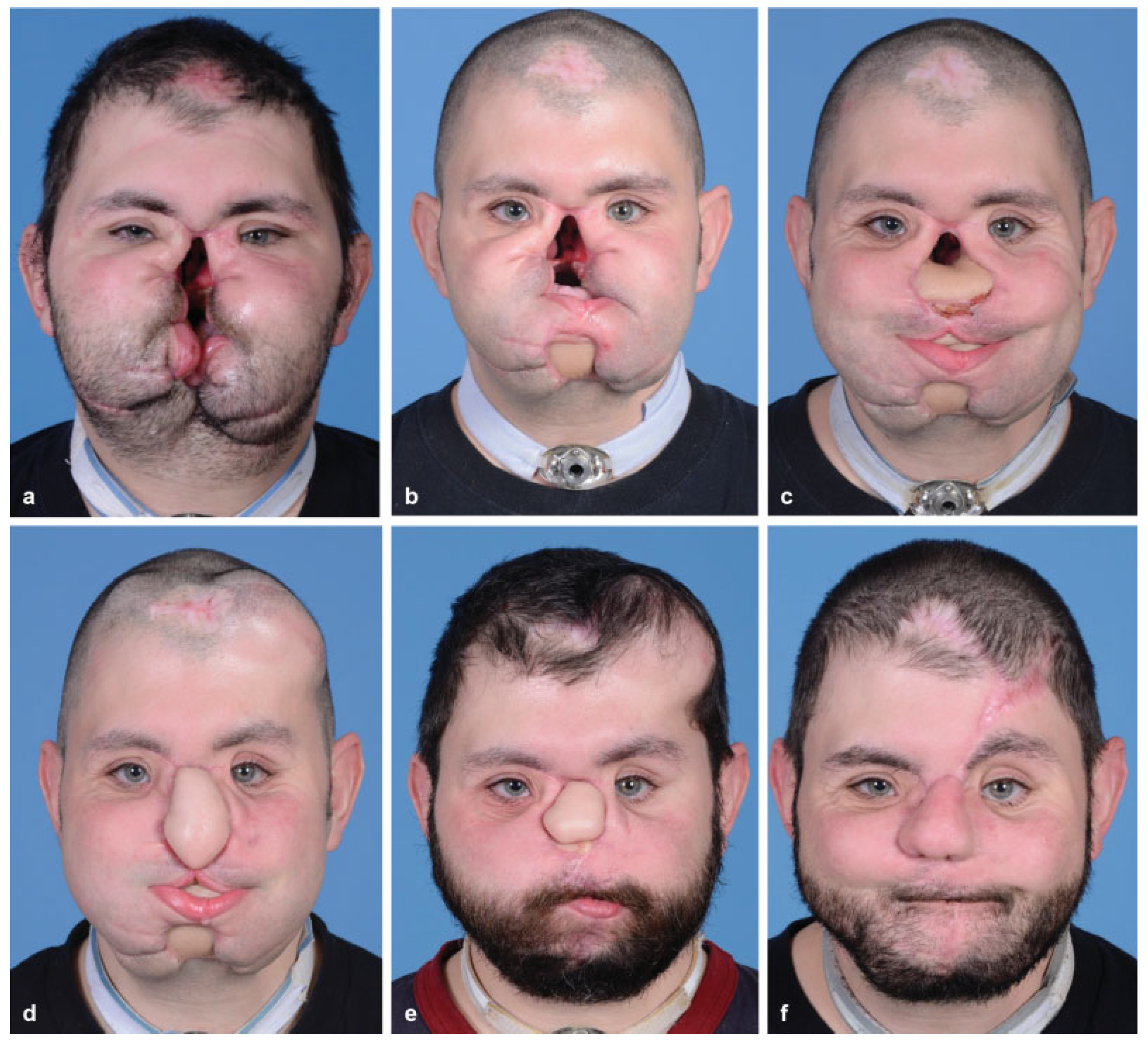
:Case Report
Discussion
Acknowledgments
References
- Yamamoto, Y.; Minakawa, H.; Sugihara, T.; et al. Facial reconstruction with free-tissue transfer. Plast. Reconstr. Surg. 1994, 94, 483–489. [Google Scholar] [PubMed]
- Rifkin, W.J.; David, J.A.; Plana, N.M.; et al. Achievements and challenges in facial transplantation. Ann. Surg. 2018, 268, 260–270. [Google Scholar] [PubMed]
- Sosin, M.; Ceradini, D.J.; Levine, J.P.; et al. Total face, eyelids, ears, scalp, and skeletal subunit transplant: A reconstructive solution for the full face and total scalp burn. Plast. Reconstr. Surg. 2016, 138, 205–219. [Google Scholar] [PubMed]
- Peled, M.; Leiser, Y.; Emodi, O.; Krausz, A. Treatment protocol for high velocity/high energy gunshot injuries to the face. Craniomaxillofac. Trauma. Reconstr. 2012, 5, 31–40. [Google Scholar] [PubMed]
- Kiwanuka, H.; Aycart, M.A.; Bueno, E.M.; Alhefzi, M.; Krezdorn, N.; Pomahac, B. Patient recruitment and referral patterns in face transplantation: A single center’s experience. Plast. Reconstr. Surg. 2016, 138, 224–231. [Google Scholar] [PubMed]
- Rodriguez, E.D.; Martin, M.; Bluebond-Langner, R.; Khalifeh, M.; Singh, N.; Manson, P.N. Microsurgical reconstruction of posttraumatic high-energy maxillary defects: Establishing the effectiveness of early reconstruction. Plast. Reconstr. Surg. 2007, 120 (Suppl. 2), 103S–117S. [Google Scholar] [PubMed]
- Clark, N.; Birely, B.; Manson, P.N.; et al. High-energy ballistic and avulsive facial injuries: Classification, patterns, and an algorithm for primary reconstruction. Plast. Reconstr. Surg. 1996, 98, 583–601. [Google Scholar] [PubMed]
- Vásconez, H.C.; Shockley, M.E.; Luce, E.A. High-energy gunshot wounds to the face. Ann. Plast. Surg. 1996, 36, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; Sawatari, Y.; Peleg, M. High-energy traumatic maxillofacial injury. J. Craniofac Surg. 2015, 26, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Roche, N.A.; Vermeersch, H.F.; Stillaert, F.B.; et al. Complex facial reconstruction by vascularized composite allotransplantation: The first Belgian case. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Cavadas, P.C.; Ibáñez, J.; Thione, A. Surgical aspects of a lower face, mandible, and tongue allotransplantation. J. Reconstr. Microsurg. 2012, 28, 43–47. [Google Scholar] [PubMed]
- Aycart, M.A.; Alhefzi, M.; Kueckelhaus, M.; et al. A retrospective analysis of secondary revisions after face transplantation: Assessment of outcomes, safety, and feasibility. Plast. Reconstr. Surg. 2016, 138, 690e–701e. [Google Scholar] [PubMed]
- Mohan, R.; Fisher, M.; Dorafshar, A.; et al. Principles of face transplant revision: Beyond primary repair. Plast. Reconstr. Surg. 2014, 134, 1295–1304. [Google Scholar] [PubMed]
- Kumnig, M.; Jowsey-Gregoire, S.G. Key psychosocial challenges in vascularized composite allotransplantation. World J. Transplant. 2016, 6, 91–102. [Google Scholar] [PubMed]
- Bergmann, T.K.; Barraclough, K.A.; Lee, K.J.; Staatz, C.E. Clinical pharmacokinetics and pharmacodynamics of prednisolone and prednisone in solid organ transplantation. Clin. Pharmacokinet. 2012, 51, 711–741. [Google Scholar] [PubMed]
- Tierce, J.C.; Porterfield-Baxa, J.; Petrilla, A.A.; Kilburg, A.; Ferguson, R.M. Impact of mycophenolate mofetil (MMF)-related gastrointestinal complications and MMF dose alterations on transplant outcomes and healthcare costs in renal transplant recipients. Clin. Transplant. 2005, 19, 779–784. [Google Scholar] [PubMed]
- Nankivell, B.J.; Borrows, R.J.; Fung, C.L.; O’Connell, P.J.; Allen, R.D.; Chapman, J.R. The natural history of chronic allograft nephropathy. N. Engl. J. Med. 2003, 349, 2326–2333. [Google Scholar] [PubMed]
- Özkan, Ö.; Özkan, Ö.; Doğan, U.; et al. Consideration of difficulties and exit strategies in a case of face allotransplantation resulting in failure. Microsurgery 2017, 37, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Rifkin, W.J.; Manjunath, A.; Kimberly, L.L.; et al. Long-distance care of face transplant recipients in the United States. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 1383–1391. [Google Scholar] [PubMed]
- Howard, J. Man’s second face transplant is a world first. Available online: https://www.cnn.com/2018/04/17/health/sec- ond-face-transplant-bn/index.html (accessed on 18 September 2018).


| Facial Transplantation | |||
| Category | Strong Indications | Strong Contraindications | Relative Contraindications |
| Surgical/ Anatomical | Extensive defects involving the majority of the surface area of the face | Sufficient tissue of the central face subunits (upper/lower eyelids, upper/lower lips, nose) to complete autologous reconstruction | |
| Extensive damage to or loss of the central face subunits (upper/lower eyelids, upper/lower lips, nose) | Adequate autologous tissue donor sites | ||
| Lack of autologous reconstructive options | |||
| Nonsurgical | Adequate support system | Inadequate support system | History of malignancy |
| No active psychiatric disorders | History of poor compliance | Blindness | |
| Active psychiatric disorder | Immunocompromised | ||
| Active malignancy | Immunosensitized | ||
| End-organ dysfunction | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2018 by the author. The Author(s) 2018.
Share and Cite
Rifkin, W.J.; Bellamy, J.L.; Kantar, R.S.; Farber, S.J.; Diaz-Siso, J.R.; Brecht, L.E.; Rodriguez, E.D. Autologous Reconstruction of a Face Transplant Candidate. Craniomaxillofac. Trauma Reconstr. 2019, 12, 150-155. https://doi.org/10.1055/s-0038-1675844
Rifkin WJ, Bellamy JL, Kantar RS, Farber SJ, Diaz-Siso JR, Brecht LE, Rodriguez ED. Autologous Reconstruction of a Face Transplant Candidate. Craniomaxillofacial Trauma & Reconstruction. 2019; 12(2):150-155. https://doi.org/10.1055/s-0038-1675844
Chicago/Turabian StyleRifkin, William J., Justin L. Bellamy, Rami S. Kantar, Scott J. Farber, J. Rodrigo Diaz-Siso, Lawrence E. Brecht, and Eduardo D. Rodriguez. 2019. "Autologous Reconstruction of a Face Transplant Candidate" Craniomaxillofacial Trauma & Reconstruction 12, no. 2: 150-155. https://doi.org/10.1055/s-0038-1675844
APA StyleRifkin, W. J., Bellamy, J. L., Kantar, R. S., Farber, S. J., Diaz-Siso, J. R., Brecht, L. E., & Rodriguez, E. D. (2019). Autologous Reconstruction of a Face Transplant Candidate. Craniomaxillofacial Trauma & Reconstruction, 12(2), 150-155. https://doi.org/10.1055/s-0038-1675844



