Beta-Catenin Mutation with Complex Chromosomal Changes in Desmoid Tumor of the Scalp: A Case Report
Abstract
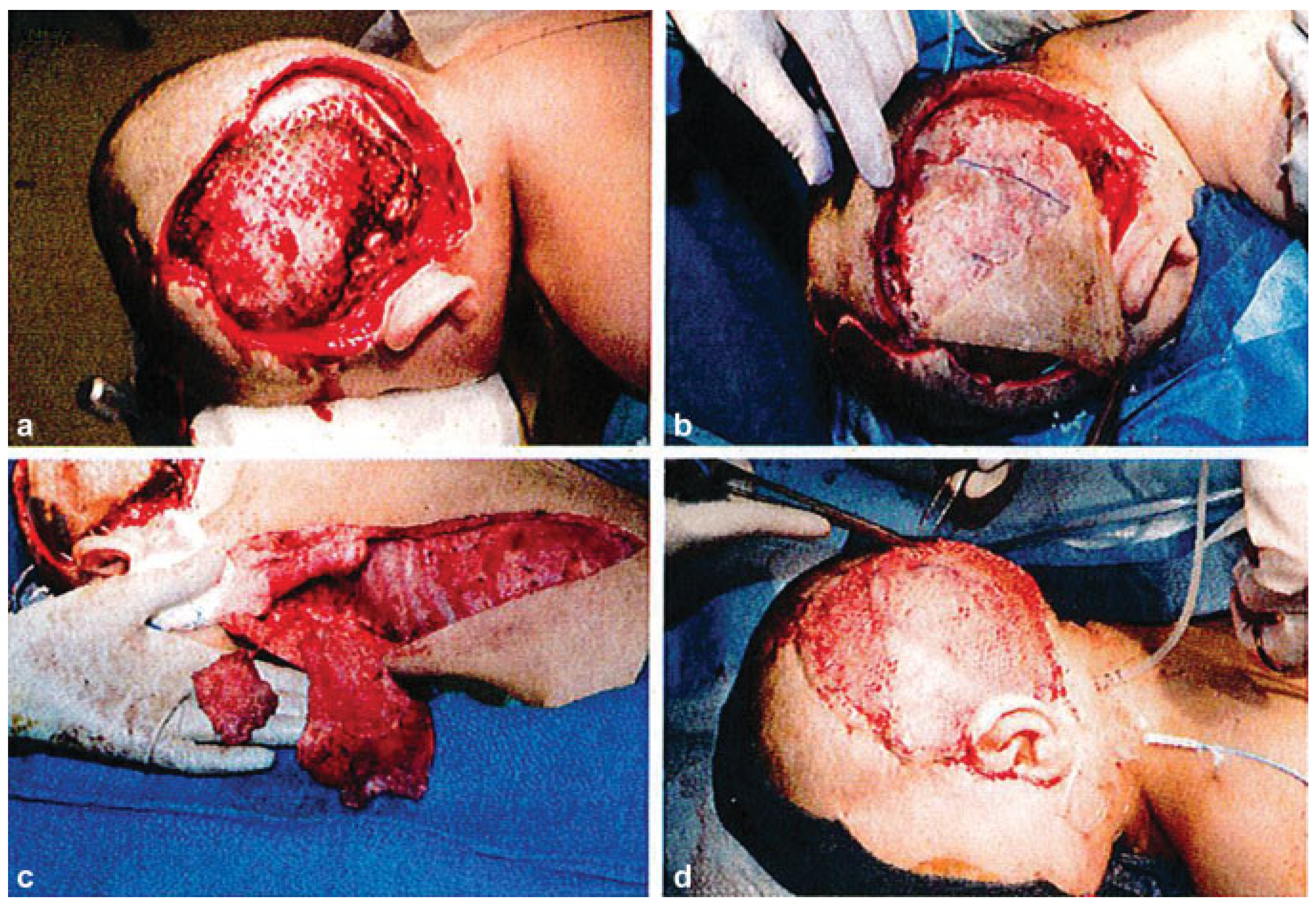
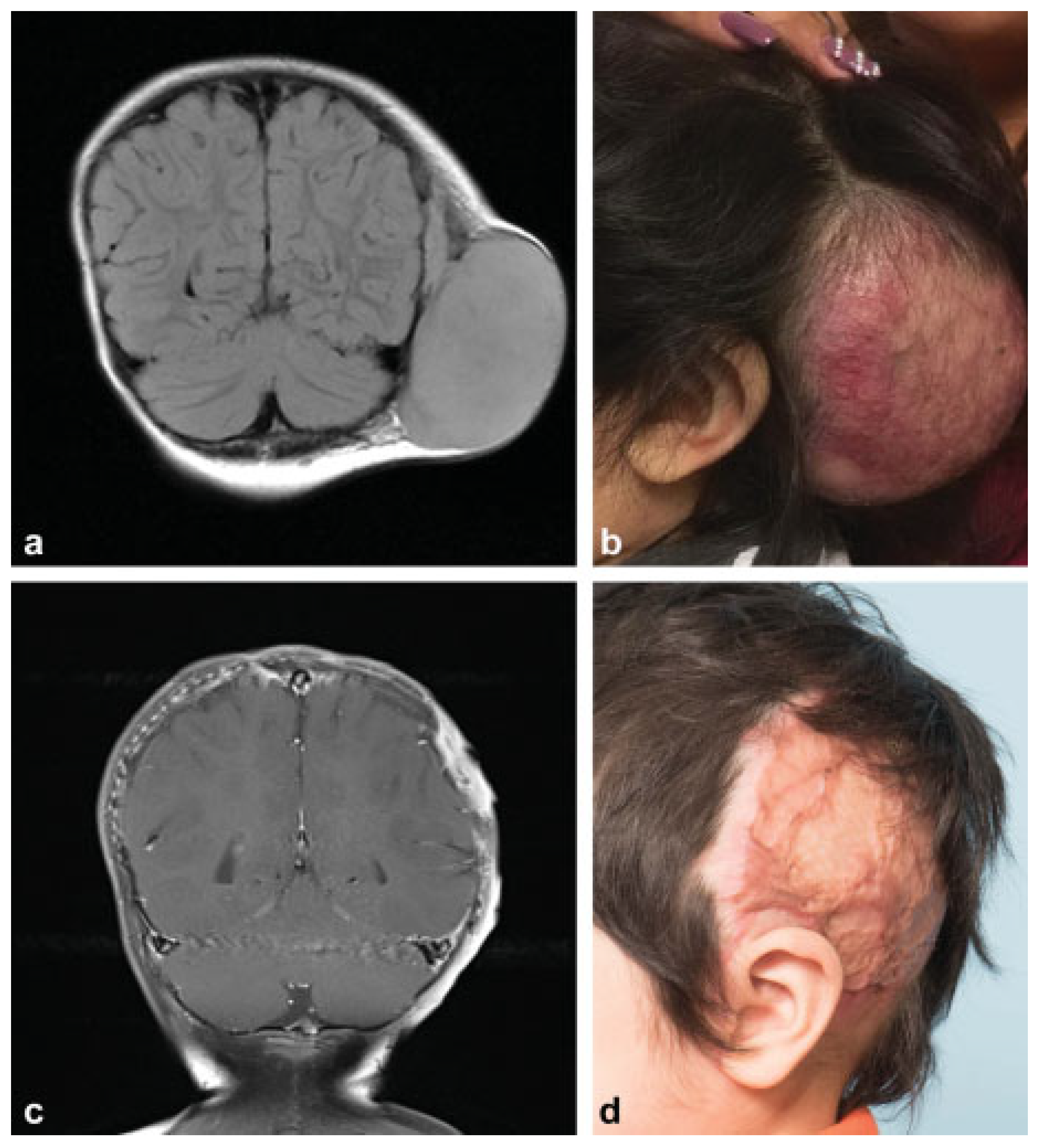
:Preoperative
Operation
Postoperative
Discussion
Conflicts of Interest
References
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [PubMed]
- Rao, C.V.; Asch, A.S.; Yamada, H.Y. Frequently mutated genes/pathways and genomic instability as prevention targets in liver cancer. Carcinogenesis 2017, 38, 2–11. [Google Scholar] [PubMed]
- Dose, M.; Emmanuel, A.O.; Chaumeil, J.; et al. β-Catenin induces T-cell transformation by promoting genomic instability. Proc. Natl. Acad. Sci. USA 2014, 111, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Alman, B.A.; Li, C.; Pajerski, M.E.; Diaz-Cano, S.; Wolfe, H.J. Increased beta-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors). Am. J. Pathol. 1997, 151, 329–334. [Google Scholar] [PubMed]
- Martínez-Lage, J.F.; Acosta, J.; Sola, J.; Poza, M. Congenital desmoid tumor of the scalp: A histologically benign lesion with aggressive clinical behavior. Childs Nerv. Syst. 1996, 12, 409–412. [Google Scholar] [PubMed]
- Le Guellec, S.; Soubeyran, I.; Rochaix, P.; et al. CTNNB1 mutation analysis is a useful tool for the diagnosis of desmoid tumors: A study of 260 desmoid tumors and 191 potential morphologic mimics. Mod. Pathol. 2012, 25, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Bodenmiller, B.; Aebersold, R. Quantitative analysis of protein phosphorylation on a system-wide scale by mass spectrometrybased proteomics. Methods Enzymol. 2010, 470, 317–334. [Google Scholar] [PubMed]
- Wang, Z.; Vogelstein, B.; Kinzler, K.W. Phosphorylation of β-catenin at S33, S37, or T41 can occur in the absence of phosphorylation at T45 in colon cancer cells. Cancer Res. 2003, 63, 5234–5235. [Google Scholar] [PubMed]
- Liu, C.; Li, Y.; Semenov, M.; et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002, 108, 837–847. [Google Scholar] [PubMed]
- Landrum, M.J.; Lee, J.M.; Benson, M.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [PubMed]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; et al. Exome Aggregation Consortium. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291. [Google Scholar] [PubMed]
- Knudson, A.G. Two genetic hits (more or less) to cancer. Nat. Rev. Cancer 2001, 1, 157–162. [Google Scholar] [PubMed]
- Bridge, J.A.; Sreekantaiah, C.; Mouron, B.; Neff, J.R.; Sandberg, A.A.; Wolman, S.R. Clonal chromosomal abnormalities in desmoid tumors. Implications for histopathogenesis. Cancer 1992, 69, 430–436. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2018 by the author. The Author(s) 2018.
Share and Cite
Liu, G.; Weiner, H.L.; Pederson, W.C.; Davies, L.; Buchanan, E.P. Beta-Catenin Mutation with Complex Chromosomal Changes in Desmoid Tumor of the Scalp: A Case Report. Craniomaxillofac. Trauma Reconstr. 2019, 12, 146-149. https://doi.org/10.1055/s-0038-1676078
Liu G, Weiner HL, Pederson WC, Davies L, Buchanan EP. Beta-Catenin Mutation with Complex Chromosomal Changes in Desmoid Tumor of the Scalp: A Case Report. Craniomaxillofacial Trauma & Reconstruction. 2019; 12(2):146-149. https://doi.org/10.1055/s-0038-1676078
Chicago/Turabian StyleLiu, Gary, Howard L. Weiner, William C. Pederson, Lesley Davies, and Edward P. Buchanan. 2019. "Beta-Catenin Mutation with Complex Chromosomal Changes in Desmoid Tumor of the Scalp: A Case Report" Craniomaxillofacial Trauma & Reconstruction 12, no. 2: 146-149. https://doi.org/10.1055/s-0038-1676078
APA StyleLiu, G., Weiner, H. L., Pederson, W. C., Davies, L., & Buchanan, E. P. (2019). Beta-Catenin Mutation with Complex Chromosomal Changes in Desmoid Tumor of the Scalp: A Case Report. Craniomaxillofacial Trauma & Reconstruction, 12(2), 146-149. https://doi.org/10.1055/s-0038-1676078


