Abstract
To clarify the conflicting recommendations for care of blowout fracture (BOF), a prospective randomized study is required. Here, we present a prospective randomized pilot study on BOF. This article aimed to evaluate which computed tomography (CT) findings predict late functional and/or cosmetic symptoms in BOF patients with ≥1.0 mL herniation of orbital content into maxillary and/or ethmoidal sinuses. It also aimed to evaluate which patients with BOF would benefit from surgical treatment or observational follow-up. Twenty-six patients with BOF ≥ 1.0 mL herniation were randomized to observational (n = 10) or surgical treatments (n = 16) and were followed up for functional and cosmetic symptoms for at least 1 year. The results from CT scan measurements were correlated to the patients’ symptoms and clinical findings which we report in this pilot study. Of the 10 patients randomized to observation, five had an inferomedial BOF with a herniation of ≥1.3 mL and all patients developed cosmetic deformities and required surgery. The remaining five patients in the observational group had inferior BOF and one of them had a distance of 3.3 cm from the inferior orbital rim to the posterior edge of the fracture and developed a cosmetic deformity but was unwilling to proceed to surgical treatment, and four patients had a median distance of 2.9 cm from the inferior orbital rim to the posterior edge of the fracture and did not develop cosmetic deformities. The median time from injury to surgery was 13 (3–17) days for the surgical group and 37 (17–170) days for the patients who underwent surgery in the observational group. The surgical results were similar for all the operated patients at the final control. Diplopia decreased and remained partly in one patient in the surgical group and in two patients in the observational group. Hypoesthesia of the infraorbital nerve decreased in nonsurgically treated patients, but surgery seemed to induce hypoesthesia. In this prospective randomized controlled pilot study on BOF, all patients in the observational group with inferomedial fractures developed visible deformity. Diplopia in BOF, without ocular motility limitation, is believed to be due to edema. Diplopia is not an indication for surgery as long as it reduces over time.
Orbital blowout fracture (BOF) is a common finding in patients with facial trauma. It is well known that entrapment of extraocular muscle is an absolute indication for immediate surgical intervention [1]. It is also known that BOF can result in considerable aesthetic deformities [2,3], hypoesthesia of the infraorbital nerve [4], and chronic diplopia [5]. Early assessment of the significance of the fracture through clinical examination and imaging and an informed decision between surgical and observational treatments are crucial for an optimal result [6]. Due to the risk of late enophthalmus, hypoglobus, and superior sulcus deformity, surgical correction has been considered a requirement in the following cases: >1.5 mL herniation [7], cranial–caudal dimension of the orbit > 0.8 cm [8], an orbital toor fracture > 1 cm2 [9], >50% fractured orbital toor [6], diplopia 2 weeks after the trauma [6], or an enophthalmus greater than 2 mm acute or after 6 weeks [9]. However, there are considerable differences in opinion regarding the management of BOF [10].
Recently, several studies have been published on different implants [11,12] used in reconstructing orbital BOFs and orbital volume restoring with sophisticated devices [13,14]. Which patient benefits from surgical or nonsurgical treatment and timing of surgical repair is still unclear and remains surgeon and institution dependent [10]. To clarify the conticting recommendations for care of orbital BOFs, a prospective randomized controlled clinical trial is required. However, such a study has been considered to be difficult due to ethical aspects and recruitment of patients [15].
In this prospective randomized controlled pilot study on BOF with a herniation of ≥1.0 mL, the authors aimed to assess (1) which patients with BOF develop functional and/or aesthetic symptoms, (2) which CT scan findings predict late visible deformity, (3) which patients with BOF benefit from surgical versus observational treatment, and (4) the importance of timing for surgical repair.
The Ethics Committee of the Karolinska Institute (EPN) Stockholm, Sweden, approved the study protocols and informed consent was obtained from each individual included in the study. The studies were conducted in adherence to the Declaration of Helsinki.
Materials and Methods
This was a prospective randomized controlled pilot study on orbital BOF with ≥ 1.0 mL herniation performed at the Department of ENT and Head and Neck Surgery at the Karolinska University Hospital in Stockholm, Sweden. Patients with facial trauma who presented with a CT scan verifying an isolated unilateral inferior or inferomedial BOF were included between 2011 and 2015. Patients with injuries threatening the eye such as ocular motility limitation were treated according to current guidelines and not included in this study.
After clinical examination and evaluation of the CT scans, patients with ≥1.0 mL herniation were asked to participate in the study. After the inclusion, patients were randomized to surgery or observation. The patients obtained the information consent prior to the randomization. Sealed envelopes were used to randomize the patients. The Envelopes were distributed in a predetermined order, concealed from the researchers.
Patients were followed up for a minimum of 1 year with five clinical examinations (2 weeks and 1, 3, 6, and 12 months postinjury). If a patient in the observational group developed symptoms in need of surgical correction, that is, persisting diplopia or visible deformity such as hypoglobus, superior sulcus deformity, or enophthalmus, surgery was offered and performed compatible to the surgical group. If surgery was performed, patients were followed up for at least 1 year after surgery. Patients in the surgical group underwent reduction of the herniated orbital content and reconstruction of the entire fractured orbital walls with orbital titanium-reinforced porous polyethylene implants.
At each visit, patients completed a self-reported questionnaire and a clinical examination was performed by a physician for functional symptoms such as ocular motility, diplopia, hypoesthesia of the infraorbital nerve, as well as cosmetic deformities such as enophthalmus, hypoglobus, and superior sulcus deformity. The measurement of enophthalmus was performed according to Hertel’s exophthalmometer [16]. Hypoglobus and superior sulcus deformity were noted if they were visible. Patients were asked if they felt satisfied with the treatment they received at each visit. The patients’ questionnaire and the physicians’ protocol were study specific and have not been validated.
The CT scans were performed with ≤2-mm slices. All CT scans of patients who completed the study were analyzed for several measurements, see Table 1. They were transferred to a workstation (GE Healthcare Advantage Workstation version 4), where the images were evaluated in axial, coronal, and sagittal planes in an osseous window level setting according to a previous study [17]. The method we used in all the measurements of the CT scans (Figure 1) is described and used again in our other study [18].
Measurements were made accordingly: Sagittal plane where the fracture was considered largest in the inferior wall:
- The distance from the inferior orbital rim to the anterior edge of the fracture (Figure 1a, i), on the same slice.
- The distance from the inferior orbital rim to the posterior edge of the fracture (Figure 1a, ii), on the same slice.
- The longest anteroposterior length of the fracture (Figure 1a, iii).
- The degree of displacement of orbital bulge in mm (Figure 1b).
Coronal plane:

Table 1.
Patients with inferior and inferomedial orbital BOF, randomized to observation and surgery and subgroups with VD vs. no VD in comparison with CT scan measurements.
Table 1.
Patients with inferior and inferomedial orbital BOF, randomized to observation and surgery and subgroups with VD vs. no VD in comparison with CT scan measurements.
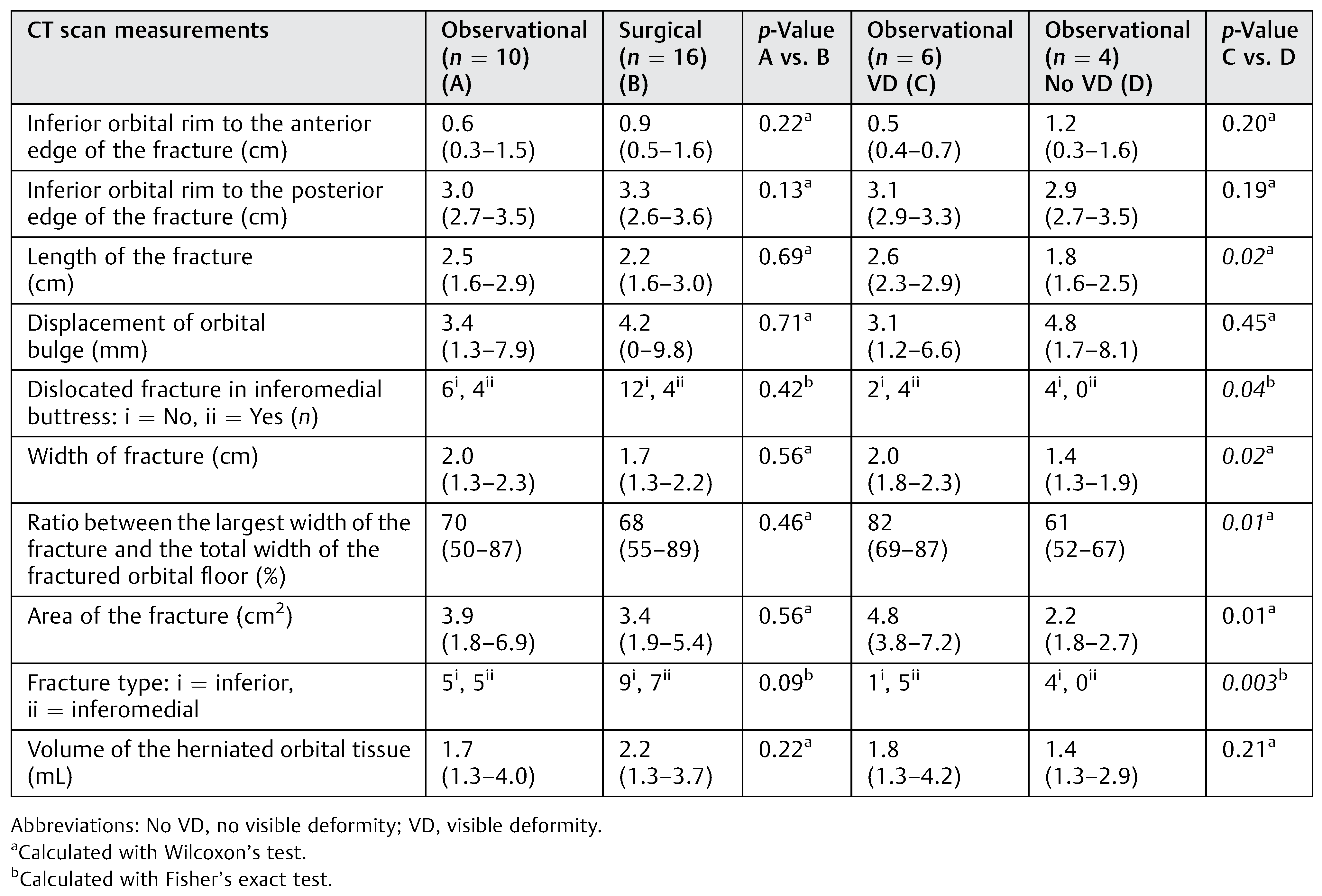 |
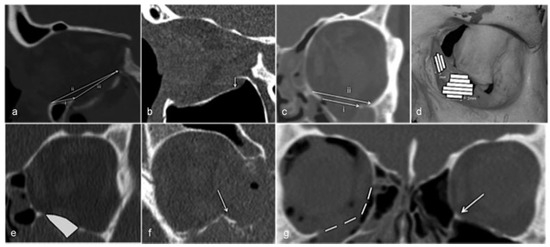
Figure 1.
(a) Inferior orbital rim to anterior edge of the fx i, posterior edge of the fx ii, and the longest anteroposterior length of the fx iii. (b) Displacement of orbital bulge. (c) Largest width of the fx i and the orbital floor ii. (d) Area of the fx. (e) Volume of the herniated orbital tissue. (f) Medial buttress fractured and dislocated. (g) Estimation of displaced inferomedial buttress in comparison with the unfractured contralateral orbit and inferomedial buttress (arrow).
Area and Volume Measurements
All measurements were performed on the initial CT scans by a second person and not by the main author, using the GE Healthcare Advantage Workstation version 4 (GE Healthcare, Milwaukee, WI).
Area
We performed a quantitative computational method for calculating the area of the fractures [19,20]. Stacks of 2-mm slices in the coronal plane were created. The width of the fractured orbital wall in each 2-mm slice was measured. This resulted in trapezoidal strips with a known area (see Figure 1d). The areas of the strips were combined to calculate the entire area of the fracture. Where the inferomedial buttress was displaced, the measurement was estimated after comparison with the unfractured contralateral orbit (Figure 1g).
Volume
The CT scans used were axial raw thin slices in a soft-tissue window setting (HU 600/1000) to distinguish blood from orbital fat and muscle tissue. The following steps were taken: “VR tools”; “Segment”; and “Quick paint” with brush size of 2 mm. The herniated content was marked green in one slice and then scrolled two to three steps posteriorly to mark the content again. This was repeated until all the content was marked in this plane. Then the same procedure was performed in the sagittal plane to fill in the gaps between the coronal slices. In the medial fractures, the coronal and axial planes were used instead. The marked area was applied and the “display tools” were used. The “Threshold” was set between −300 and 200 to exclude bone and air. To measure volume, the “Globe” function was used.
Statistical Analyses
All variables are expressed as median (10th and 90th percentiles) or percentages, as appropriate. Statistical significance was set at the level of p < 0.05. Comparisons between two groups were assessed with the nonparametric Wilcoxon test for continuous variables and Fisher’s exact test or Chisquare test for nominal variables. We did not take into consideration the multiple comparisons; all significance must be taken as descriptive. All statistical analyses were performed using statistical software SAS version 9.4 (SAS Campus Drive, Cary, NC).
Results
Clinical Characteristics
Twenty-nine patients were recruited in this study. Three patients from the observational group dropped out of the trial: one chose not to complete the study, one moved to another country, and one patient died from unrelated causes. Twenty-six patients completed the study, 10 in the observational group and 16 in the surgical group. None of the patients had ocular motility limitation at inclusion or at final control.
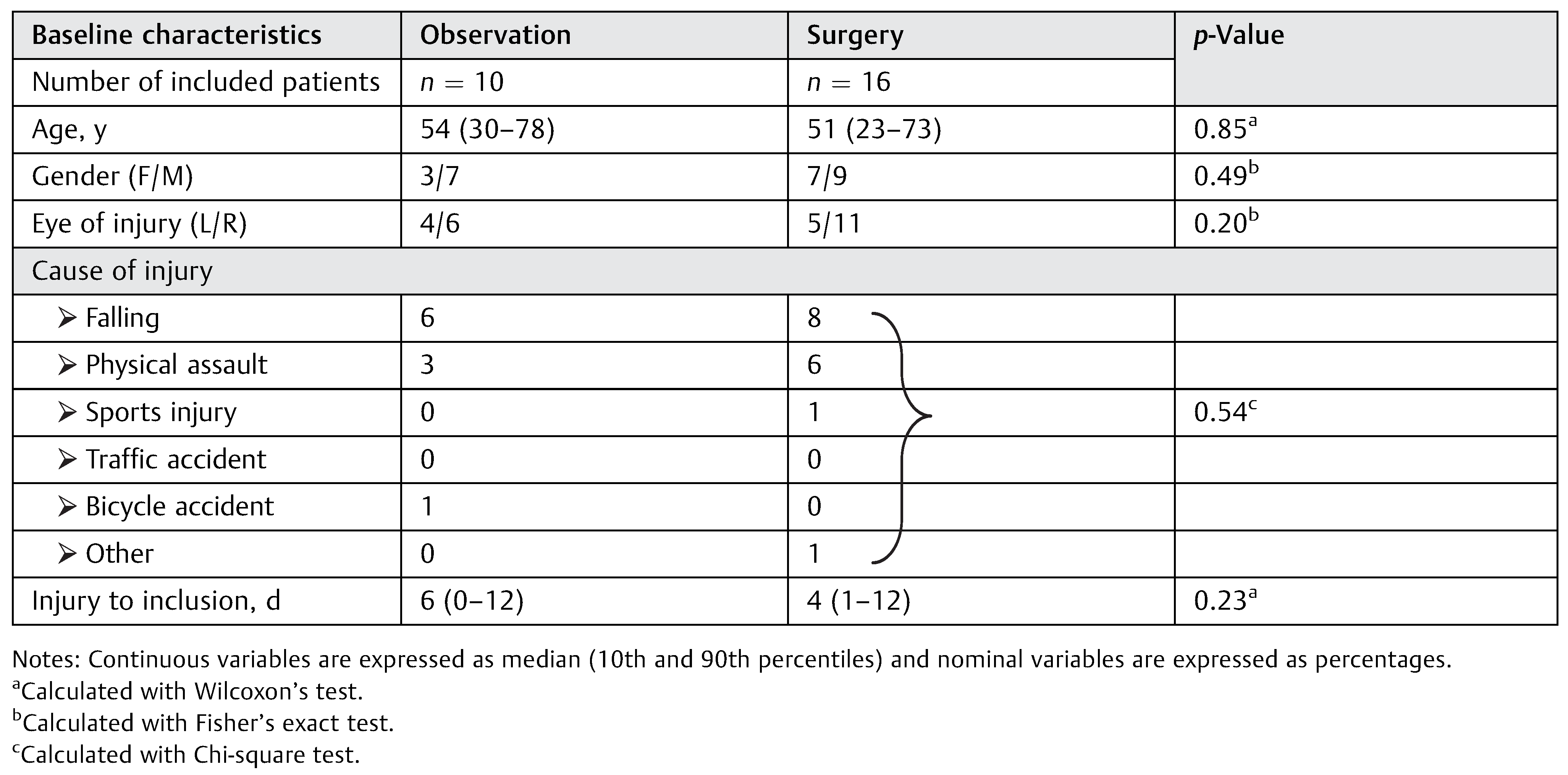
For clinical characteristics of the patients, see Table 2. There was no significant difference between the patients in the observational and the surgical groups in baseline characteristics including gender, injured side, age, cause of injury, and time to inclusion.
The most common cause of injury was falling followed by physical assault. All patients in both groups were satisfied with the treatment that they received at the final control.
All the 26 patients presented in this study had been at an inclusion visit, a 1-year follow-up visit, and at least one more visit in between; 16 patients were at the first visit (1–3 weeks postinjury); 19 patients at the second visit (3–7 weeks postinjury), 19 patients were at the third visit (10–16 weeks postinjury), 14 patients were at fourth visit (22–32 weeks postinjury); and 26 patients were at the 5th and final visit or final control (49–103 weeks postinjury). For all included patients, the median time from injury to inclusion in the trial was 5 days (0–12). In the observational group, six patients developed a visible deformity with a median of 34 (16–150) days after the injury and five of these patients chose to proceed to surgery which was performed 37 (17–170) days after the injury. In the surgical group, the median time from injury to surgery was 13 (3–17) days.

Table 2.
Two groups of patients: observation and surgery.
Table 2.
Two groups of patients: observation and surgery.
 |
Diplopia
Observational Group
According to the reports of both patients and physicians, 50% (n = 5) of the patients had diplopia at inclusion and this remained with down gaze in 20% (n = 2) of the patients at the final control. Due to hypoglobus and enophthalmus, these two patients proceeded to surgery 17 and 37 days after injury, respectively. The patient who proceeded to surgery at 17 days postinjury reported diplopia in up gaze, while the patient who underwent surgery at 37 days postinjury had no diplopia at the 1-year follow-up. No patient needed surgery due to diplopia.
Surgical Group
Patients reported diplopia in 56% (n = 9) of the cases and physicians in 50% (n = 8) of the cases at inclusion. Diplopia remained in 6% (n = 1) of the patients, which was observed in lateral gaze at the final control.
Hypoesthesia
Observational Group
According to the reports of both patients and physicians, 60% (n = 6) of the patients had hypoesthesia of the inferior orbital nerve at inclusion and it remained in 40% (n = 4) of the patients at final control. One patient developed hypoesthesia after undergoing surgery.
Surgical Group
According to the reports of both patients and physicians, 50% (n = 8) of the patients had hypoesthesia of the inferior orbital nerve at inclusion. At final control, four of them still had hypoesthesia and another four developed hypoesthesia after undergoing surgery.
Visible Deformity
Observational Group
Visible deformity (superior sulcus deformity and/or hypoglobus and/or 3-mm enophthalmus) was found by the physicians in 60% (n = 6) of the patients, 34 (16–150) days after the injury. Eighty-three percent (n = 5) of the patients with visible deformity had inferomedial BOF and they all chose to proceed with surgery. Surgery was performed 37 (17–170) days after the injury. Visible deformity was resolved in all patients underwent surgery. Seventeen percent (n = 1) of the patients with a visible deformity had an inferior BOF and chose not to undergo surgery.
Surgical Group
None of the operated patients had a visible deformity at final control. The surgery was performed 13 (3–17) days after injury. In two patients, a slightly scleral show was found at the final control and the patients were not interested in surgical correction.
CT Scan Measurements
We found no statistically significant differences for CT scan measurements between the observational group and surgical group as expected. There were no statistically significant differences between observational group with inferior BOF and surgical group with inferior BOF, also between observational group with inferior BOF who developed visible deformity and those who did not. There were statistically significant differences between observational group who developed visible deformity and those who did not (Table 1).
Observational Group
Five patients had inferior BOF and five patients had inferomedial BOF. Of the six patients who developed visible deformity, one patient had inferior BOF and five patients had inferomedial wall fractures. Patients with inferomedial BOF had a herniation of 1.6 mL (1.3–4.2). This finding is in line with findings of our earlier studies which showed that with inferomedial BOF visible deformity is expected when the herniation is ≥0.9 mL [18].
With inferior BOF, the median volume of the herniation was 1.8 mL (1.3–2.9) and the distance from the inferior orbital rim to the posterior edge of the fracture was 2.9 mm (2.7–3.5). One patient with an inferior BOF had a 3.3-cm distance from the inferior orbital rim to the posterior edge of the fracture and developed a visible deformity. Three patients with inferior BOF had a <3.0 cm distance from the inferior orbital rim to the posterior edge of the fracture and did not develop a visible deformity. One patient with an inferior BOF had 3.5-cm distance and did not develop visible deformity. This observation is also in line with findings of our earlier study [18] that with inferior BOF visible deformity is expected when the distance from the inferior orbital rim to the posterior edge of the fracture is ≥ 3.0 cm.
We found a statistically significant difference when comparing the patients in the observational group who developed visible deformity with those who did not in type of fracture (p = 0.003), the length of the fracture (p = 0.02), the width of the fracture (p = 0.02), the ratio between the largest width of the fracture and the total width of the fractured orbital toor (p = 0.01), dislocated fracture in inferomedial buttress (p = 0.04), and the area of the fracture (p = 0.01). All these significant differences were observed only in patients with inferomedial fracture.
Surgical Group
Nine patients had inferior BOF and seven patients had inferomedial BOF. The median volume of the herniation was 2.2 (1.3–3.7) mL and the distance from the inferior orbital rim to the posterior edge of the fracture was 3.3 (2.6–3.6) mm.
Discussion
We present a prospective randomized pilot study on orbital BOF with ≥1.0 mL herniation. To the best of our knowledge, this is the first prospective randomized study on orbital BOF.
Although this is a pilot study, with a limited number of patients, we found significant differences between inferomedial and inferior BOFs (p = 0.003) when comparing patients who developed visible deformities to those who did not. All patients with inferomedial BOF who were randomized to observation developed visible deformities and they all proceeded to surgery, whereas one-fifth of the patients with an inferior BOF developed a deformity but chose not to have an operation. This later group needs to be studied further.
As described in our other study [18], we found that diplopia without ocular muscle entrapment will, to a large extent, resolve. Diplopia is believed to be due to edema and as long as it decreases, there is no indication for surgery. A remaining diplopia can be due to nerve injury [21], which may not be addressed with a surgical intervention. We disagree with the suggestion that a BOF with diplopia, not due to ocular motility limitation, requires surgical intervention within 2 weeks [22].
Hypoesthesia of the infraorbital nerve remains both in the observational and the surgical groups and may persist after 1 year. However, we found that surgery could induce hypoesthesia which therefore should be a part of patient informed consent. Our recommendation is that hypoesthesia should not be an indication for surgery.
This randomized controlled study of BOF is unique most probably due to a long history of surgical intervention on all patients with substantial BOF. From earlier studies we concluded that the importance of the degree of herniation in the development of enophthalmus was unclear [17]. Regarding the design of the study, we considered an orbital BOF with ≥1.0 mL herniation as at risk for the development of enophthalmos [20,23]. Furthermore, we hypothesized that several patients in this study with BOF would develop visible deformity if they were observed over time. From an ethical perspective, it was therefore necessary to design the study so that if a visible deformity was discovered it would be corrected as soon as it developed. Our intention was that these patients would receive the same end result as if the patient was operated prior to the development of a visible deformity. Therefore, we designed the study with continuous clinical controls with short time periods in between and patients were thoroughly informed about the importance of follow-up. We do not feel that we have unnecessarily put patients at risk because there is lack of evidence-based knowledge in the treatment of BOF. However, surgery was performed 37 (17–170) days after the injury in the patients in the observational group who developed visible deformity and chose surgical treatment. Visible deformity was resolved in all patients who had surgical intervention. Seventeen percent (n = 1) of the patients with a visible deformity had an inferior BOF and refused to undergo surgery.
In earlier studies, it has been described that inferomedial BOF has a high risk for the development of visible deformity [5]. In this study, we found that all patients in the observational group with inferomedial fractures had a herniation of ≥1.0 mL and developed visible deformity. They all opted for surgical treatment and at the 1-year follow-up, none of them showed visible deformity. This finding supports our previous findings in our observational study that with inferomedial BOF, visible deformity is expected when the herniation is ≥0.9 mL [18]. Therefore, we hypothesize that there is a fundamental difference between inferomedial and inferior BOF.
In our previous study, we found that the size of the herniation alone could not predict the visible deformity in inferior BOF. In inferior BOF fractures with a herniation of ≥1.0 mL, we also found that a distance from the inferior orbital rim to the posterior edge of the fracture ≥ 3.0 cm was important for the patients’ outcome, whereas in patients with fractures with <1.0 mL herniation, the area of the fracture ≥ 2.3 cm [2] was associated with visible deformity [18].
In the present study, 80% (4/5) of patients with inferior BOF in the observational group did not develop visible deformity. Three of these patients had a fracture where the posterior fracture edge was <3.0 cm from the inferior rim. This is in accordance with our earlier findings that a cosmetic deformity can be predicted in more than 80% with this type of fracture [18]. Two patients with inferior BOF, where the fracture was ≥3.0 cm from the inferior orbital rim, were included in the study. One of the patients developed visible deformity, whereas the other patientdid not. The reason for this is unclear and needs to be studied further. In this pilot study, the number of patients with inferior BOF is still insufficient to clarify this issue. In the literature, it has earlier been proposed that in BOF, a fracture > 50% of the orbital toor [6], an area of the fracture [9] > 1.0 cm2, as well as a herniation of >1.5 mL [7] can be predictive for patient outcome. This could not be confirmed in the present study. However, in our earlier observational study, these parameters have not been shown to correlate with the development of a visible deformity [18].
In the literature, early surgical intervention in patients with BOF has been proposed to be important for patient outcome [6]. In our study, the timing of surgery naturally differed between the observational and the surgical groups. In the surgical group, surgical correction was performed 13 (3–17) days after the injury, while in the observational group surgical correction was performed 37 (17–170) days after the injury. In spite of this, the surgical result was found to be satisfactory by the patients and also the physician in both groups. This may be interpreted that the surgical result from a late correction appears to be the same as in early corrections if the surgical correction is performed immediately after the visible deformity is discovered.
Strength and Limitations of this Study
The strength of this study is that, as far as we are aware, this is the first prospective randomized study on BOF. Furthermore, in our opinion we have addressed the problems and difficulties with surgical randomized controlled studies. All the clinical examinations were performed by one attending physician and the CT scans measurements by another physician, making the results reliable.
Since this is a pilot study, a limitation would be the low number of patient analyses. A limitation of this study is that nonvalidated clinical protocols were used for physician’s and patient’s questionnaires. Hertel’s exophthalmometry has limitations and mismeasurements cannot be ruled out. Another limitation is 10% drop out of patients who were included in the study.
Finally, all the patients in this study expressed satisfaction with the treatment they have received.
Conclusion
In this prospective randomized controlled pilot study on BOF, we found that all patients in the observational group with inferomedial fractures had a herniation of ≥ 1.3 mL and they all developed visible deformity, but this was based on only five patients. Diplopia in BOF, without motility limitation, is believed to be due to edema and it is not an indication for surgery as long as it decreases. Hypoesthesia of inferior orbital nerve may remain and surgery may increase the risk for the development of hypoesthesia. Although ethically challenging, we feel that randomized controlled studies of BOF are appropriate if one follows up patients closely.
References
- Bansagi, Z.C.; Meyer, D.R. Internal orbital fractures in the pediatric age group: Characterization and management. Ophthalmology 2000, 107, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, B.W.; Scawn, R.L.; Korn, B.S.; Kikkawa, D.O. Secondary orbital reconstruction in patients with prior orbital fracture repair. Ophthal Plast Reconstr Surg 2016, 32, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Putterman, A.M.; Stevens, T.; Urist, M.J. Nonsurgical management of blow-out fractures of the orbital toor. Am J Ophthalmol 1974, 77, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Boush, G.A.; Lemke, B.N. Progressive infraorbital nerve hypesthesia as a primary indication for blow-out fracture repair. Ophthal Plast Reconstr Surg 1994, 10, 271–275. [Google Scholar] [CrossRef]
- Ellis, E., III. Orbital trauma. Oral Maxillofac Surg Clin North Am 2012, 24, 629–648. [Google Scholar] [CrossRef]
- Hawes, M.J.; Dortzbach, R.K. Surgery on orbital toor fractures. Intuence of time of repair and fracture size. Ophthalmology 1983, 90, 1066–1070. [Google Scholar] [CrossRef]
- Manson, P.N.; Grivas, A.; Rosenbaum, A.; Vannier, M.; Zinreich, J.; Iliff, N. Studies on enophthalmos: II. The measurement of orbital injuries and their treatment by quantitative computed tomography. Plast Reconstr Surg 1986, 77, 203–214. [Google Scholar] [CrossRef]
- Mansour, T.N.; Rudolph, M.; Brown, D.; Mansour, N.; Taheri, M.R. Orbital blowout fractures: A novel CT measurement that can predict the likelihood of surgical management. Am J Emerg Med 2017, 35, 112–116. [Google Scholar] [CrossRef]
- Rinna, C.; Ungari, C.; Saltarel, A.; Cassoni, A.; Reale, G. Orbital toor restoration. J Craniofac Surg 2005, 16, 968–972. [Google Scholar] [CrossRef]
- Alinasab, B.; Ryott, M.; Stjärne, P. Still no reliable consensus in management of blow-out fracture. Injury 2014, 45, 197–202. [Google Scholar] [CrossRef]
- Alonso-Rodriguez, E.; Cebrián, J.L.; Nieto, M.J.; Del Castillo, J.L.; Hernández-Godoy, J.; Burgueño, M. Polyetheretherketone custommade implants for craniofacial defects: Report of 14 cases and review of the literature. J Craniomaxillofac Surg 2015, 43, 1232–1238. [Google Scholar]
- Gander, T.; Essig, H.; Metzler, P.; et al. Patient specific implants (PSI) in reconstruction oforbital toor and wall fractures. J Craniomaxillofac Surg 2015, 43, 126–130. [Google Scholar] [PubMed]
- Schmelzeisen, R.; Gellrich, N.C.; Schoen, R.; Gutwald, R.; Zizelmann, C.; Schramm, A. Navigation-aided reconstruction of medial orbital wall and toor contour in cranio-maxillofacial reconstruction. Injury 2004, 35, 955–962. [Google Scholar] [PubMed]
- Zizelmann, C.; Gellrich, N.C.; Metzger, M.C.; Schoen, R.; Schmelzeisen, R.; Schramm, A. Computer-assisted reconstruction of orbital toor based on cone beam tomography. Br J Oral Maxillofac Surg 2007, 45, 79–80. [Google Scholar]
- Burnstine, M.A. Clinical recommendations for repair of orbital facial fractures. Curr Opin Ophthalmol 2003, 14, 236–240. [Google Scholar] [PubMed]
- Cole, H.P., III; Couvillion, J.T.; Fink, A.J.; Haik, B.G.; Kastl, P.R. Exophthalmometry: A comparative study of the Naugle and Hertel instruments. Ophthal Plast Reconstr Surg 1997, 13, 189–194. [Google Scholar]
- Alinasab, B.; Beckman, M.O.; Pansell, T.; Abdi, S.; Westermark, A.H.; Stjärne, P. Relative difference in orbital volume as an indication for surgical reconstruction in isolated orbital toor fractures. Craniomaxillofac Trauma Reconstr 2011, 4, 203–212. [Google Scholar]
- Alinasab, B.; Borstedt, K.J.; Rudström, R.; et al. New algorithm for management of orbital blowout fracture based on prospective study. Craniomaxillofac Trauma Reconstr, 2018; Epub ahead of print. [Google Scholar]
- Holtmann, H.; Eren, H.; Sander, K.; Kübler, N.R.; Handschel, J. Orbital toor fractures–shortand intermediate-term complications depending on treatment procedures. Head Face Med 2016, 12, 1. [Google Scholar]
- Ploder, O.; Klug, C.; Voracek, M.; Burggasser, G.; Czerny, C. Evaluation of computer-based area and volume measurement from coronal computed tomography scans in isolated blowout fractures of the orbital toor. J Oral Maxillofac Surg 2002, 60, 1267–1272, discussion 1273–1274. [Google Scholar] [PubMed]
- Neovius, E.; Fransson, M.; Matthis, S.P.; et al. Persistent diplopia after fractures involving the orbit related to nerve injury. J Plast Reconstr Aesthet Surg 2015, 68, 219–225. [Google Scholar]
- Yu, D.Y.; Chen, C.H.; Tsay, P.K.; Leow, A.M.; Pan, C.H.; Chen, C.T. Surgical timing and fracture type on the outcome of diplopia after orbital fracture repair. Ann Plast Surg 2016, 76 (Suppl. S1), S91–S95. [Google Scholar] [PubMed]
- Whitehouse, R.W.; Batterbury, M.; Jackson, A.; Noble, J.L. Prediction of enophthalmos by computed tomography after ‘blow out’ orbital fracture. Br J Ophthalmol 1994, 78, 618–620. [Google Scholar] [PubMed]
© 2018 by the author. The Author(s) 2018.