Bone Allograft: An Option for Total Mandibular Reconstruction
Abstract
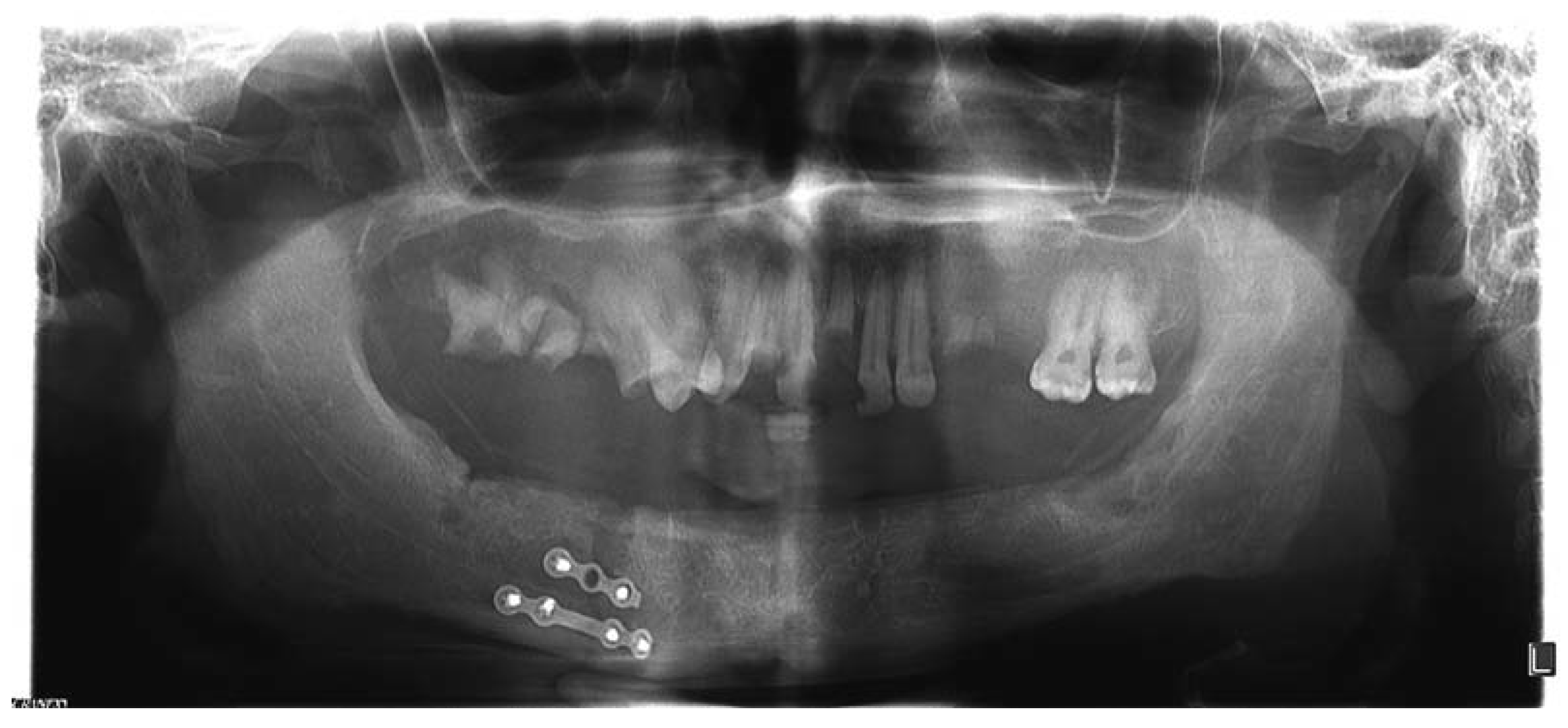
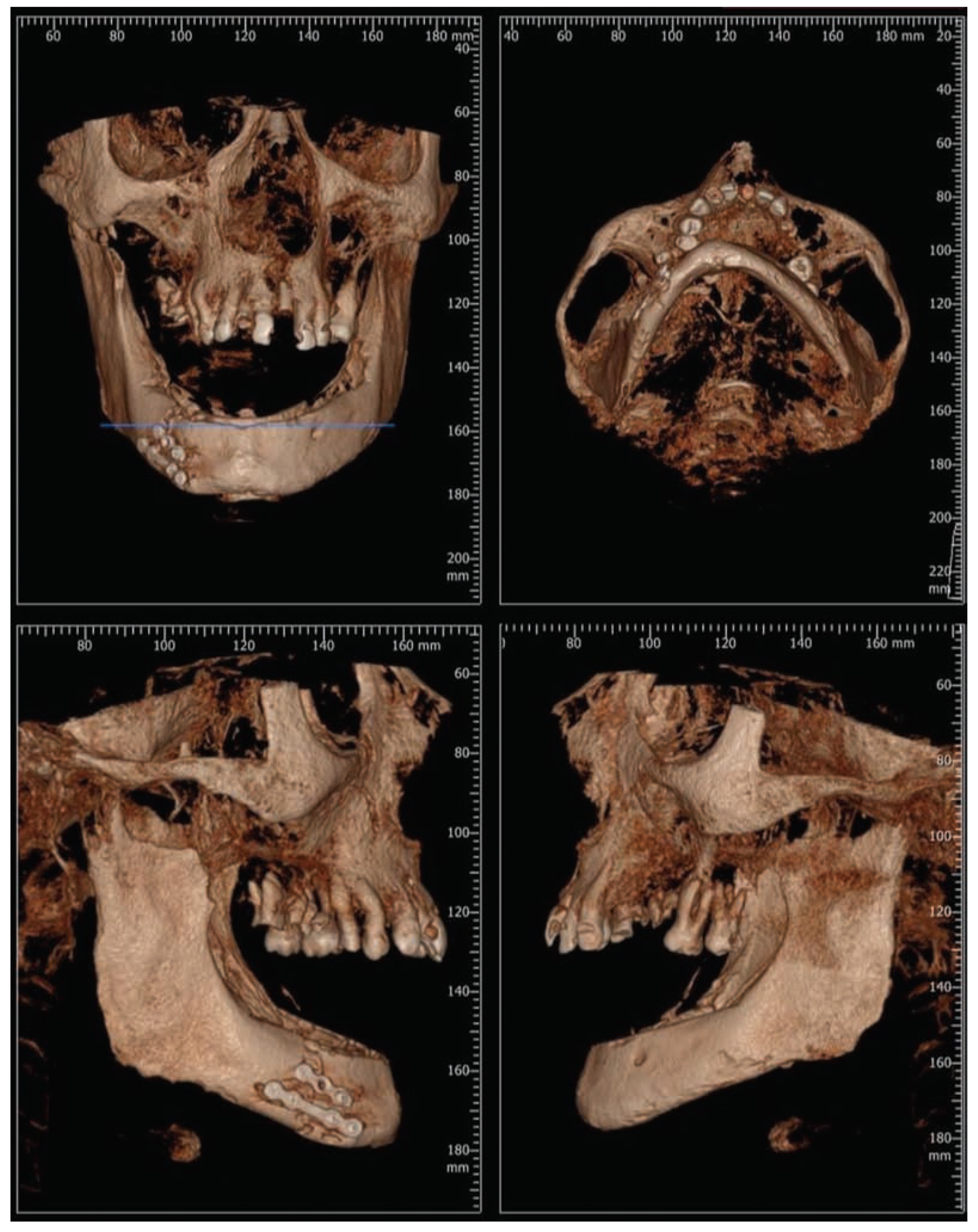
:Case Presentation
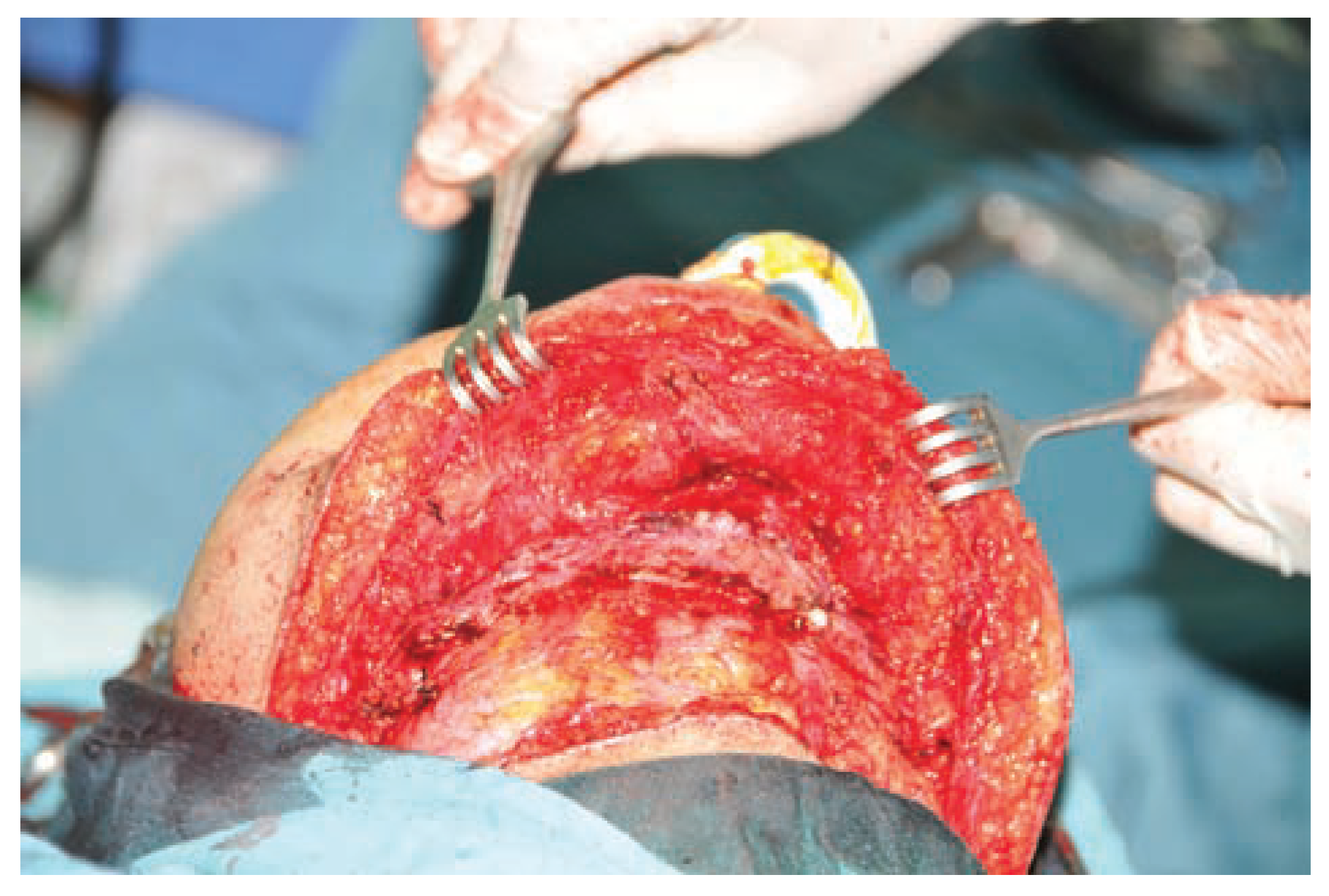
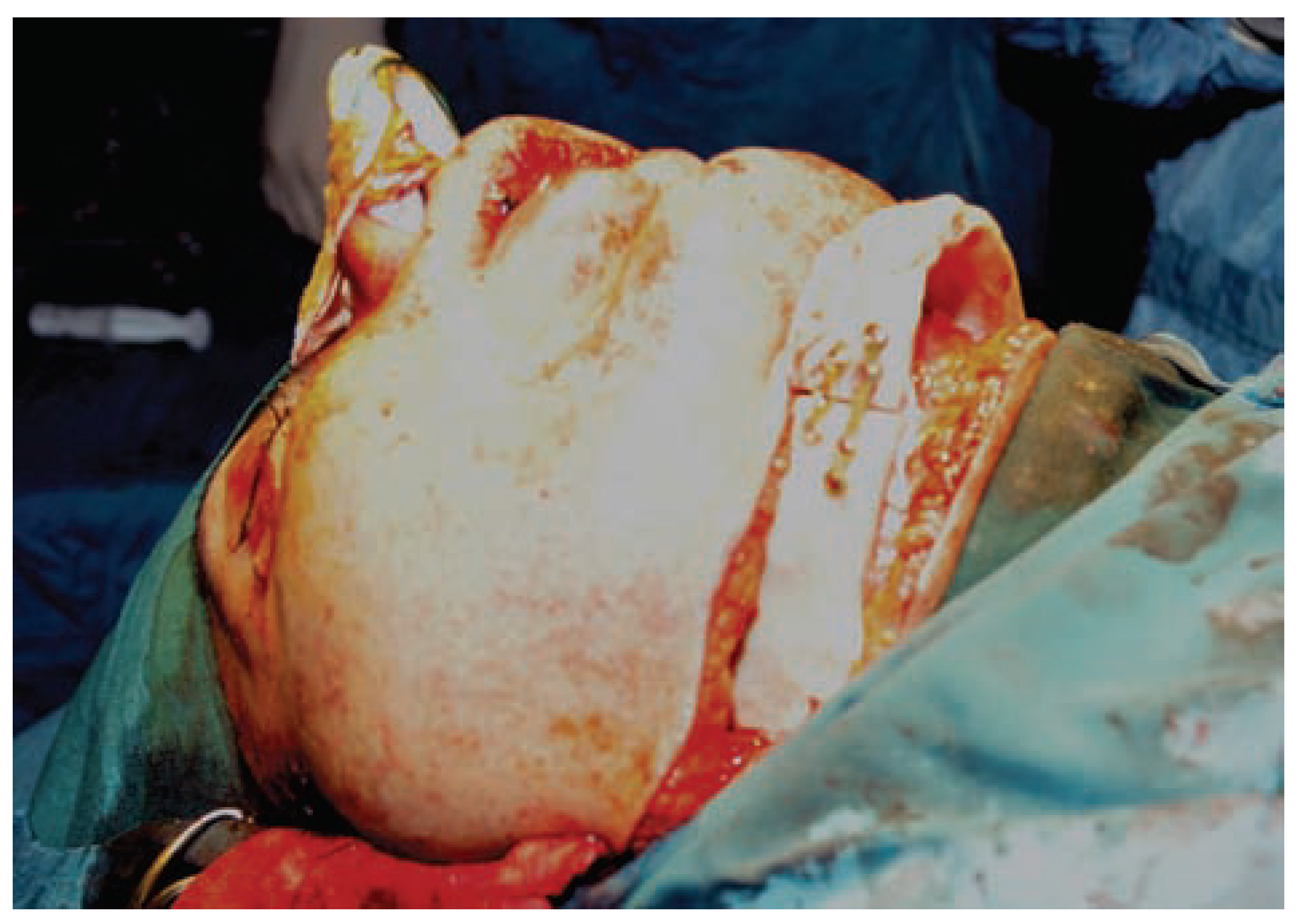
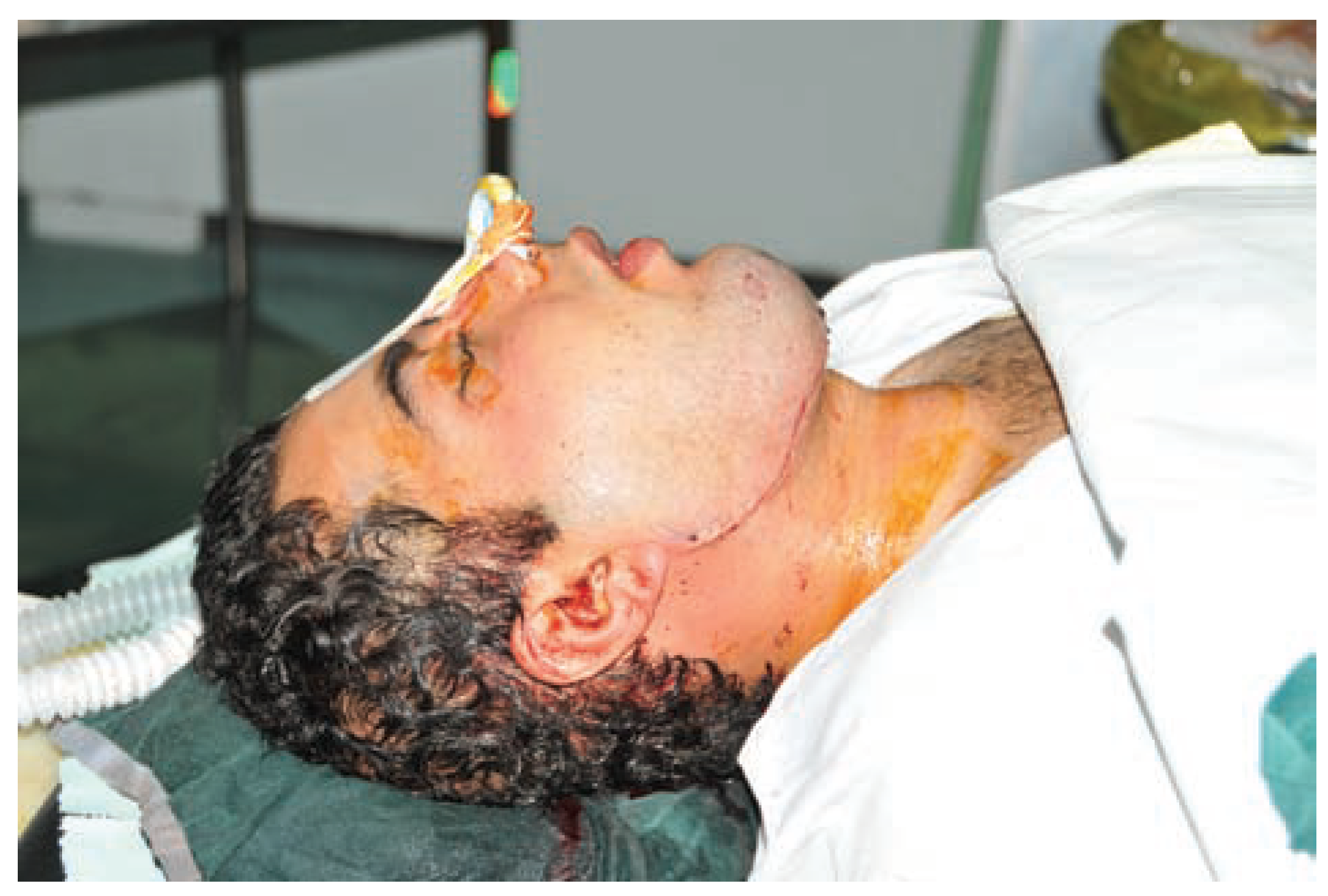
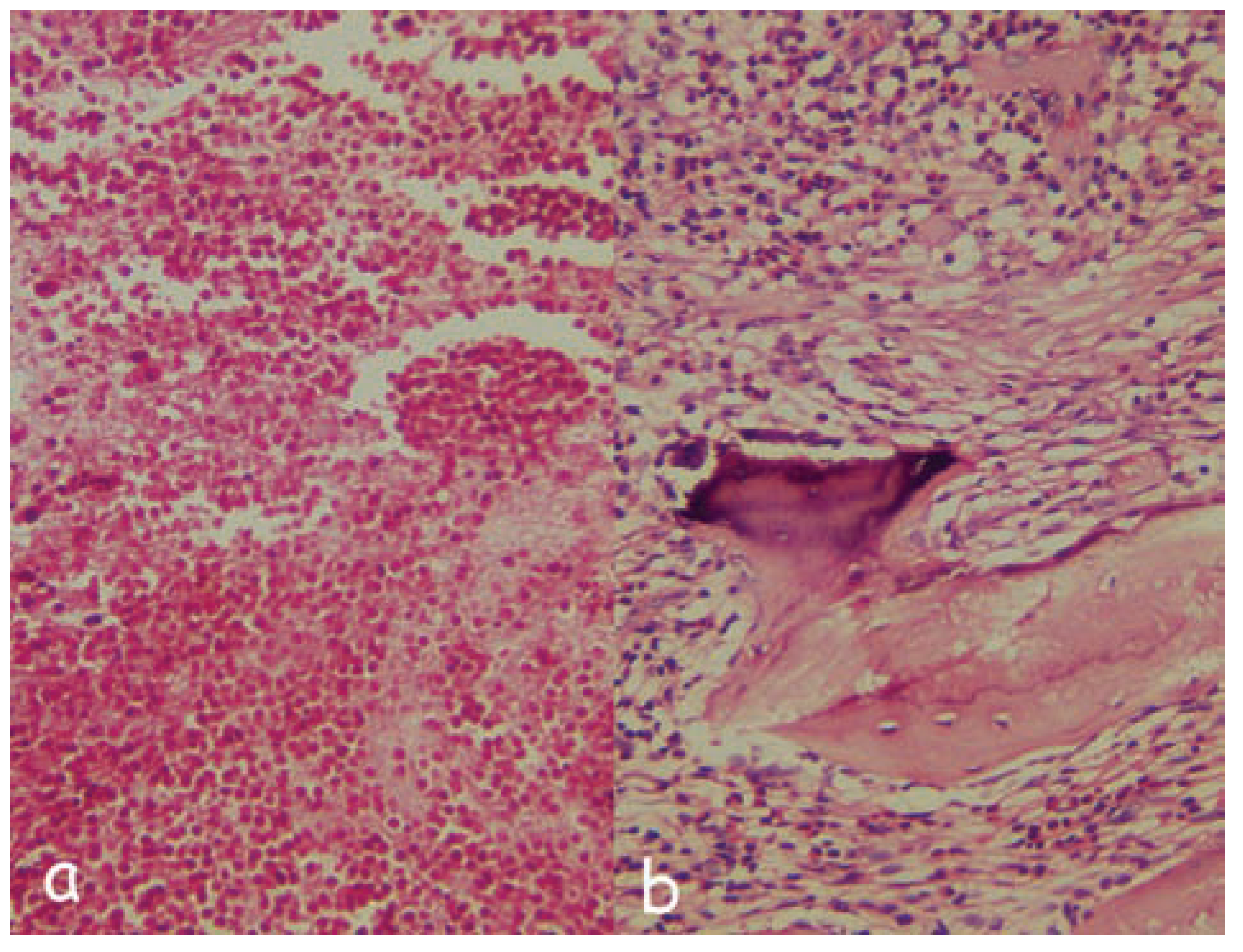
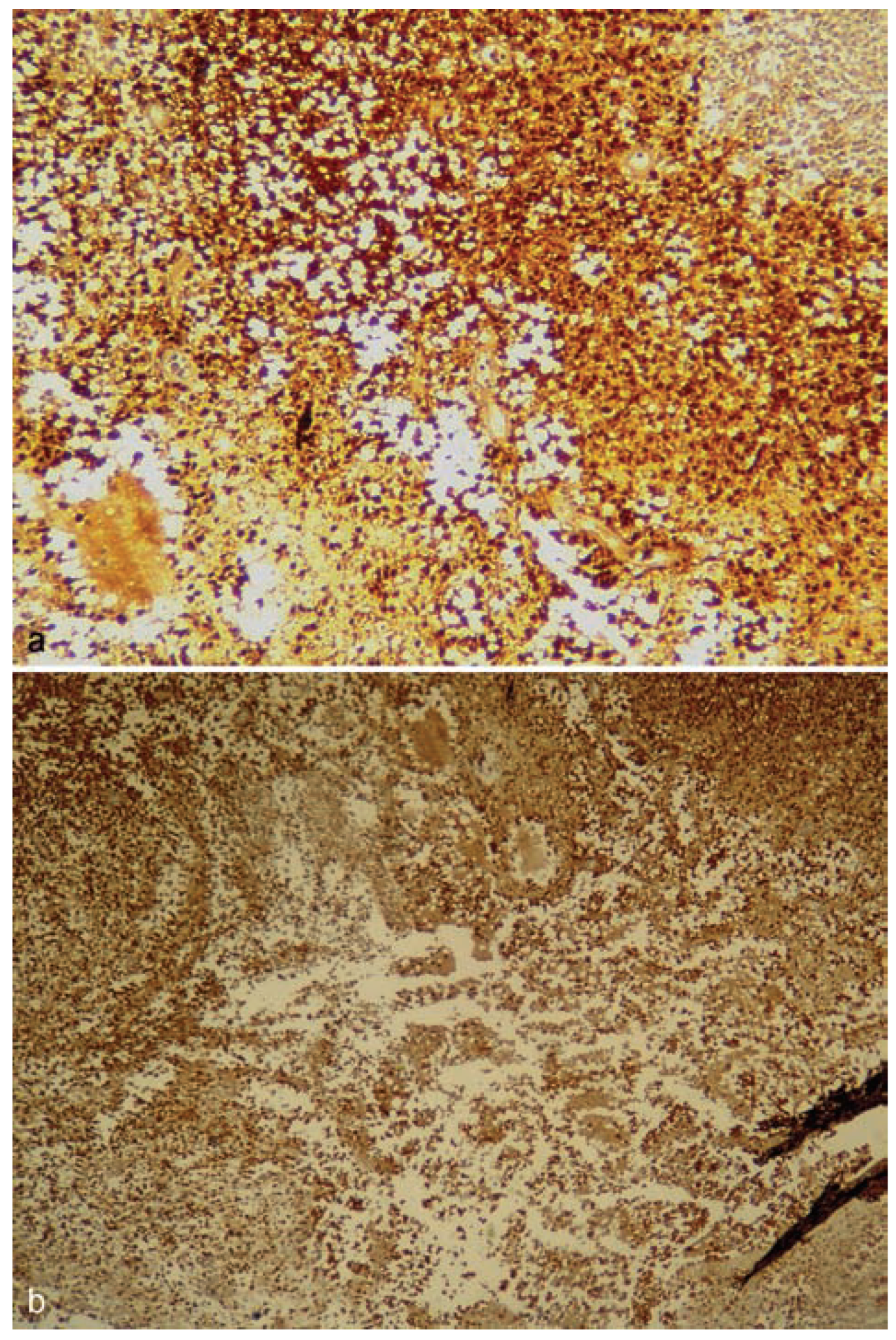

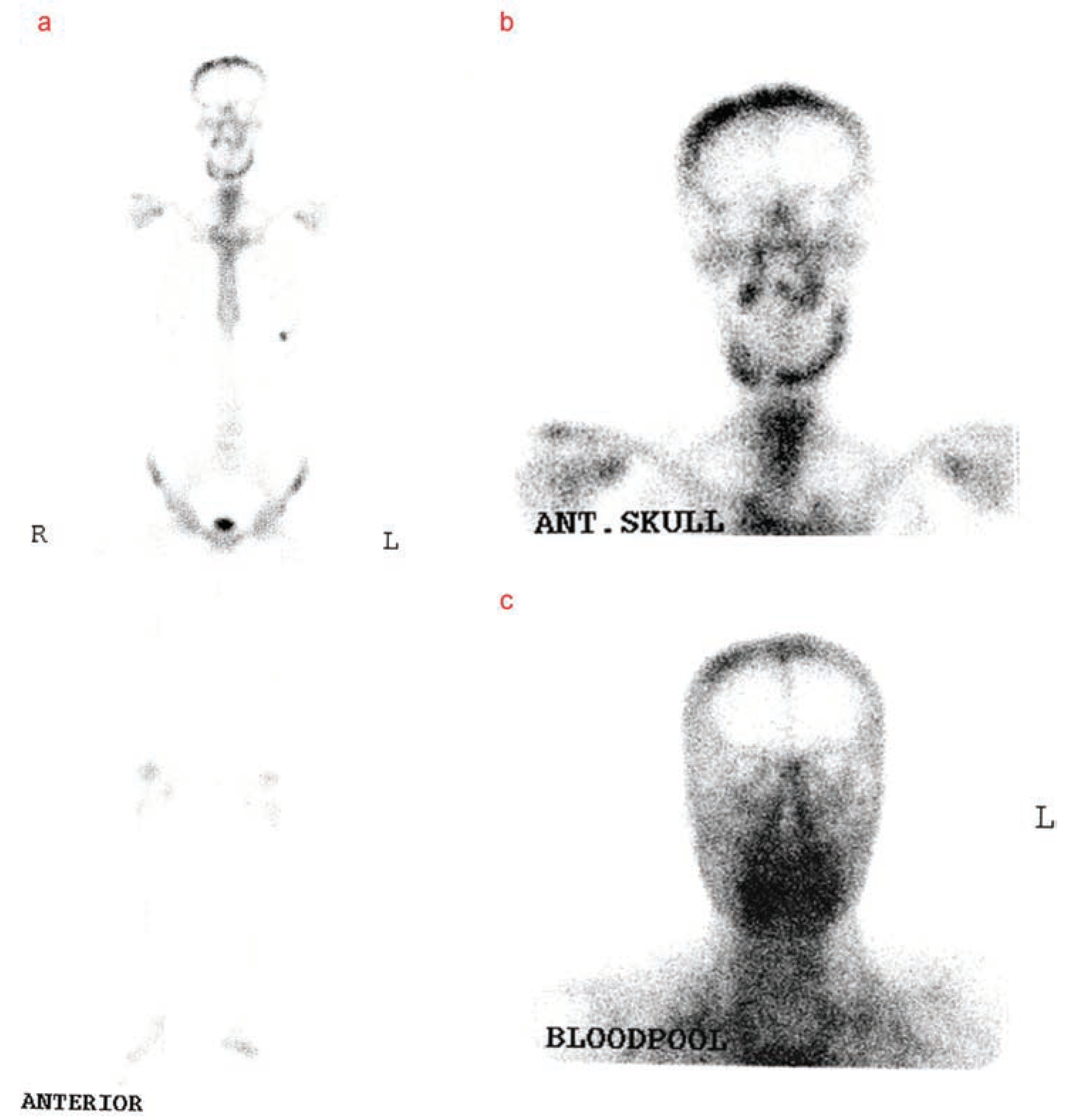
Discussion
- Donors from high-risk groups, as determined by medical testing and/or behavioral risk assessments
- Donors with positive test for HIV antibody confirmed by ELISA
- Donors whose autopsy reveals occult disease
- Donors with positive bone tests for bacterial contamination
- Donors with positive bone test for HBsAg or HCV
- Donor with positive tests for syphilis
 |
Funding
Conflicts of Interest
Acknowledgments
References
- Genden, E.; Haughey, B.H. Mandibular reconstruction by vascularized free tissue transfer. Am J Otolaryngol 1996, 17, 219–227. [Google Scholar] [CrossRef]
- Rana, M.; Warraich, R.; Kokemüller, H.; et al. Reconstruction of mandibular defects - clinical retrospective research over a 10- year period. Head Neck Oncol 2011, 3, 23. [Google Scholar] [CrossRef]
- Younger, E.M.; Chapman, M.W. Morbidity at bone graft donor sites. J Orthop Trauma 1989, 3, 192–195. [Google Scholar] [CrossRef]
- Banwart, J.C.; Asher, M.A.; Hassanein, R.S. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine 1995, 20, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Sàndor, G.K.; Rittenberg, B.N.; Clokie, C.M.; Caminiti, M.F. Clinical success in harvesting autogenous bone using a minimally invasive trephine. J Oral Maxillofac Surg 2003, 61, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Maeda, H. Recent developments of functional scaffolds for craniomaxillofacial bone tissue engineering applications. Sci- entificWorldJournal 2013, 2013, 863157. [Google Scholar] [CrossRef]
- Burwell, R.G. Studies in the transplantation of bone. VII. The fresh composite homograft-autograft of cancellous bone; an analysis of factors leading to osteogenesis in marrow transplants and in marrow-containing bone grafts. J Bone Joint Surg Br 1964, 46, 110–140. [Google Scholar] [CrossRef] [PubMed]
- Bolander, M.E.; Balian, G. The use of demineralized bone matrix in the repair of segmental defects. Augmentation with extracted matrix proteins and a comparison with autologous grafts. J Bone Joint Surg Am 1986, 68, 1264–1274. [Google Scholar] [CrossRef]
- Huggins, C. The formation of bone under the influence of epithelium of the urinary tract. Arch Surg 1931, 22, 377–408. [Google Scholar] [CrossRef]
- Clokie, C.M.; Sándor, G.K. Reconstruction of 10 major mandibular defects using bioimplants containing BMP-7. J Can Dent Assoc 2008, 74, 67–72. [Google Scholar]
- Urist, M.R. Bone: formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar]
- Huggins, C.; Wiseman, S.; Reddi, A.H. Transformation of fibroblasts by allogeneic and xenogeneic transplants of demineralized tooth and bone. J Exp Med 1970, 132, 1250–1258. [Google Scholar] [PubMed]
- Neigel, J.M.; Ruzicka, P.O. Use of demineralized bone implants in orbital and craniofacial reconstruction and a review of the literature. Ophthal Plast Reconstr Surg 1996, 12, 108–120. [Google Scholar] [PubMed]
- Mulliken, J.B.; Glowacki, J.; Kaban, L.B.; Folkman, J.; Murray, J.E. Use of demineralized allogeneic bone implants for the correction of maxillocraniofacial deformities. Ann Surg 1981, 194, 366–372. [Google Scholar]
- American Association of Tissue Banks. In 1998 Edition Standards for Tissue Banking; American Association of Tissue Banks: McLean, VA, USA, 1998.
- Horowitz, M.C.; Friedlaender, G.E. Immunologic aspects of bone transplantation. A rationale for future studies. Orthop Clin North Am 1987, 18, 227–233. [Google Scholar] [PubMed]
- Burchardt, H. Biology of bone transplantation. Orthop Clin North Am 1987, 18, 187–196. [Google Scholar]
- Saunders, J.R., Jr.; Hirata, R.M.; Jaques, D.A. Definitive mandibular replacement using reconstruction plates. Am J Surg 1990, 160, 387–389. [Google Scholar]
- Davidson, J.; Birt, B.D.; Gruss, J. A-O plate mandibular reconstruction: a complication critique. J Otolaryngol 1991, 20, 104–107. [Google Scholar]
- García García, A.; Suarez Quintanilla, D.; Diz Dios, P. Total mandibular replacement with a titanium plate after mandibulectomy for osteosarcoma. J Oral Maxillofac Surg 1994, 52, 863–867. [Google Scholar]
- Taylor, G.I.; Miller, G.D.; Ham, F.J. The free vascularized bone graft. A clinical extension of microvascular techniques. Plast Reconstr Surg 1975, 55, 533–544. [Google Scholar]
- Hidalgo, D.A. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg 1989, 84, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Bianchi, B.; Savi, A.; et al. Fibula free flap with endosseous implants for reconstructing a resected mandible in bisphosphonate osteonecrosis. J Oral Maxillofac Surg 2008, 66, 999–1003. [Google Scholar] [PubMed]
- Head, C.; Alam, D.; Joel, A.; et al. Microvascular flap reconstruction of the mandible: a comparison of bone grafts and bridging plates for restoration of mandibular continuity. Otolaryngology -Head Neck Surg 2003, 129, 48–54. [Google Scholar]
- Klokkevold, P.R.; Jovanovic, S.A. Newman, M.G., Takei, H.H., Carranza, F., Eds.; Advanced implant surgery and bone grafting techniques. In Carranza’s Clinical Periodontology, 9th ed.; W.B. Saunders: Philadelphia, PA, USA, 2002; pp. 907–908. [Google Scholar]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J 2001, 10 (Suppl 2), S96–S101. [Google Scholar] [PubMed]
- Goldring, S.R.; Roelke, M.; Glowacki, J. Multinucleated cells elicited in response to implants of devitalized bone particles possess receptors for calcitonin. J Bone Miner Res 1988, 3, 117–120. [Google Scholar] [CrossRef]
- Linde, F.; Sørensen, H.C. The effect of different storage methods on the mechanical properties of trabecular bone. J Biomech 1993, 26, 1249–1252. [Google Scholar] [CrossRef]
- Rummelhart, J.M.; Mellonig, J.T.; Gray, J.L.; Towle, H.J. A comparison of freeze-dried bone allograft and demineralized freeze-dried bone allograft in human periodontal osseous defects. J Periodontol 1989, 60, 655–663. [Google Scholar] [CrossRef]
- Francis, J.; Brunsvold, M.; Prewett, A.; Mellonig, J. Clinical evaluation of an allogeneic bone matrix in the treatment of periodontal osseous defects. J Periodontol 1995, 66, 1074–1079. [Google Scholar] [CrossRef]
- Nguyen, H.; Morgan, D.A.; Forwood, M.R. Sterilization of allograft bone: is 25 kGy the gold standard for gamma irradiation? Cell Tissue Bank 2007, 8, 81–91. [Google Scholar]
- Campbell, D.G.; Li, P. Sterilization of HIV with irradiation: relevance to infected bone allografts. Aust N Z J Surg 1999, 69, 517–521. [Google Scholar] [CrossRef]
- Dziedzic-Goclawska, A.; Kaminski, A.; Uhrynowska-Tyszkiewicz, I.; Stachowicz, W. Irradiation as a safety procedure in tissue banking. Cell Tissue Bank 2005, 6, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Cornu, O.; Banse, X.; Docquier, P.L.; Luyckx, S.; Delloye, C. Effect of freeze- drying and gamma irradiation on the mechanical properties of human cancellous bone. J Orthop Res 2000, 18, 426–431. [Google Scholar] [CrossRef]
- Nguyen, H.; Morgan, D.A.; Forwood, M.R. Sterilization of allograft bone: effects of gamma irradiation on allograft biology and biomechanics. Cell Tissue Bank 2007, 8, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Hamer, A.J.; Stockley, I.; Elson, R.A. Changes in allograft bone irradiated at different temperatures. J Bone Joint Surg Br 1999, 81, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control (CDC). Transmission of HIV through bone transplantation: case report and public health recommendations. MMWR Morb Mortal Wkly Rep 1988, 37, 597–599. [Google Scholar]
- Simonds, R.J.; Holmberg, S.D.; Hurwitz, R.L.; et al. Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N Engl J Med 1992, 326, 726–732. [Google Scholar] [CrossRef]
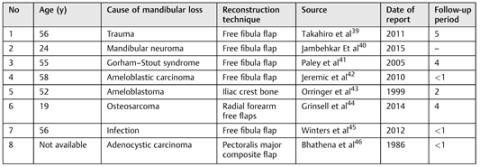
- Takahiro, A.; Hideaki, K.; Jun, S.; et al. Successful total mandibular reconstruction using a fibular graft with long-term follow-up: A case report. Asian Journal of Oral and Maxillofacial Surgery 2011, 23, 196–200. [Google Scholar]
- Jambhkare, S.S.; Mohit, J.K.; Satyahit, D.; et al. Total mandibular reconstruction and rehabilitation: A case report. J Oral Implantol. [Epub ahead of print]. 2014. [Google Scholar] [CrossRef]
- Paley, M.D.; Lloyd, C.J.; Penfold, C.N. Total mandibular reconstruction for massive osteolysis of the mandible.( Gorham-StoutSyndrome). Br J Oral Maxillofac Surg 2005, 43, 166–168. [Google Scholar] [CrossRef]
- Jeremic, J.V.; Nikolic, Z.S.; Boricic, I.v.; et al. Total mandibular reconstruction after resection of rare honeycomb-like ameloblastic carcinoma: A case report. [CrossRef]
- Orringer, J.S.; Shaw, W.W.; Borud, L.J.; et al. Total mandibular and lower lip reconstruction with a prefabricated osteocutaneous free flap. Plast Reconstr Surg 1999, 104, 793–797. [Google Scholar] [CrossRef]
- Grinsell, D.; Ting You, B.Y.; Ferris, S.I. Total mandibular reconstruction using four free flap transfers in a patient with large mandibular osteosarcoma. Eur J Plast Surg 2014, 37, 563–566. [Google Scholar]
- Winters, R.; Saad, A.; Beahm, D.D.; Wise, M.W.; St Hilaire, H. Total autogenous mandibular reconstruction using virtual surgical planning. J Craniofac Surg 2012, 23, e405–e407. [Google Scholar] [PubMed]
- Bhathena, H.; Kavarana, N.H. One-stage total mandibular reconstruction with rib, pectoralis major osteomyocutaneous flap. Head & Neck Surg 1986, 8, 211–215. [Google Scholar]

© 2016 by the author. The Author(s) 2016.
Share and Cite
Motlagh, M.F.; Bayat, M.; Naji, S. Bone Allograft: An Option for Total Mandibular Reconstruction. Craniomaxillofac. Trauma Reconstr. 2017, 10, 306-313. https://doi.org/10.1055/s-0036-1593474
Motlagh MF, Bayat M, Naji S. Bone Allograft: An Option for Total Mandibular Reconstruction. Craniomaxillofacial Trauma & Reconstruction. 2017; 10(4):306-313. https://doi.org/10.1055/s-0036-1593474
Chicago/Turabian StyleMotlagh, Masoud Fallahi, Mohamad Bayat, and Siamak Naji. 2017. "Bone Allograft: An Option for Total Mandibular Reconstruction" Craniomaxillofacial Trauma & Reconstruction 10, no. 4: 306-313. https://doi.org/10.1055/s-0036-1593474
APA StyleMotlagh, M. F., Bayat, M., & Naji, S. (2017). Bone Allograft: An Option for Total Mandibular Reconstruction. Craniomaxillofacial Trauma & Reconstruction, 10(4), 306-313. https://doi.org/10.1055/s-0036-1593474



