Orbital wall fractures, termed in this study for convenience’s sake, represent a significant subset of orbital fractures in the sense that fractures of the zygoma and of the maxilla are excluded. And orbital wall fracture, mostly blowout fractures in our study, refers to a traumatic deformity that has affected the medial wall and/or floor of the oculus. In a pure-type orbital blowout fracture, bone fragments with torn periosteal are forced out of the original bony orbit into the maxillary and/or ethmoidal sinus.
The basic objective of reconstruction of an orbital defect is to support the orbital contents by freeing the entrapped tissue and then restoring the orbital volume, function, and esthetics. Orbital wall and floor reconstruction has been achieved with a wide variety of materials including biological materials. Materials represent autogenous bone, cartilage, fascia, periosteum, allografts, xenografts, metals, and alloplastic materials. [
1]
Autogenous bone graft remains gold standard and is a well-established and relatively successful method of repairing orbital defects. Grafting of autogenous bone is mostly preferred because the method causes fewer complications. The long-standing problem associated with this method is the morbidity at donor site.
Among various materials used for orbital wall and floor defects, we have experientially found that autogenous bone grafts offer, overall, the best possible characteristics. And we use autogenous rib bone that is thinly split in situ as graft material. This in situ splitting method from rib bone was established first by Johnson and Raftopoulos in 1999. [
2] This donor site is primarily considered to be one of the better choices for craniofacial surgery, and the use of rib bone graft in that field was reported in 1980s as a schema in
Atlas of Craniomaxillofacial Surgery [
3] (
Figure 1). Currently, however, there are few reviews of the method; so, in what follows, we shall review and describe this method for the reconstruction of orbital floor and medial wall. We would like to describe this in situ splitting technique using moving images, which we hope will make the technique more tangible to the reader of this article. We believe we will succeed in convincing the reader of the advantage our technique offers.
Patients and Methods
We conducted a retrospective data review of all patients inflicted by acute orbital fracture. All the patients were treated at the Saga University Hospital (Saga, Japan) during the January 2005–July 2011 period. Patients were identified from a clinical database. Data collected included patient’s name, age, hospital number, sex, diagnosis, location of defect (i.e., floor or medial wall), duration of follow-up, and surgical details including length and width of bone graft, time to disappearance of postoperative diplopia, and postoperative complications and/or sequelae, if any.
Before surgery, computed tomographic (CT) scan and radiograph imaging were conducted which showed orbital floor and/or medial wall defects. Specifically, whether or not there was loss of bony integrity of the orbital floor and/or medial wall, and displacement of soft tissue into the maxillary and/or ethmoidal sinus. In addition, postoperative data regarding pain were collected before discharge and at an early stage of follow-up, and data consisted of visual analog scale (VAS) scores for local pain at the harvest site of rib bone graft. VAS was assessed on a continuous scale from 0 (no pain) to 10 (worst pain imaginable).
Harvesting Procedure at Donor Site
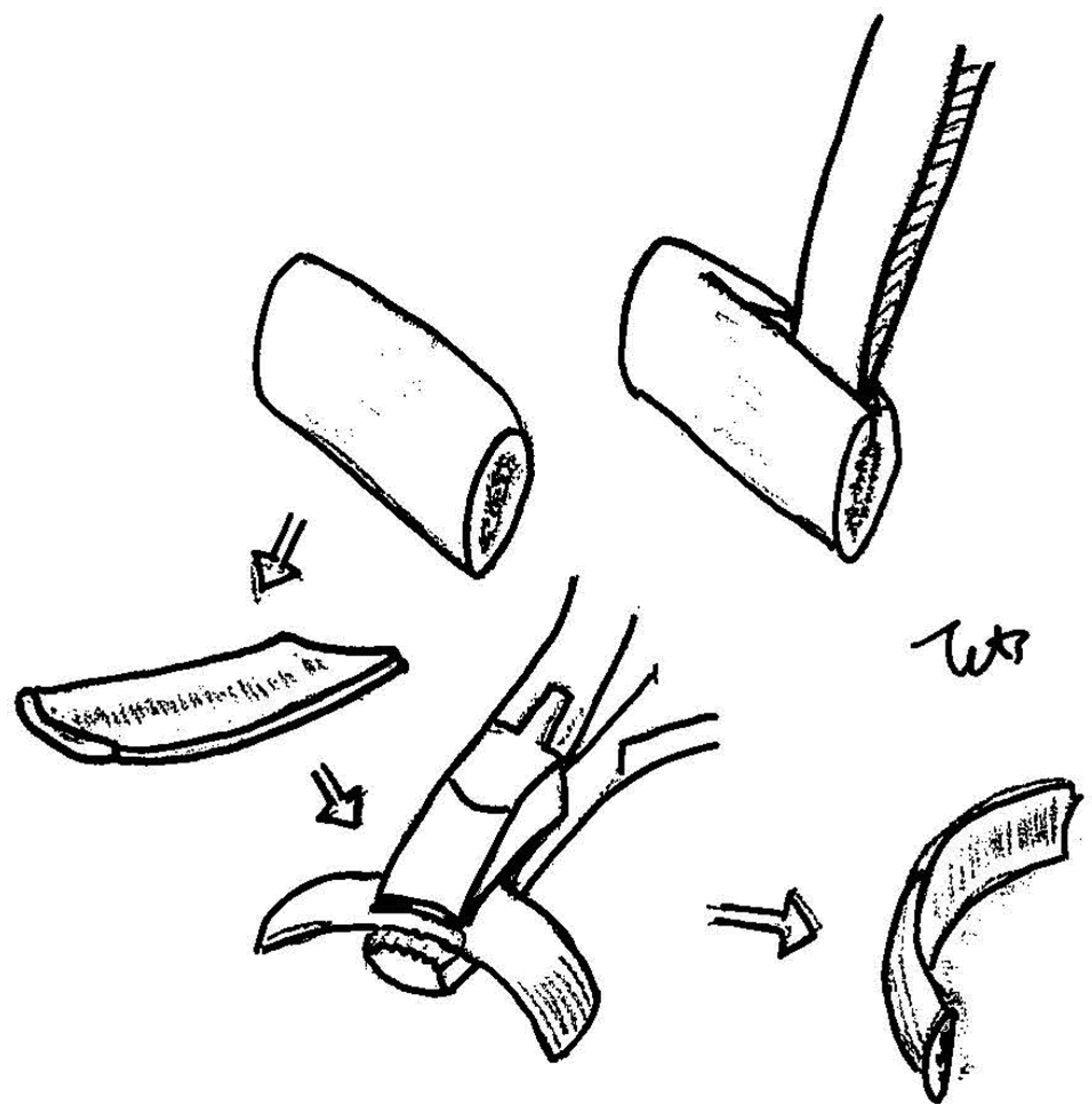
A bone graft is harvested by a 4- to 5-cm oblique incision along the anterior axillary line over the sixth or seventh rib. The incision line is often placed within the inframammary crease. Muscle and periosteal over the anterior surface of the rib are elevated to the superior and inferior edge over the length of the bone required. The outer surface of bone is chiseled by small cutting bar, then a 10-mm-width, gentlecurved Obwegeser osteotome is introduced by hand pressure only along the cutting line of superior and inferior cortex. The anterior surface of the rib is then elevated and split from the deep intact remaining cortex, splitting posteriorly. The inner deep cortex is left intact, and the pleura is not exposed. After taking out the outer table of the rib, the rib periosteum, muscle, and skin are closed in layers (Movie 1).
Movie 1
Harvesting procedure in donor site.
Results
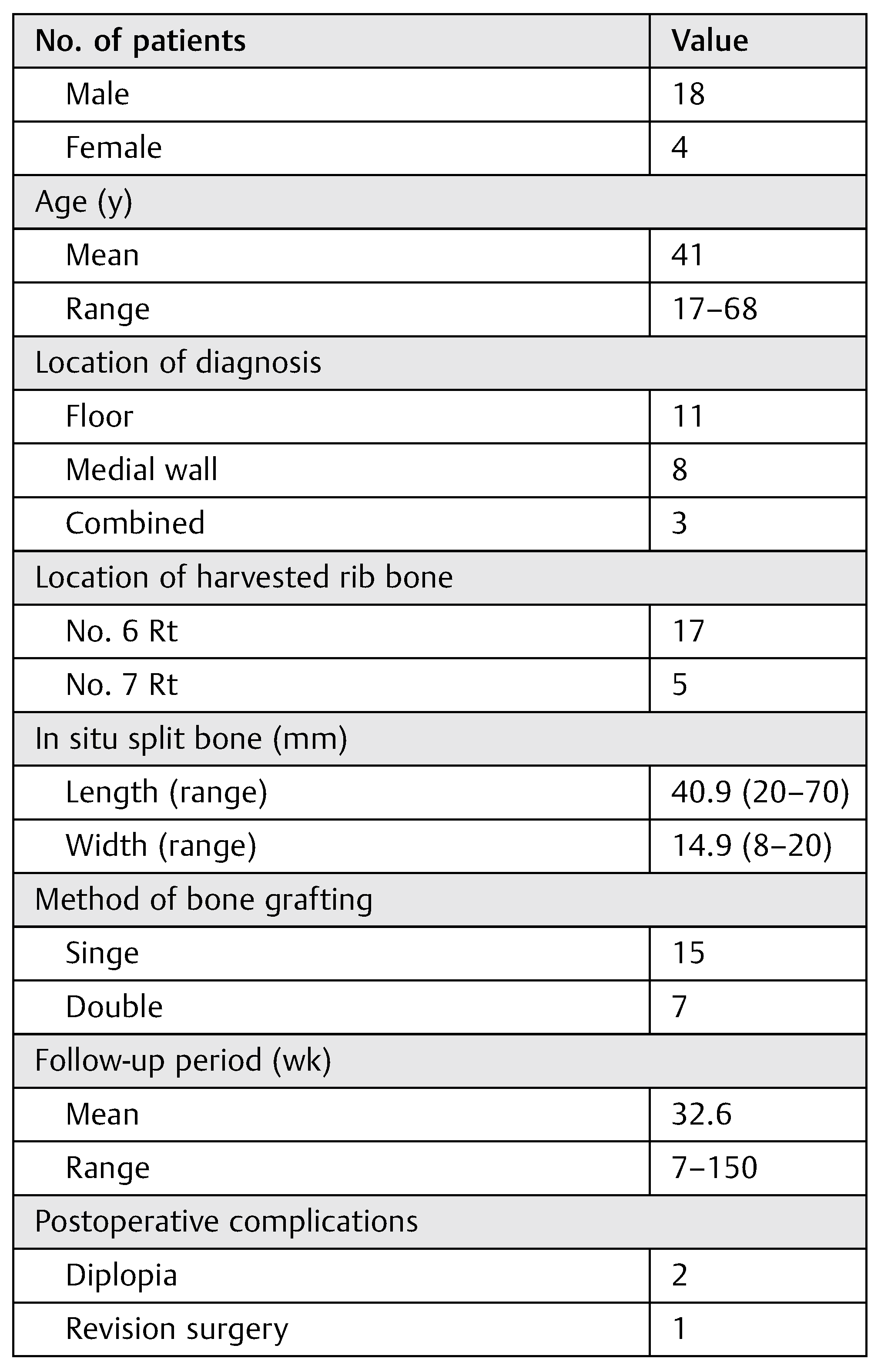
Mean age of the 22 patients at the time of surgery was 41 years (range, 17–68 years). The sex ratio among them was 18 males to 4 females. All operative procedures were performed with the repair and bone grafting of orbital floor or medial wall, and combined fracture with diplopia. The repair of the orbital floor and medial wall was successful in all cases. Ten patients had bone grafting onto the orbital floor, 8 had it done onto medial wall, and 4 onto both floor and wall after reduction. The mean length of in situ rib bone grafts was 40.9 mm (range, 20–70 mm); the mean width of those was 14.9 mm (range, 8–20 mm). Grafting was done by a single leaf for 15 cases and by a double leaf for 7 cases to the size of defects. Of all 22 patients with diplopia, 20 improved and 2 remained stable at the last follow-up after surgery. Postoperative CT scans in 17 patients revealed an adequate maintenance of orbital volume without any evidence of resorption of the graft. There was one case that needed revision surgery, in which iliac bone was the alternative donor site. The harvested graft was grafted again onto both floor and medial wall as necessitated by postoperative endophthalmitis as well as by large defects in both the floor and medial wall (
Table 1).
Follow-up periods ranged from 7 to 150 weeks (~3 years) with a median of 32.6 weeks (~8 months).
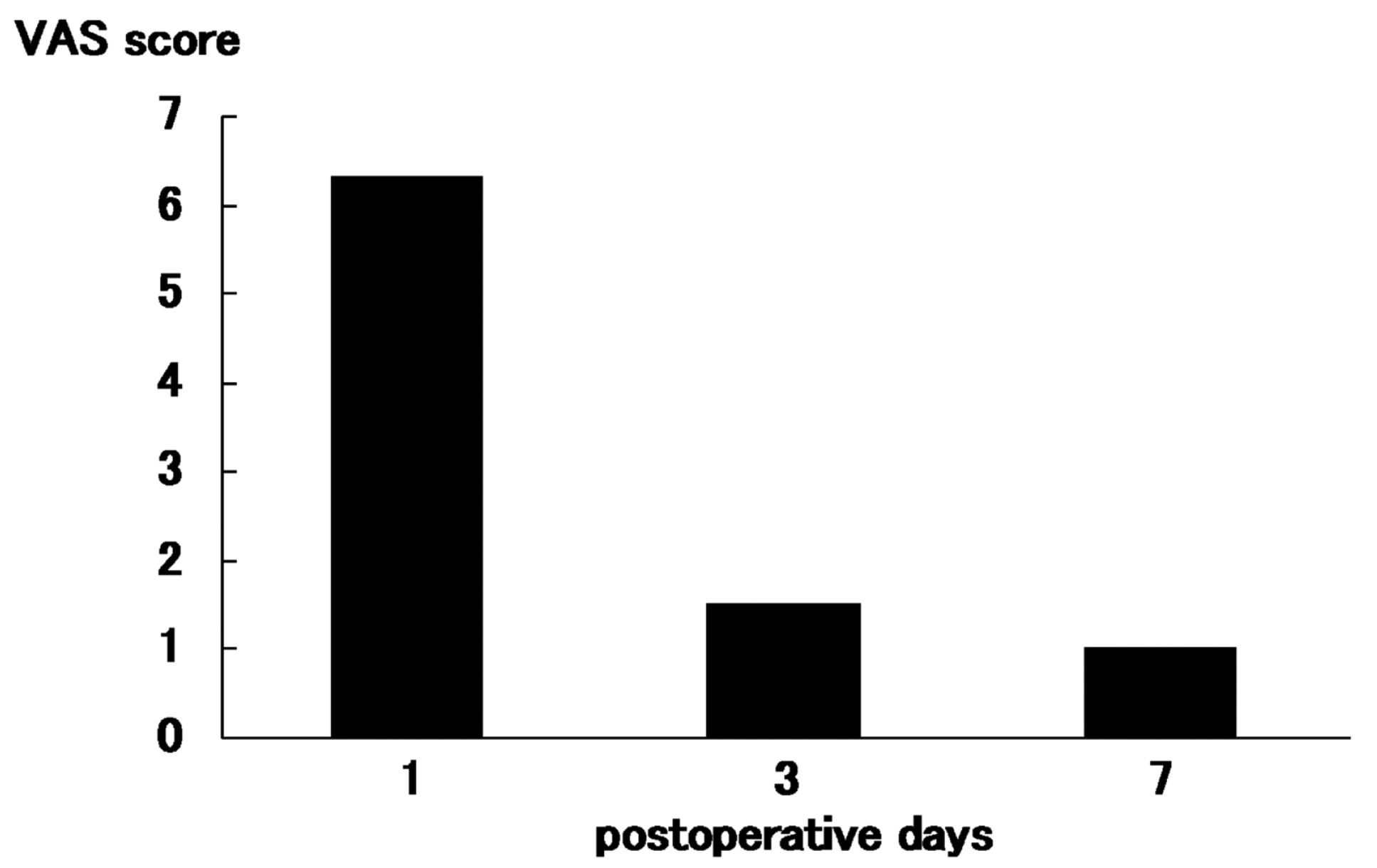
Postoperative VAS scores for local pain at the graft harvest site in 16 patients were 6.3 (range, 4–8) in 1 day after surgery, 1.5 (range, 1–4) in 3 days after surgery, and 1.0 (range, 0–3) in 7 days after surgery (
Figure 2). The medial time taken to walk normally was 1.2 days (range, 1–2; first day in 12 patients and second day in 4 patients).
Case 12
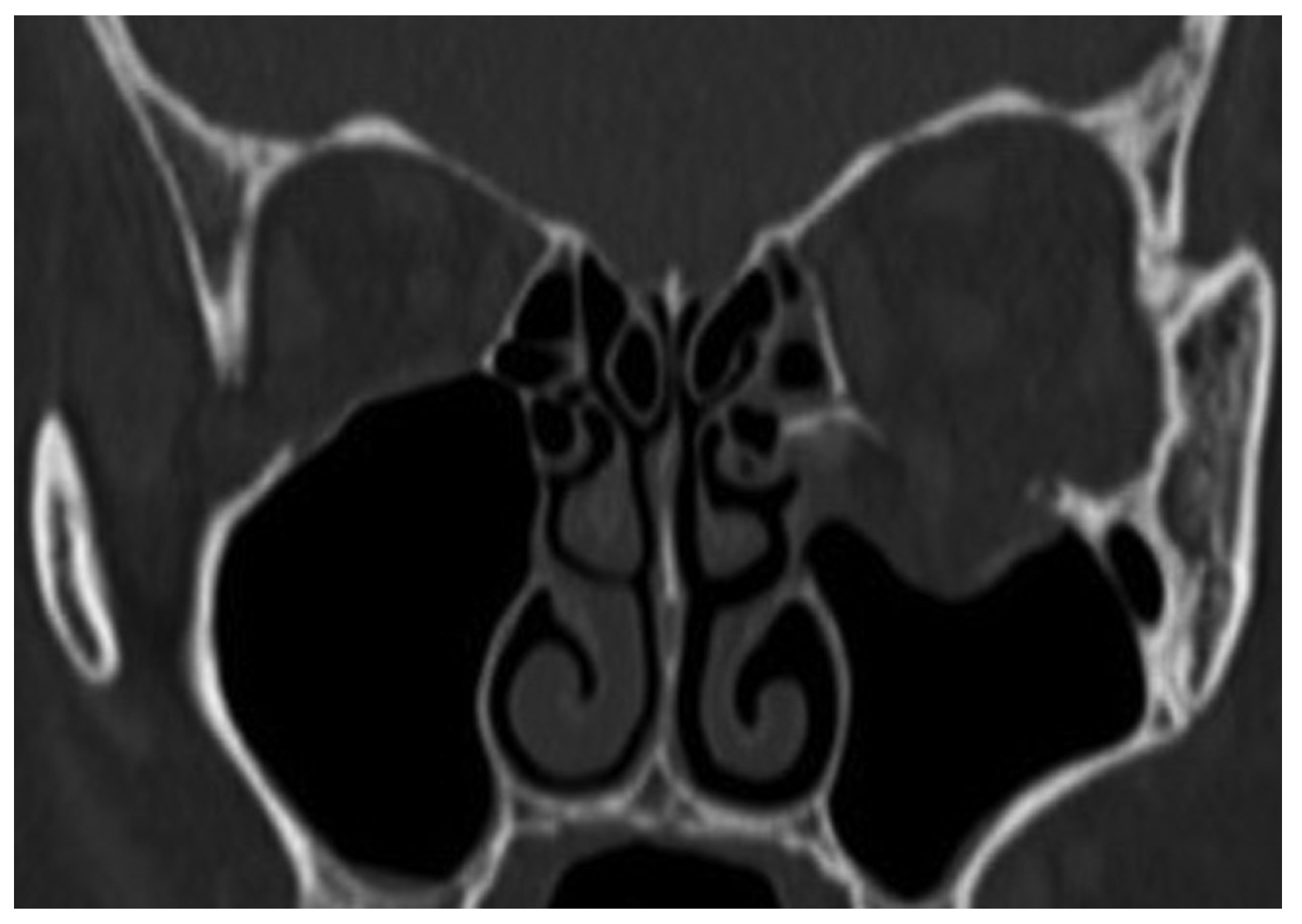
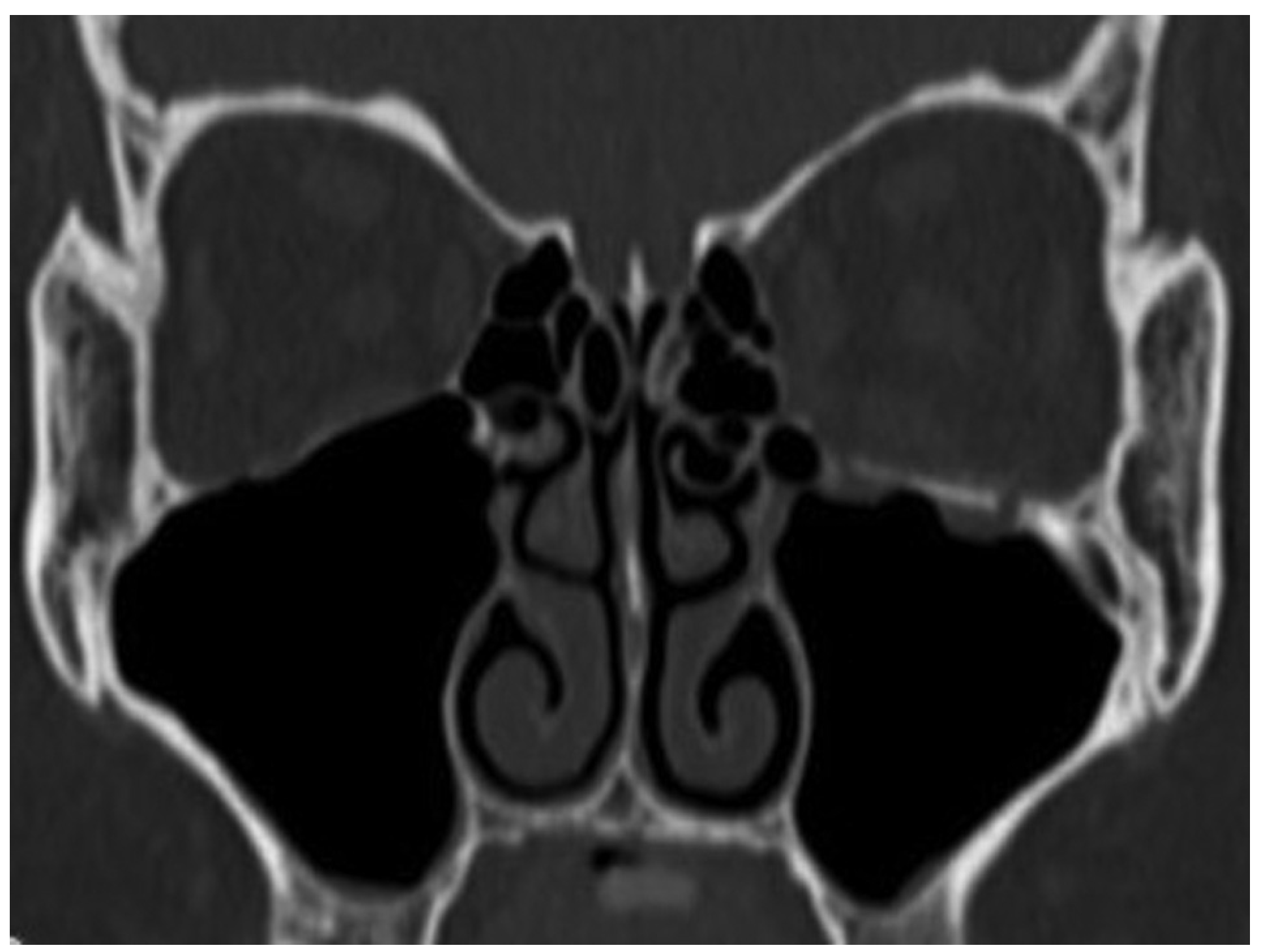
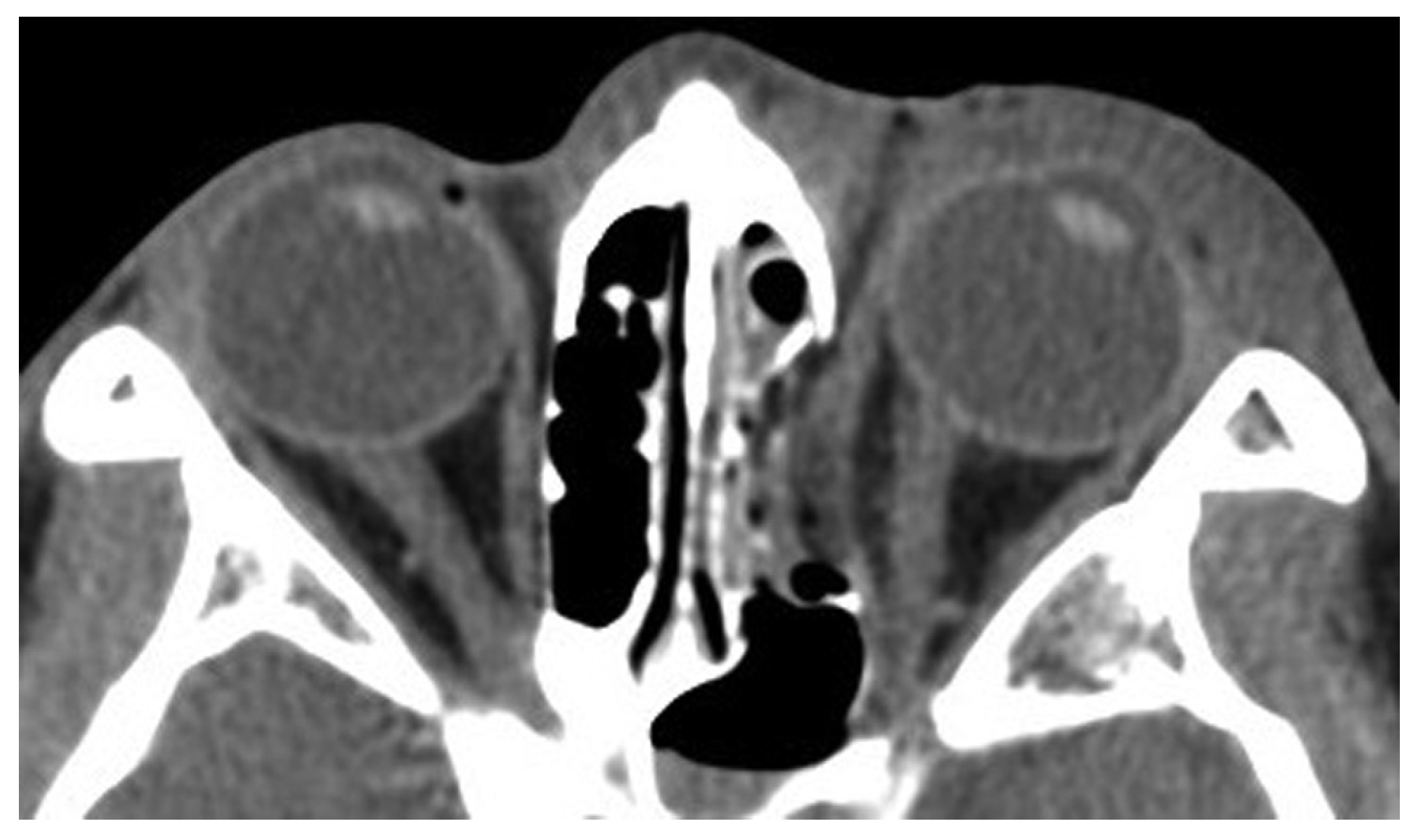
A 39-year-old woman sustained facial injuries, and presented with diplopia. She was referred to our clinic by an ophthalmologist for a suspected blowout fracture at 3 weeks after injury. A CT scan revealed bone defect of orbital floor and the displacement of soft tissue into the maxillary (
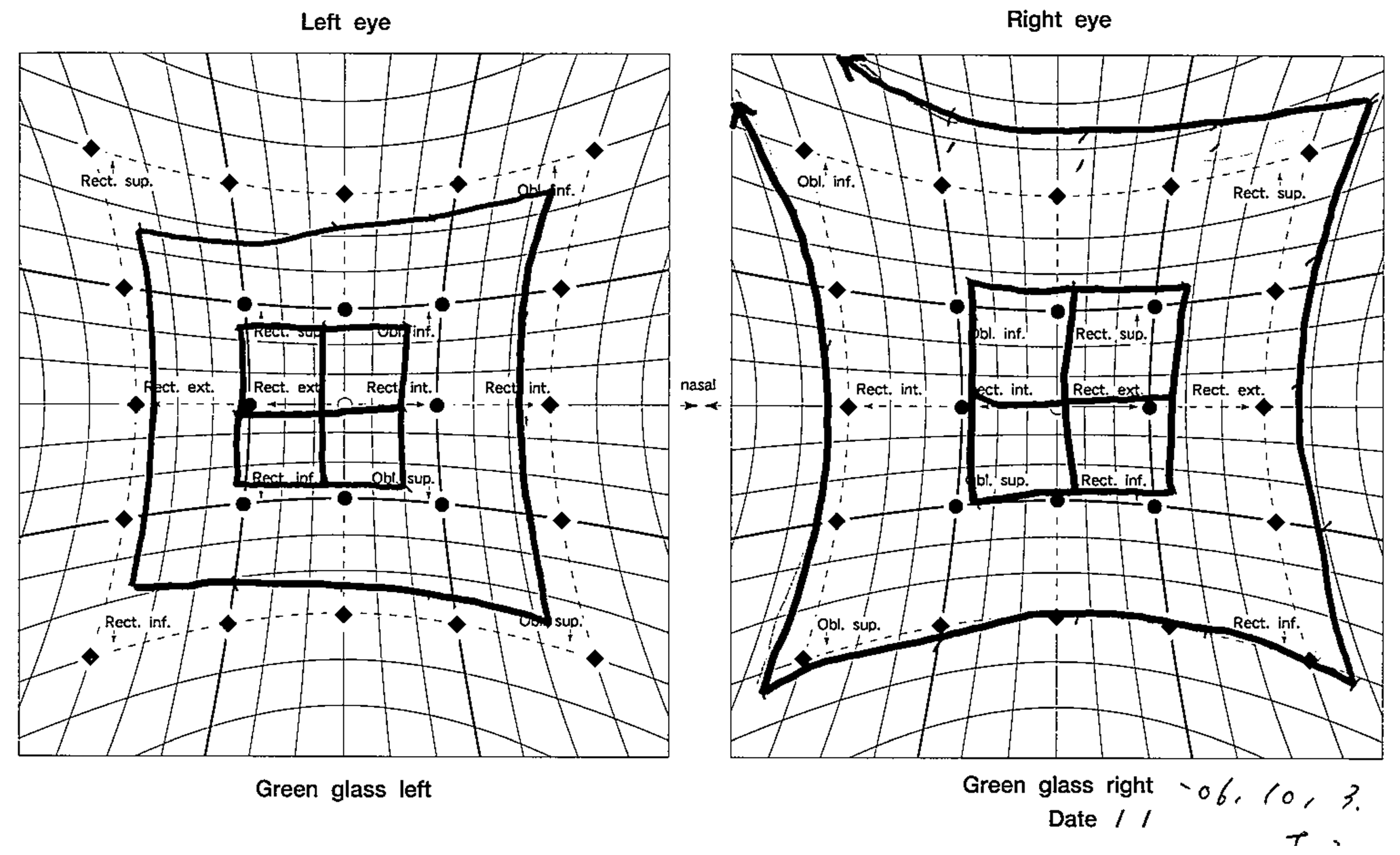
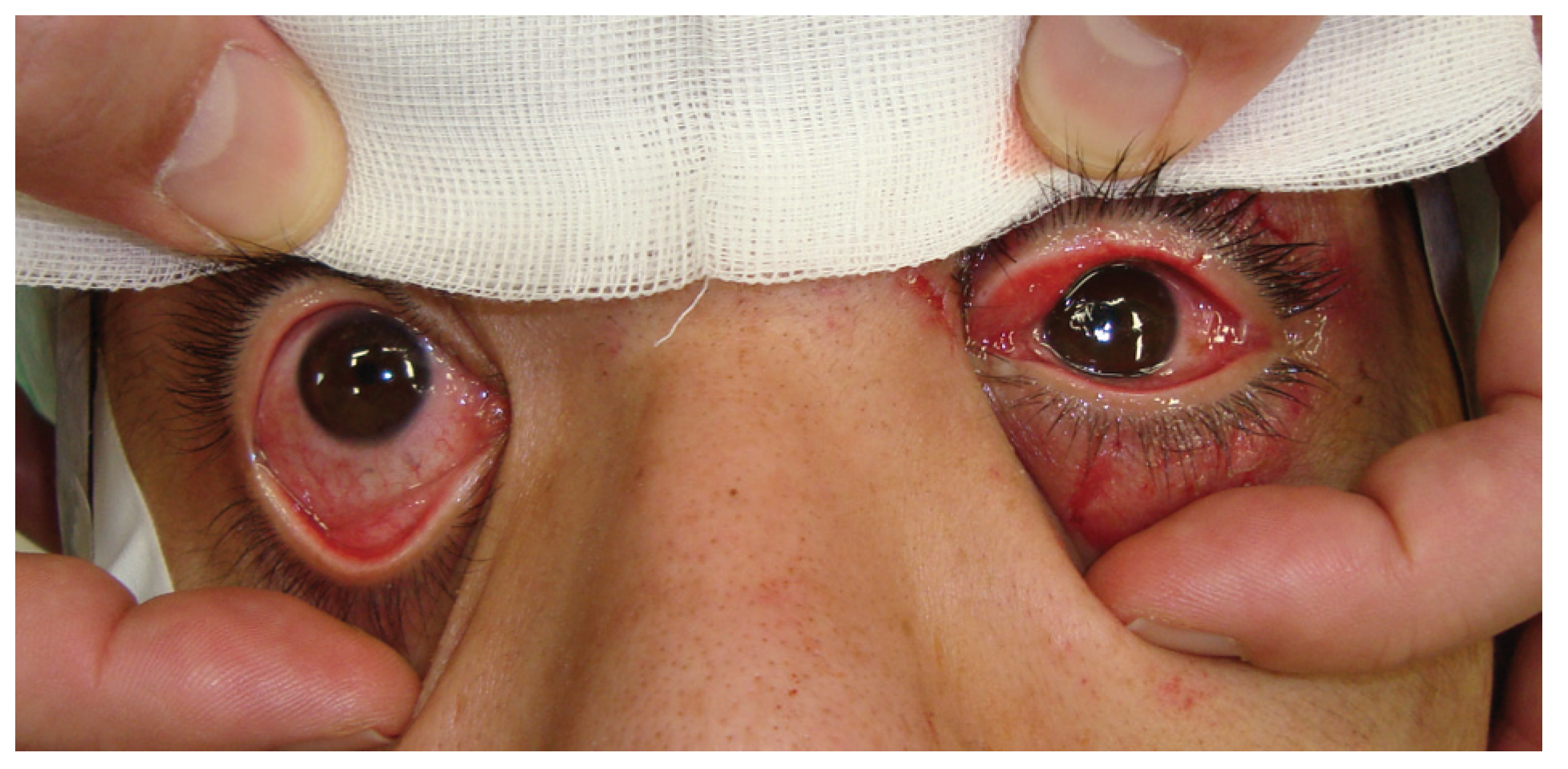
Figure 3). The patient’s left eye movements—up gaze and down gaze—were severely restricted (
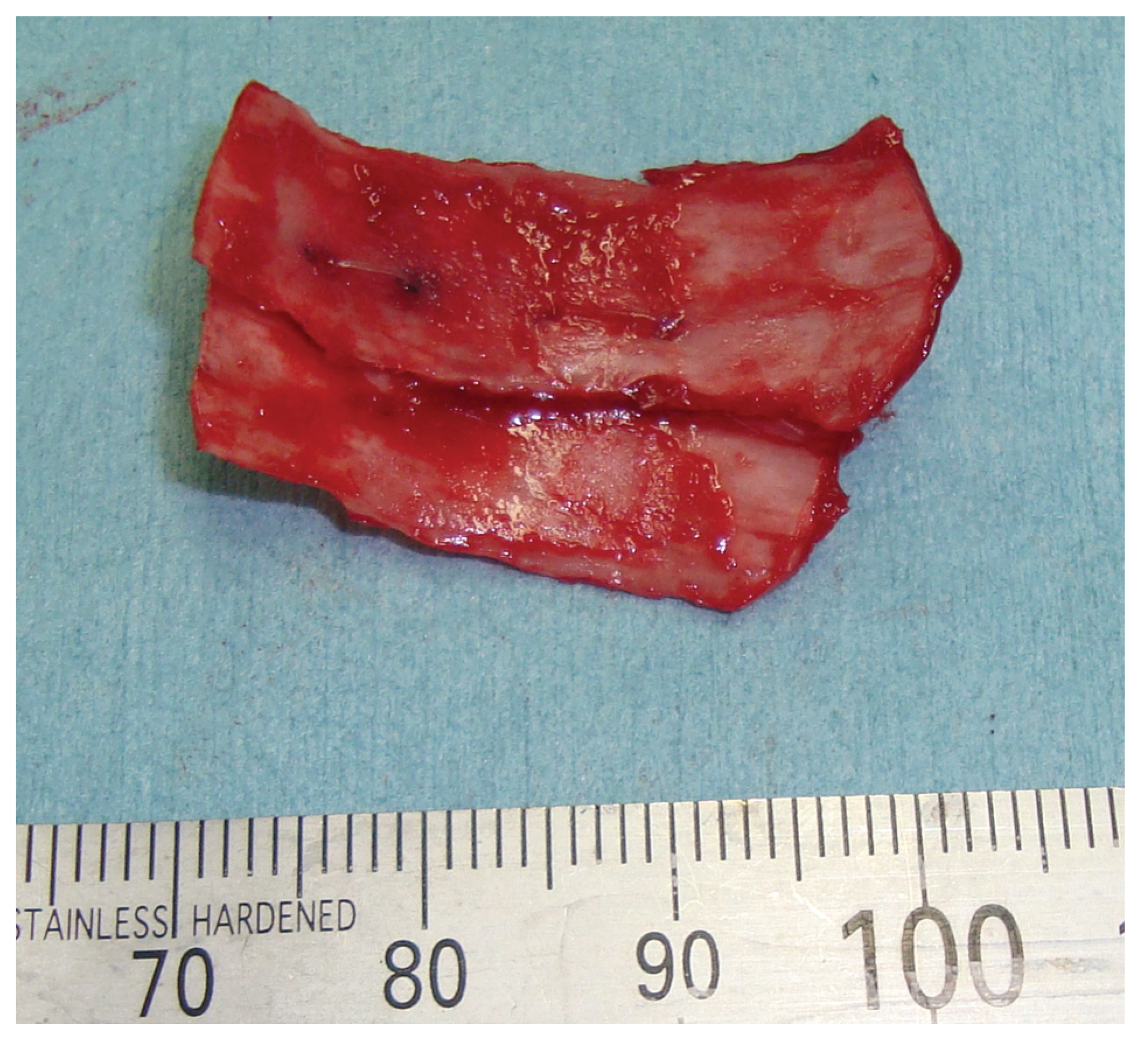
Figure 4). The diagnosis was a blowout fracture with the orbital floor punched out. Forced duction testing was performed at the beginning of the case to document any preoperative globe restriction. The surgery was performed to reconstruct the orbital floor at 27 days after injury. By transconjunctival approach, the orbital fat and muscle was pulled into the floor cavity, and a 35 mm × 20 mm defect of the orbital floor was seen. A graft was harvested from the sixth right-side rib by splitting it in situ at the size of 50 mm × 15 mm. The split bone was cut and remade into a double leaf using absorbable suture, and gently inserted into the orbital floor without fixation.
Tarsorrhaphy was done and kept for 1 day to prevent the swelling of conjunctiva, and the exercise of eye movements started soon. Coronal CT after surgery revealed good position of the bone graft to orbital floor at 4 weeks after surgery (
Figure 5). The diplopia was rectified completely in 7 weeks after surgery (
Figure 6).
Case 24
A 26-year-old man was found in need of an urgent surgery with missing medial rectus muscle with nausea and vomiting. He sustained facial injuries in a car accident, and presented with diplopia with headache. He was referred to our emergency clinic and was charged to our department for a suspected blowout fracture. CT imaging revealed a fracture of the medial wall of the punched-out type and also the absence of medial rectus muscle on the orbital medial wall (
Figure 7). The patient’s left eye movements—medial gaze and lateral gaze—were severely restricted. The diagnosis was a blowout fracture with the punched-out type of the orbital medial wall. Immediate surgery was performed to release the strangulated muscle at 11 hours after injury. His left eye was locked to medial side under general anesthesia (
Figure 8).
We opted for the retroseptal transconjunctival approach, in which we made a medially extended incision and a medial W-shaped skin incision to retract the orbital fat and muscle from the medial wall to the anterior side. As expected, we confirmed a fracture with entrapment of the orbital content in the ethmoidal sinus. The herniated tissue was carefully restored without additional osteotomy, and 35 mm × 25 mm defects of the orbital medial wall were seen. Rib bone split in situ with a size of 70 mm × 15 mm was harvested from the seventh right-side rib. The in situ split bone was cut and remade to a double leaf using absorbable suture (
Figure 9), and inserted, gently, into the orbital floor without fixation.
Tarsorrhaphy was done and kept for 2 days to prevent the swelling of conjunctiva, and the exercise of eye movements started soon. His headache began to disappear immediately after surgery, and diplopia was rectified completely in 13 weeks after surgery (
Figure 10 and
Figure 11).
Discussion
The goal of surgical repair of an orbital wall fracture is to restore the traumatized wall so as to prevent herniation of the contents of the ocular globe into the maxillary and/or ethmoidal sinus, as it causes undesirable fat atrophy, disturbance in the ocular motility, and related complications. Repair of orbital wall fractures puts a surgeon in a situation not without drawbacks in terms of the reconstruction materials and the possibility of technical errors. Various autogenous, alloplastic, and allogenic materials have been tried for reconstruction of the orbital wall.
In recent years, an increasing interest in the use of alloplasts for orbital reconstruction has become apparent in the literature. [
1] This apparent preference for alloplastic materials is likely the result of their ease of use, technological advancements, absence of donor site morbidity, and an increasing level of evidence of the safety and efficacy of synthetic materials as indicated in the literature. [
4]
A recent increase in the interest in alloplastic materials with enough publication notwithstanding, there are surgeons in the field for whom the use of autogenous grafts is a well-established and fairly successful method of repairing orbital defects. [
1] Autologous bone grafts are used in orbital surgery because they offer strength, rigidity, vascularization potential, and they also incorporate well into orbital tissues with minimal acute and chronic immune reactivity. [
5]
Calvarial bone appears to be a superior option in orbital reconstruction because of its accessibility, the various graft sizes that can be harvested, the hidden nature of the scar as a result of its location in a hair-bearing area, and the occurrence of little or no postoperative pain. Donor-site morbidity remains a general drawback for autologous bone harvesting.
In full-thickness calvarial harvesting, care has to be taken not to tear the dura. If injured, it might result in an iatrogenic subarachnoid hemorrhage or even intracerebral hemorrhage and might require reconstruction. [
1,
6]
As to the harvest site of an autogenous graft, iliac bone is probably the standard for orbital defect repairs. The advantages of using autogenous bone as reconstruction material are its biocompatibility and osteogenic, osteoconductive, and osteoinductive capacity. [
7] Morespecifically, iliac bone is easy to harvest, and the medial cortexof the anterior iliac crest is relatively easy to shape to fit the internal orbital wall. [
8] A disadvantage of iliac bone graft lies in the problems of bone resorption and donorsite complications. The iliac crest graft is said to be bulky without trimming and it may resorb unpredictably. [
9]
In contrast, rib bone is easy to harvest by splitting it in situ to obtain a thin graft. Rib bone is considered to be better than iliac bone in that it requires little additional trimming once harvested. It is true that it may resorb unpredictably as with iliac bone, but this does not pose a major problem in the case of orbital wall reconstruction for a fresh fracture.
Repositioning is by far the most important procedure for fresh fractures including the blowout type of fractures and the outcome of the procedure is not dependent on the kind of bone graft adopted. We surmise, though, that rib bone is more amenable to splitting technique, which allows the harvesting surgeon to resect the endochondral space more thoroughly. The result would probably mean less resorption. All surgeons aim to see their patients cured with as little complication as possible, no matter what method or repair material may have been adopted.
The main criticism about harvesting a graft from iliac crest is the kind of local pain that the patient has after surgery. The pain is often so severe that the patient becomes unable to walk and ends up being hospitalized for a prolonged period before recovery. All patients treated with an iliac bone– harvested graft stated, without any exception, that they had difficulty walking during the first week of healing, which was attested to by their mean VAS value of 5.5 (range, 3–8) at 1 week after surgery. [
10]
In contrast, our data about postoperative VAC scores for local pain at the rib bone graft harvest site was 1.5 (range, 1– 4) at 3 days after surgery and 1.0 (range, 0–3) at 7 days after surgery. Every patient we treated could walk during the first week postoperatively. Especially, those who had undergone in situ split rib bone harvesting experienced less pain when they walked and were saved from a long stay in hospital.
Another donor-site problem has to do with nerve and blood vessel injuries. [
11,
12] At this point, iliac bone harvesting probably poses a greater problem than rib bone harvesting due to the presence of a major sensory nerve in the thigh. Consequences of an injury to the nerve, there would be of far more serious nature.
To begin with, rib bone graft as one of the autogenous materials was first reported in craniofacial surgery in the reconstruction of malar and orbital defects. This graft was certainly not a well-established method of repairing orbital wall defects. Johnson and Raftopoulos first reported on split rib bone graft for orbital floor in 1999 as part of the Idea and Innovation section in
Plastic and Reconstructive Surgery. [
1] But no articles followed their 1999 report on split rib bone graft for orbital floor and wall. Instead, a great deal of attention was paid to the variety of alloplastic and allogenic materials being tried for the reconstruction of the orbital wall.
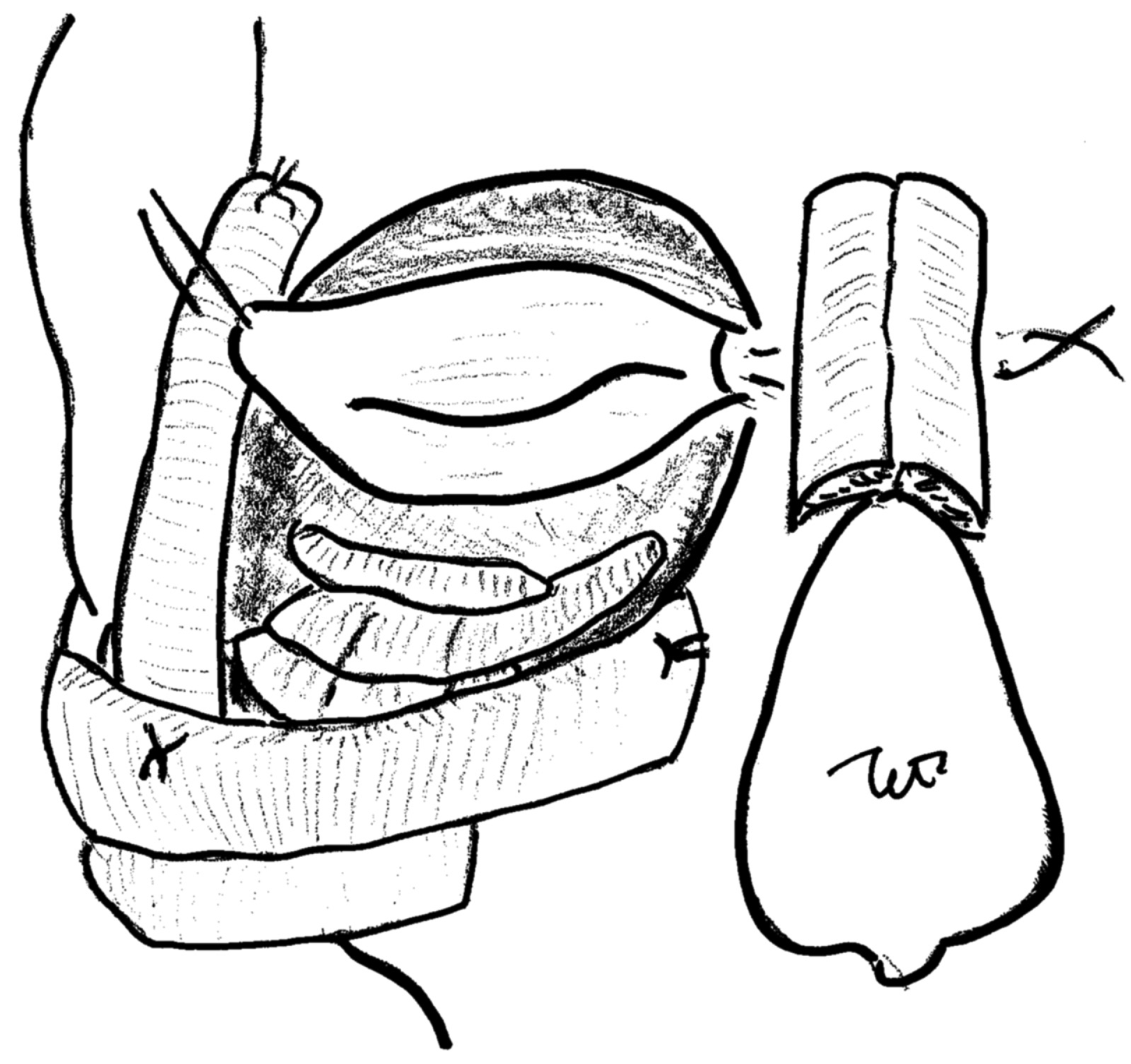
The use of rib bone graft in craniofacial surgery was schematically reported in 1980s in the
Atlas of Craniomaxillofacial Surgery by Mosby. [
3]
Onlay split rib grafts were used across the depressed frontal segment and onto the nasal bones and wired into place. Onlay grafts were placed on the orbital floors (
Figure 12). Though the usefulness of onlay split rib graft was well known, the results have not been sufficiently described in craniofacial surgery.
The average width of rib bone lamellae harvested in situ was 15 mm, which was obviously too narrow to repair defects whose width exceeded that. In such a case, we had to adopt a double-leafed lamella as a graft, and it required more delicate workmanship in forming a satisfactory graft. A defect just in the floor or in the medial wall was not difficult to repair with an improvised double-leafed graft. However, when it came to repairing an oversized “three-dimensional” defect covering both floor and medial wall, we found it more difficult to address than we thought. Indeed, the reason that we had a case that required revision surgery was precisely that. You might argue, in fact, that the technique we are presenting in this study is good for rather narrow and localized “two-dimensional” defects alone. We grant that larger and “three-dimensional” defects might be repaired better with some other technique.
In conclusion, as for the complications that might ensue, one might compare in situ splitting of a rib bone for a graft with iliac bone in primary repair of an orbital wall injury. It is easier for sure to harvest a graft if it is from a rib bone, particularly in the case of in situ splitting. Also the outer table of rib bone is probably easier to shape to fit the curvature of the internal orbital wall than iliac bone. We believe good aesthetic and functional results can be achieved as well with less physical invasion against the patient. All patients we treated with our technique stated they had no serious trouble walking 1 or 2 days after surgery. They also experienced less pain than patients who underwent iliac bone harvesting, as judged from reports by other research groups.
Our experiences and the depiction in the Atlas of Craniomaxillofacial Surgery tell us that the technique of in situ splitting of a rib bone graft for the repair of the orbital floor and medial wall is a simple and safe procedure. A surgeon can easily take out the rib graft that is split in situ, and it causes less pain in donor site. This technique has proved to be an optimal choice in craniofacial reconstruction, especially in uncomplicated defects of orbital floor and medial wall.