Primary Leiomyosarcoma of the Mandibular Alveolar Mucosa of a 12-Year-Old Child from Ethiopia: A Case Report
Abstract
:Case Report
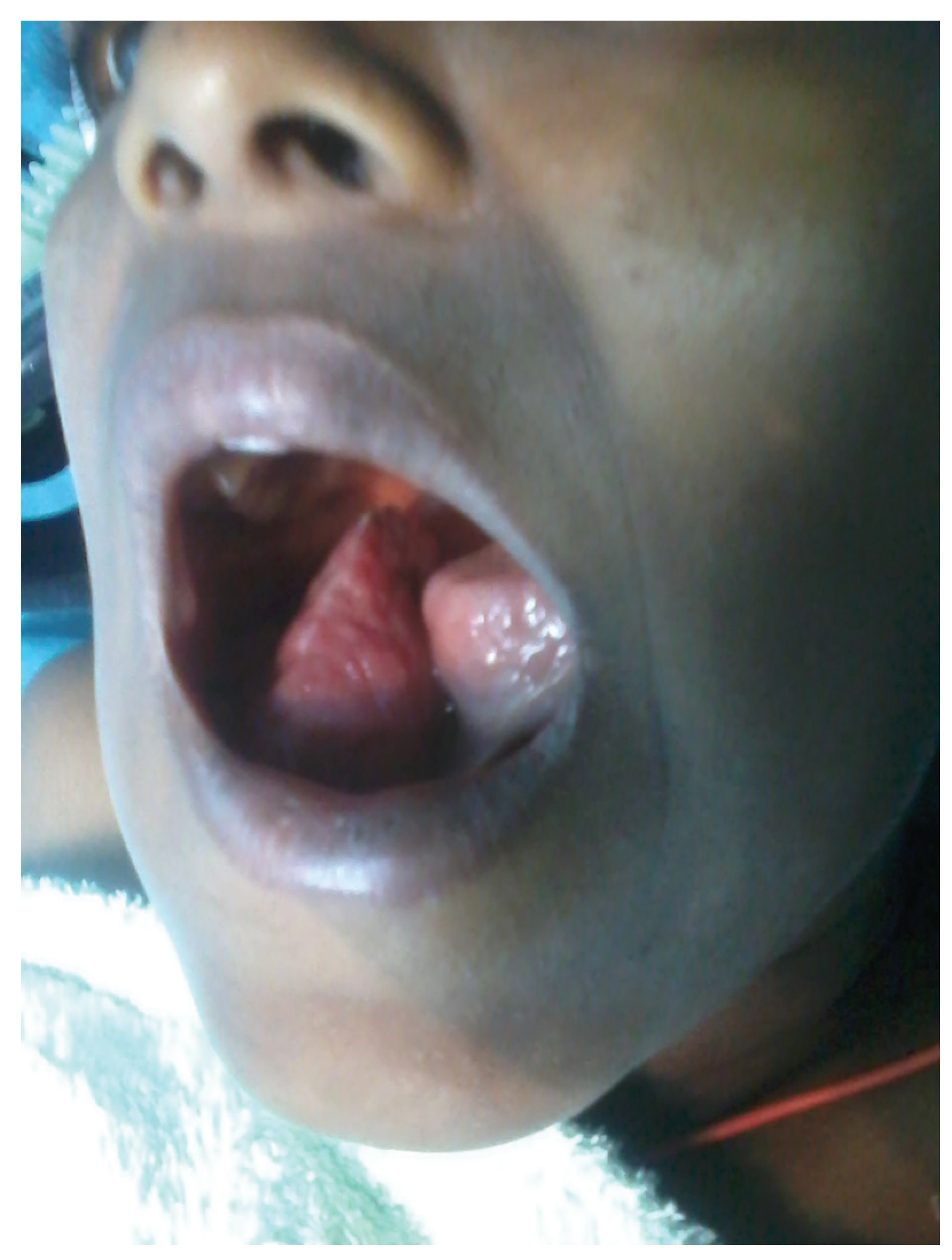
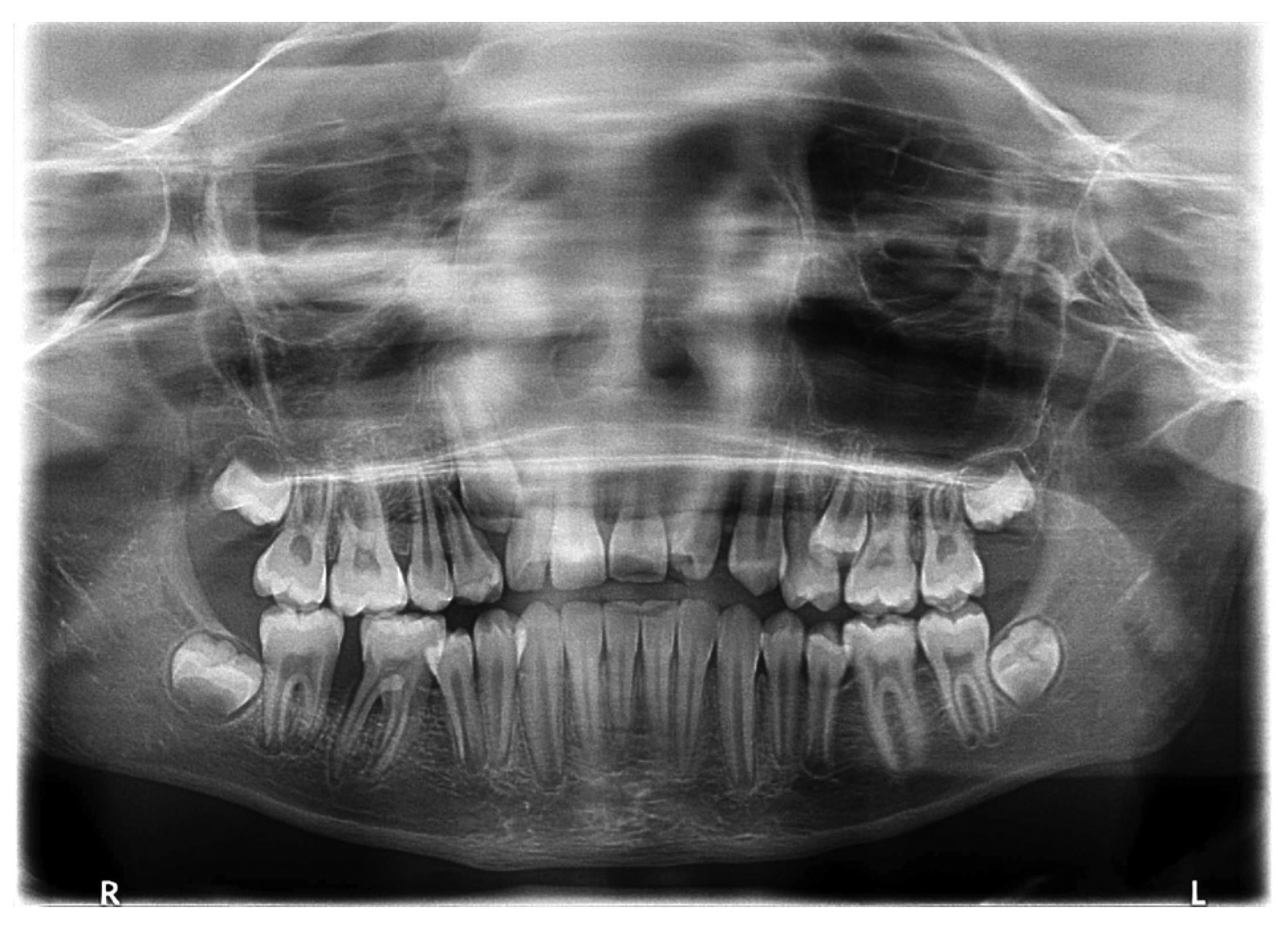
Discussion

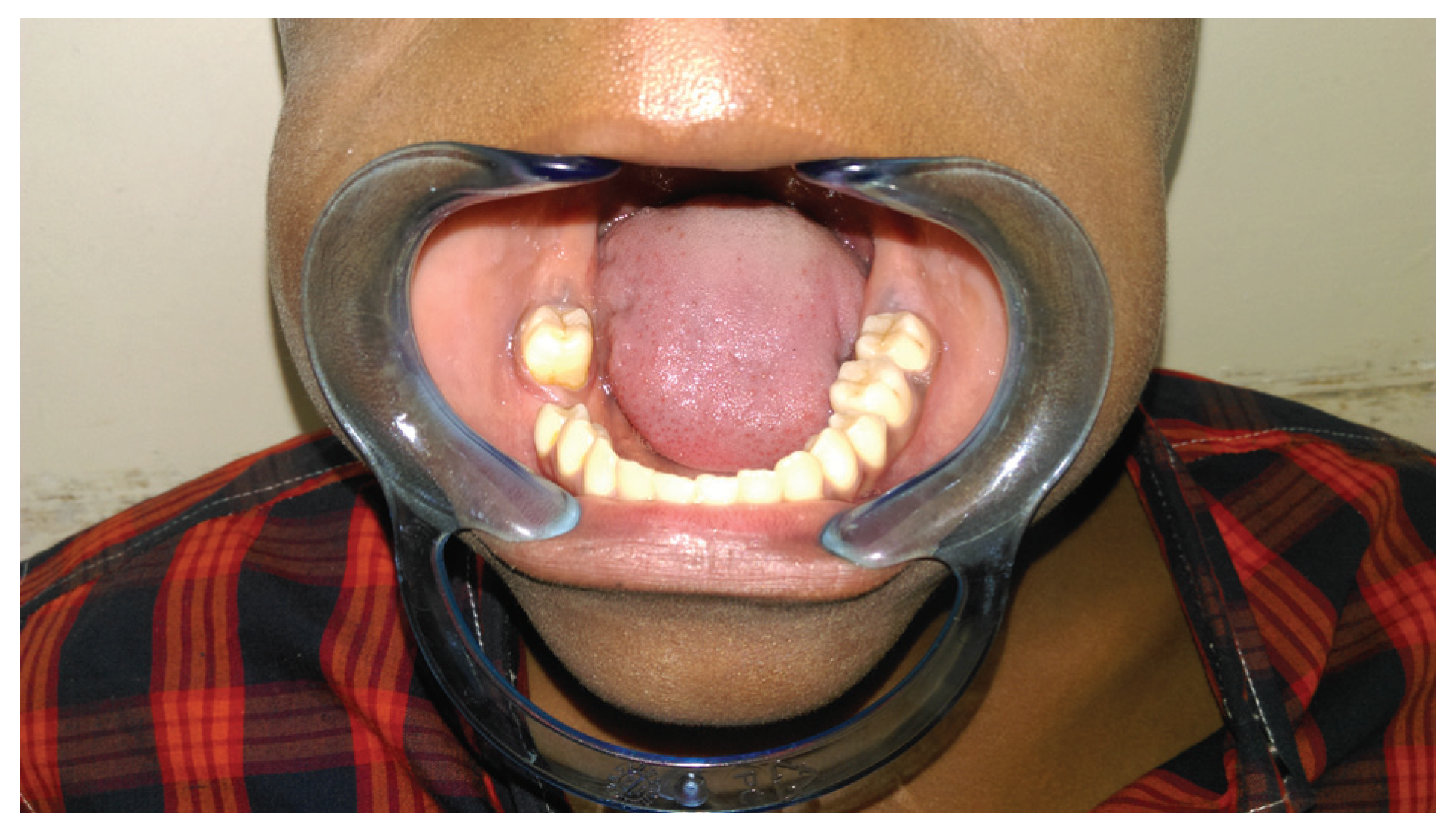
Conclusion
References
- Ezinger, F.M.; Weiss, S. Soft Tissue Tumors, 2nd ed.; C. V. Mosby Company: St. Louis, 1988; pp. 402–421. [Google Scholar]
- Gustafson, P.; Willén, H.; Baldetorp, B.; Fernö, M.; Åkerman, M.; Rydholm, A. Soft tissue leiomyosarcoma. A population-based epidemiologic and prognostic study of 48 patients, including cellular DNA content. Cancer 1992, 70, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Skoulakis, C.; Chimona, T.S.; Tsirevelou, P.; Papadakis, C.E. Subcutaneus leiomyosarcoma of the neck: A case report. Cases J 2010, 3, 52. [Google Scholar] [CrossRef] [PubMed]
- Farman, A.G.; Kay, S. Oral leiomyosarcoma. Report of a case and review of the literature pertaining to smooth-muscle tumors of the oral cavity. Oral Surg Oral Med Oral Pathol 1977, 43, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Freedman, P.D.; Jones, A.C.; Kerpel, S.M. Epithelioid leiomyosarcoma of the oral cavity: Report of two cases and review of the literature. J Oral Maxillofac Surg 1993, 51, 928–932. [Google Scholar] [CrossRef]
- Miles, A.E.; Waterhouse, J.P. A leiomyosarcoma of the oral cavity with metastasis to lymph-glands. J Pathol Bacteriol 1962, 83, 551–555. [Google Scholar] [CrossRef]
- Poon, C.K.; Kwan, P.C.; Yin, N.T.; Chao, S.Y. Leiomyosarcoma of gingiva: Report of a case and review of the literature. J Oral Maxillofac Surg 1987, 45, 888–892. [Google Scholar] [CrossRef]
- Kissane, J.M. Anderson’s Pathology, 9th ed.; C. V. Mosby Company: St. Louis, 1861. [Google Scholar]
- Piattelli, A.; Artese, L. Leiomyosarcoma of the tongue: A case report. J Oral Maxillofac Surg 1995, 53, 698–701. [Google Scholar] [CrossRef]
- Ethunandan, M.; Stokes, C.; Higgins, B.; Spedding, A.; Way, C.; Brennan, P. Primary oral leiomyosarcoma: A clinico-pathologic study and analysis of prognostic factors. Int J Oral Maxillofac Surg 2007, 36, 409–416. [Google Scholar] [CrossRef]
- Vilos, G.A.; Rapidis, A.D.; Lagogiannis, G.D.; Apostolidis, C. Leiomyosarcomas of the oral tissues: Clinicopathologic analysis of 50 cases. J Oral Maxillofac Surg 2005, 63, 1461–1477. [Google Scholar] [CrossRef]
- Stout, A.P.; Hill, W.T. Leiomyosarcoma of the superficial soft tissues. Cancer 1958, 11, 844–854. [Google Scholar] [CrossRef]
- Fu, Y.S.; Perzin, K.H. Nonepithelial tumors of the nasal cavity, paranasal sinuses, and nasopharynx: A clinicopathologic study. IV. Smooth muscle tumors (leiomyoma, leiomyosarcoma). Cancer 1975, 35, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Phillips, H.; Brown, A. Leiomyosarcoma: Report of case. J Oral Surg 1971, 29, 194–195. [Google Scholar] [PubMed]
- Izumi, K.; Maeda, T.; Cheng, J.; Saku, T. Primary leiomyosarcoma of the maxilla with regional lymph node metastasis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995, 80, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Kratochvil, F.J.D., III; MacGregor, S.D.; Budnick, S.D.; Hewan-Lowe, K.; Allsup, H.W. Leiomyosarcoma of the maxilla. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol 1982, 54, 647–655. [Google Scholar] [CrossRef]
- Martin-Hirsch, D.P.; Habashi, S.; Benbow, E.W.; Farrington, W.T. Postirradiation leiomyosarcoma of the maxilla. J Laryngol Otol 1991, 105, 1068–1071. [Google Scholar] [CrossRef]
- Sanerkin, N.G. Primary leiomyosarcoma of the bone and its comparison with fibrosarcoma. Cancer 1979, 44, 1375–1387. [Google Scholar] [CrossRef]
- Neville, B.W.; Damm, D.D.; Allen, C.M.; Bouquout, J.E. Oral and Maxillofacial Pathology; W. B. Saunders: Philadelphia, PA, 1995; pp. 406–407. [Google Scholar]
- Rodini, C.O.; Pontes, F.S.C.; Pontes, H.A.R.; Santos, P.S.S.; Magalhães, M.G.; Pinto, D.S., Jr. Oral leiomyosarcomas: Report of two cases with immunohistochemical profile. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007, 104, e50–e55. [Google Scholar] [CrossRef]
- Sandruck, J.; Escobar, P.; Lurain, J.; Fishman, D. Uterine leiomyosarcoma metastatic to the sphenoid sinus: A case report and review of the literature. Gynecol Oncol 2004, 92, 701–704. [Google Scholar] [CrossRef]
- Fields, J.P.; Helwig, E.B. Leiomyosarcoma of the skin and subcutaneous tissue. Cancer 1981, 47, 156–169. [Google Scholar] [CrossRef]
- Martis, C. Leiomyosarcoma of the maxilla: Report of two cases. J Oral Surg 1978, 36, 62–65. [Google Scholar]
- Wile, A.G.; Evans, H.L.; Romsdahl, M.M. Leiomyosarcoma of soft tissue: A clinicopathologic study. Cancer 1981, 48, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D., Jr. A classification of marginal tissue recession. Int J Periodontics Restorative Dent 1985, 5, 8–13. [Google Scholar] [PubMed]
- Sumida, T.; Hamakawa, H.; Otsuka, K.; Tanioka, H. Leiomyosarcoma of the maxillary sinus with cervical lymph node metastasis. J Oral Maxillofac Surg 2001, 59, 568–571. [Google Scholar] [CrossRef]
- Izumi, K.; Maeda, T.; Cheng, J.; Saku, T. Primary leiomyosarcoma of the maxilla with regional lymph node metastasis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995, 80, 310–319. [Google Scholar] [CrossRef]
- Gabbiani, G.; Kapanci, Y.; Barazzone, P.; Franke, W.W. Immunochemical identification of intermediate-sized filaments in human neoplastic cells. A diagnostic aid for the surgical pathologist. Am J Pathol 1981, 104, 206–216. [Google Scholar] [PubMed]
- Schenberg, M.E.; Slootweg, P.J.; Koole, R. Leiomyosarcomas of the oral cavity. Report of four cases and review of the literature. J Craniomaxillofac Surg 1993, 21, 342–347. [Google Scholar] [CrossRef]
- Kuruvilla, A.; Wenig, B.M.; Humphrey, D.M.; Heffner, D.K. Leiomyosarcoma of the sinonasal tract. A clinicopathologic study of nine cases. Arch Otolaryngol Head Neck Surg 1990, 116, 1278–1286. [Google Scholar] [CrossRef]
- Chang, A.E.; Rosenberg, S.A.; Glatstein, E.; Antman, K.H. Sarcomas of soft tissues. In Cancer Principles and Practice of Oncology; DeVita, V.T., Hellmann, S., Rosenberg, S.A., Eds.; Lippincott: Philadelphia, PA, 1989; pp. 1345–1398. [Google Scholar]
- Josephson, R.L.; Blair, R.L.; Bedard, Y.C. Leiomyosarcoma of the nose and paranasal sinuses. Otolaryngol Head Neck Surg 1985, 93, 270–274. [Google Scholar] [CrossRef]
© 2016 by the author. The Author(s) 2016.
Share and Cite
Kenea, T.T.; Kebede, B.A.; Gozjuze, F.M.; Kiros, H.; Wilde, F. Primary Leiomyosarcoma of the Mandibular Alveolar Mucosa of a 12-Year-Old Child from Ethiopia: A Case Report. Craniomaxillofac. Trauma Reconstr. 2017, 10, 56-59. https://doi.org/10.1055/s-0036-1582459
Kenea TT, Kebede BA, Gozjuze FM, Kiros H, Wilde F. Primary Leiomyosarcoma of the Mandibular Alveolar Mucosa of a 12-Year-Old Child from Ethiopia: A Case Report. Craniomaxillofacial Trauma & Reconstruction. 2017; 10(1):56-59. https://doi.org/10.1055/s-0036-1582459
Chicago/Turabian StyleKenea, Tewodros Tefera, Betel Abebe Kebede, Fekadu Mesele Gozjuze, Hagos Kiros, and Frank Wilde. 2017. "Primary Leiomyosarcoma of the Mandibular Alveolar Mucosa of a 12-Year-Old Child from Ethiopia: A Case Report" Craniomaxillofacial Trauma & Reconstruction 10, no. 1: 56-59. https://doi.org/10.1055/s-0036-1582459
APA StyleKenea, T. T., Kebede, B. A., Gozjuze, F. M., Kiros, H., & Wilde, F. (2017). Primary Leiomyosarcoma of the Mandibular Alveolar Mucosa of a 12-Year-Old Child from Ethiopia: A Case Report. Craniomaxillofacial Trauma & Reconstruction, 10(1), 56-59. https://doi.org/10.1055/s-0036-1582459


