Abstract
Attempts at reconstruction of posttraumatic craniofacial defects (PTCDs) can be a challenge in low-resource practice areas of the world where the needed biomaterials are logistically beyond reach. A simple low-profile technique of autologous osteosynthesis for PTCD using the titanium clamps is presented in this report. In addition, a 6-year prospective database on a consecutive cohort of patients who underwent this procedure was analyzed for clinical, functional, and aesthetic outcomes, both in-hospital and at midterm follow-up. The clinical data of 18 patients, all males, mean age 31.3 years (standard deviation, 9.7), were analyzed. Road traffic accidents (RTAs) were the cause of trauma in 14 of 18 patients (78%) and motorcycle crash, none helmeted, in 10 of the 18 patients (71% of RTAs). Out of 18 cases, 17 were open fractures; 89% suffered mild head injury, and associated brain injury on CT scan included pneumocephalus in 6 (5 of them significant); acute extradural hematoma in 4 and subdural in 2, and brain contusions in 9. The surgery was successful in all the cases: operative time <3 hours in 10 cases (56%), the in-hospital outcome was good in 95%. The median follow-up time was 24 months, in 6 of the 18 cases for ≥36 months. There was no case of surgical site infection in the perioperative or the follow-up period to date. The aesthetic outcome was also acceptable. This surgical technique for the reconstruction of PTCD appears effectual. Although its low cost makes it very attractive therein, it appears to be actually also recommendable even outside the low-resource developing countries.
Restoring the premorbid form and function following posttraumatic craniofacial skeletal deformities (PTCD) requires a good understanding of craniofacial symmetry [1], and some working knowledge of the principles of orthopedic surgical management of open fractures. This can be challenging especially when there is severe fracture comminution [2]. Although autologous bone is the gold standard for reconstruction of craniofacial defects, the usual practice of discarding fracture fragments in comminuted craniofacial fractures, especially the open (compound) types, usually results in bone defects that calls for cranioplasty with alloplastic materials [2].
Largely due to socioeconomic limitations in a developing country, surgical reconstruction of PTCD is usually done with the autologous fracture fragments in our practice. This is greatly facilitated by the functionally stable internal fixation that is afforded by the titanium clamp, CranioFix (CranioFix, B Braun, Aesculap, Germany). In this report, we present a surgical description of the use of this vise-like titanium clamp for the autologous osteosynthesis of posttraumatic craniofacial convexital skeletal disruption in a prospective cohort of patients in a Nigerian neurosurgery practice. As far as we know from the global literature, it also appears that, apart from a recent work by Li et al., [3] this patient cohort we present is the largest series to date on the use of the titanium clamps for the reconstruction of PTCD.
Methods
- Titanium Clamps (CranioFix) in Craniofacial Posttraumatic Reconstruction
For this implant neurosurgery, we exercise a heightened concern for surgical site infection (SSI). Hence, extra efforts are made to maintain surgical asepsis all the way. Our choice of perioperative prophylactic antimicrobial is ceftriaxone, a third-generation cephalosporin. A high dose, 2 g in adults, is given at induction of anesthesia, and continued till 48 hours postoperatively. The same cephalosporin, as well as gentamycin, is mixed with the intraoperative surgical field irrigation fluid. In cases with associated dirty open wound, a social toileting with soapy lotions such as chlorhexidine and generous plain-water irrigation are first applied, mopped dry before the proper surgical site prepping is done, and the surgical incision line is isolated with surgical sterile drapes. Figure 1, Figure 2 and Figure 3 illustrate the essential elements of the surgical steps for the use of the titanium clamps in this surgical procedure. First, standard scalp flaps (Figure 1 and Figure 2) or extension of overlying scalp lacerations (Figure 3) are raised to expose the cranial-facial fracture lines. For elevation of the fracture fragments, attempts are made to achieve osteotomies and calvarial craniotomies that include intact bone segments to facilitate the reassembly of the fracture fragments (Figure 1 and Figure 2) [3,4]. Loose fracture fragments that are sizeable enough to handle are harvested, while very tiny fragments are discarded. Then generous debridement of the fracture fragments to be reassembled is performed either onsite for those still attached, or side table for the loose ones. The debridement involves scrupulous scraping, especially of any exposed diploeic layer, with appropriate size bone curettes and wet sponges, and simultaneous generous irrigation with antibiotic-laden normal saline solutions. The bone fragments, usually pristine after this scraping and scrubbing, are then soaked in the same antimicrobial-laden solutions (Figure 2c) while other necessary intracranial work, extra- and/or intradural, is performed. The latter includes repair of dural tears and evacuation of traumatic brain lesions. Finally, the reassembling of the fragments is done, the aim always being autologous osteosynthesis. The bone fragments are brought on-site, sometimes actually force-impacted into position, and are held together by the titanium clamps. The latter are positioned between the bone fragments, and a single 16-mm CranioFix clamp is sometimes able to hold some four bone fragments together by so doing [3,5]. The whole construct of the assembled bone fragments is then held to the adjoining craniotomy flap or the rest of the cranium with another piece of the titanium clamp. This way, on average approximately three clamps are required to complete the reconstruction. We are very conservative in the number of the clamps used due to economic reasons. The operative field is finally given another generous irrigation, and the surgical wound is closed as usual.
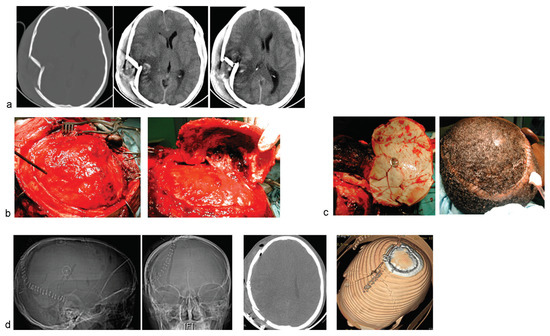
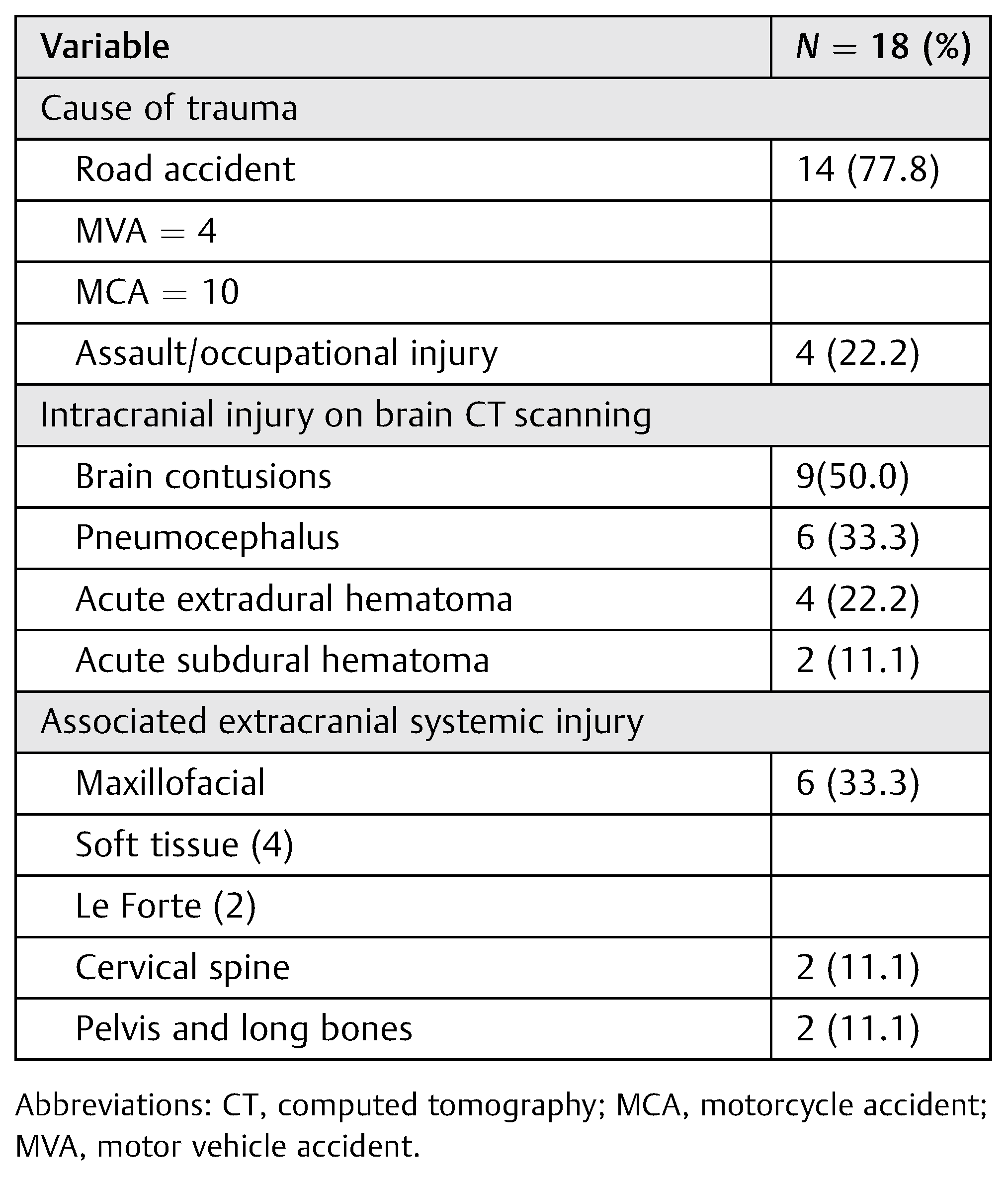
Figure 1.
A young man presented 2 days following moderate head injury sustained out of state; he was referred from another neurosurgery unit. (a) Bone and brain windows of cranial CTshowed comminuted depressed right temporoparietal skull fracture. There were associated brain contusions and cerebral edema, with midline shift. (b) Exposure and elevation of a craniotomy flap incorporating the fracture fragments. (c) Thorough debridement of the fracture site, refixation of the fracture fragments with the 16-mm cranioFix clamp is shown, as well as the operative wound ontable, after closure. (d) Postoperative radiological images showing excellent restoration of the craniofacial symmetry, as well as the titanium implants, low profile, in situ.
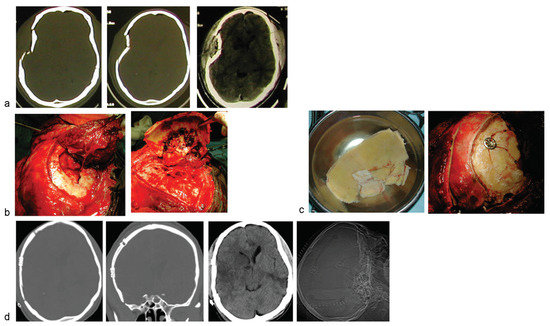
Figure 2.
Another case of comminuted right temporofacial skull fracture. (a) Cranial CT showing the depressed, comminuted skull fracture. There is only minimal contusion of underlying brain. (b) The fracture lines were exposed, and a craniotomy flap incorporating the fragments was raised. (c) The fracture fragments are first given thorough debridement, soaked in saline impregnated with antimicrobial, and finally refixed with the titanium clamp. (d) Postoperative cranial CT images showing excellent restoration of the craniofacial symmetry.
Figure 3.
A 3-year-old child suffered severe head injury and open skull fracture from pedestrian road accident in another city. (a) Left frontal-facial scalp lacerations with evisceration of brain tissue. (b) Cranial CT revealed severe comminuted, craniofacial skeletal disruption, and associated marked brain contusion. (c) Surgical exposure of the fracture lines by extending the scalp lacerations. The wound at closure is also shown. (d) Postoperative skull X-ray study (cannot afford post-op CT) showed the titanium clamps, low-profile, facilitating the good reconstruction of the cranial-facial skeletal disruption. (e) Clinical photo of the child at about a year post-op.
Materials
An observational study of a prospective consecutive cohort of patients who underwent this surgical procedure in our academic neurosurgical practice over a75-month period (August 2009 till November 2015) is presented. Patients who suffered traumatic bone loss preoperatively that defied attempts at reconstruction were excluded from the analysis. For the study patients, the data analyzed included some clinical-demographic information (age, gender, cause of trauma, and pattern of associated head and extracranial injuries) and clinical outcome, in-hospital and at follow-up. The primary outcome measure was the in-hospital disposition at discharge using the Glasgow outcome scale (GOS). Secondary outcomes were SSI perioperatively or at follow-up, and any need for reoperation as well as the subjective assessment of the aesthetic outcome.
The data have been analyzed with the SPSS software, version 21 (SPSS Inc., Chicago, IL) and are presented in descriptive statistics: frequencies and proportions; mean (standard deviation, SD) and median (range).
Results
Eighteen cases of posttraumatic complex craniofacial fractures had the CranioFix clamps used to facilitate their reconstruction in the period of this study. They were all males, mean age 31.3 years (SD, 9.7) and median 33.3 years (range, 3–43). Some of their clinical and radiological information is depicted in Table 1. Road traffic accidents (RTAs) were the cause of the trauma in 14 of 18 patients (78%) motorcycle crash, none helmeted, in 10 of these patients (71% of RTA).

Table 1.
CranioFix for posttraumatic craniofacial reconstruction: some clinical and radiological demographics of the patients presented.
The site of accident was out of state in 13 cases (72%). Some of them were from far flung regions of our country; one case from a neighboring country in West Africa. As many as 15 (83%) were referrals from other health facilities, including other university teaching hospitals with their own neurosurgeon staff. Seven of these (47%) had been seen in two or more of these other health facilities. Hence, presentation in our service was delayed in most cases, median trauma duration being 72 hours (range, 1–504): 9 cases (50%) greater than 24 hours, one case after 3 weeks. Also, the time from trauma to surgery was delayed, median 90 hours (range, 48–2,160): in all the cases, greater than 48 hours, and after 4 days in half of the patient population.
Only one case was a closed fracture, and the rest were compound craniofacial skeletal disruption, with significant, ragged lacerations in three cases (Figure 3). Most (16/18, 88.8%) of the patients presented with mild HI, Glasgow coma scale of 13 to 15; one case each with moderate and severe HI, respectively, and 14 (85.7%) had history of loss of consciousness that ranged in duration from 10 minutes to 5 days (median, 48 hours). This is in keeping with their delayed presentation to us: some of them were referred from the peripheral health facilities to our neurosurgical service for the operative treatment of their skull/brain injury after regaining some level of consciousness. Associated intracranial injuries included pneumocephalus in six (five of them significant); acute extradural hematoma in four and subdural in two, and brain contusions in nine (Figure 1 and Figure 3). Extracranial-associated injuries were pelvic fracture in two; femoral fracture in two, one open and the other one closed; and maxillofacial skeletal injuries in six: four simple nonsurgical fractures and two more complex ones.
Outcome
The surgical procedure was successfully completed in all patients, operative time less than 3 hours in 10 cases (56%). The median in-hospital length of stay was 12 days and the outcome was “normal” (GOS, 1) in 15 patients (83%), and moderate deficit (GOS 2) in two patients. One patient died from extracranial cause. Of the 17 surviving patients, 2 were lost to follow-up since hospital discharge. The rest have been followed up for a median of 24 months (range, 2–73 months), and as much as one-third (6/18) for more than 36 months. There was no case of SSI in the perioperative or the follow-up period to date. There has been no concerning issue of aesthetics in any of the patients (Figure 1, Figure 2 and Figure 3).
Discussion
In the face of the shortage of the appropriate technological backup, and an overall poor health infrastructure in general, there are usually strong concerns, indeed reservations, about instrumentation neurosurgery in developing countries [6,7]. This is specifically as regards possible postoperative complications not the least of which is SSI. But the situation needs not remain so permanently, and certainly not absolutely. Increasingly, an understanding of the issues at play and an application of appropriate preemptive measures to forestall these challenges have since begun to open up new horizons of possibilities in this regard [6]. This study is a practical demonstration of this fact from a developing subSahara African country.
A biomaterial, the titanium clamp (CranioFix), has been successfully implanted for the reconstruction of PTCD in a prospective, consecutive series of patients over a period of more than 6 years. The technique has greatly helped facilitate a highly functional and aesthetic reconstruction of these otherwise disfiguring, and possibly distressing, defects [3,5,8].
The postoperative follow-up period has ranged from 2 to 73 months, at least a third of the patients for longer than 36 months. The procedure has also been, mercifully, free of SSI to date.
The traditional operative treatment for many of the PTCD, especially when they are open fractures as in this series, is a staged procedure [9]. The first stage involves debridement and damage control of the traumatic brain lesions. Usually, the bone fragments are simply discarded creating bone defects that need subsequent repair. Although some workers achieve this repair in the same sitting [9], most others perform secondary cranioplasty later on [10,11,12,13]. But whether the cranioplasty is done as a primary or secondary surgery, there is usually the need to make good the cranial-facial defects left after discarding the “contaminated” fracture fragments.
The substitute material for this cranioplasty ideally is autologous bone. This can be harvested from the cranium or other parts of the body including the ribs. This process involves another complex, time-consuming tissue violation, and the bone so harvested is usually hardly enough for the defect [2,14]. Increasing number of alloplastic materials are now in use to circumvent the limitations of the use of autologous bone. They include polymethylmethacrylate (PMMA), polyetheretherketone (PEEK), hydroxyapatite, titanium mesh, and so on [2,14,15,16]. But these are costly implants, obviously not an attractive option in low-resource regions of the world like our own. Their use is also fraught with higher rates of SSI [2].
The simple technique used by us in this patient series obviates many of the demerits of these biomaterials. It salvages as much of the native fracture fragments as possible, and utilizes the titanium clamp for their fixation to reconstruct the craniofacial defects. We have found the 16-mm clamp big enough to hold as many as four fracture splinters. This way the average number of clamps we use per case is three: we have to be as judicious as possible in the use of these clamps for economic reasons. What is more, the titanium clamp is fast to apply on-site, with much less cumbersome appliances than most other biomaterials. It also appears the cheapest of all [8,17]. Not only are plates and screws more cumbersome, somewhat, to handle, compared with the CranioFix clamps, they are also much more expensive. They may also produce scalp irritation [17].
In essence, this simple low-profile technique, autologous osteosynthesis with CranioFix in PTCD, is an effectual one in this craniofacial surgery. Two reasons can be adduced for this. One, not only is autologous bone the gold standard in craniofacial reconstruction, bone grafts are also known to be at less risk of infections compared with alloplastic materials in cranioplasty [2]. It also appears that although thorough debridement of the bone fragments and the fracture sites is key in this treatment paradigm, even so may be the rigid fixation of the fracture fragments which the CranioFix clamps have been known to be the most versatile for, of all the biomaterials used for that purpose [3,17,18]. Sutures provide only low-grade connection of the bone fragments, and that by friction only leading to a fairly common complication of skull flap settling in their use. This lack of locking connection between the bone fragments when held by sutures encourage micromovements of the fragments transmitted through rapid changes in the intracranial pressure [17]. This obviously has adverse effect on consolidation of the callus in the bone healing process. Steel wires are stronger than sutures, but are not so biocompatible. They are even more prone to causing scalp irritation being more prominent under the scalp. Worst of all, they create significant, obtrusive metallic artifacts on follow-up imaging studies. Miniplates and screws come closest to the titanium clamps in their utility for cranialfacial bone fixation but have the drawbacks alluded to above: more time consuming to apply, more expensive, and may be more prone to producing scalp irritation [8,17].
Conclusions
A surgical series of autologous reconstruction of PTCD using the titanium clamp (CranioFix) is presented in this study. The technique was successful, free of complications including SSI, in a 6-year prospective consecutive cohort of patients in a neurosurgery practice from a developing country. Based on the scientific principles of orthopedic surgery in the management of open fractures (generous debridement/irrigation [19] and rigid fixation of fracture fragments), it appears the technique can be applied even outside the low-resource developing countries [5,8].
Funding
No external funding was received to execute this project.
Conflicts of Interest
We firmly declare that we have no conflict of interest with any of the materials and devices reported in this article.
References
- Habal, M.B. The cranial vault configuration is an integral part of the practice of craniofacial surgery. J Craniofac Surg 2015, 26, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Neovius, E.; Engstrand, T. Craniofacial reconstruction with bone and biomaterials: Review over the last 11 years. J Plast Reconstr Aesthet Surg 2010, 63, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qian, C.; Yang, S.; Chen, Y.; Sun, W.; Wang, Y. Cranial reconstruction with titanium clamps in frontal comminuted depressed skull fractures. J Craniofac Surg 2013, 24, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Burstein, F.; Cohen, S.; Hudgins, R.; Boydston, W. Frontal basilar trauma: Classification and treatment. Plast Reconstr Surg 1997, 99, 1314–1321; discussion 1322–1323. [Google Scholar] [CrossRef]
- Ebel, H.; Schillinger, G.; Walter, C.; Brockhagen, H.G.; Klug, N. Titanium clamps for refixation of bone fragments in the repair of depressed skull fractures: Technical note. Minim Invasive Neurosurg 2000, 43, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Njoku, I.; Wanin, O.; Assey, A.; et al. Minimally invasive 2D navigation-assisted treatment of thoracolumbar spinal fractures in East Africa: A case report. Cureus 2016, 8, e507. [Google Scholar] [PubMed]
- Adeleye, A.O.; Fasunla, J.A.; Young, P.H. Skull base surgery in a large, resource-poor, developing country with few neurosurgeons: Prospects, challenges, and needs. World Neurosurg 2012, 78, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wörner, B.; Lange, M.; Herzog, A.; Fink, U.; Oeckler, R. A new method for surgical repair of impression fractures of the cranial vault and frontal sinus with rivet-like titanium clamps. Neurosurg Rev 2001, 24, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Marbacher, S.; Andres, R.H.; Fathi, A.R.; Fandino, J. Primary reconstruction of open depressed skull fractures with titanium mesh. J Craniofac Surg 2008, 19, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Imola, M.J.; Ducic, Y.; Adelson, R.T. The secondary correction of posttraumatic craniofacial deformities. Otolaryngol Head Neck Surg 2008, 139, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Mokal, N.J.; Desai, M.F. Secondary correction of post-traumatic craniofacial deformities. J Craniofac Surg 2014, 25, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Bussieres, M.; Tatum, S.A. Secondary craniofacial surgery for trauma. Facial Plast Surg 2000, 16, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Velho, V.; Kharosekar, H.U.; Thukral, J.S.; Valsangkar, S.; Survashe, P. Management strategies for comminuted fractures of frontal skull base: An institutional experience. Indian J of Neurosurgery 2015, 4, 80–84. [Google Scholar] [CrossRef][Green Version]
- Kuttenberger, J.J.; Hardt, N. Long-term results following reconstruction of craniofacial defects with titanium micro-mesh systems. J Craniomaxillofac Surg 2001, 29, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Burstein, F.D.; Cohen, S.R.; Hudgins, R.; Boydston, W.; Simms, C. The use of hydroxyapatite cement in secondary craniofacial reconstruction. Plast Reconstr Surg 1999, 104, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.T.; Somers, P.C.; Kjar, J.G. The use of Champy miniplates for osteosynthesis in craniofacial deformities and trauma. Plast Reconstr Surg 1986, 77, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Lerch, K.D. Reliability of cranial flap fixation techniques: Comparative experimental evaluation of suturing, titanium miniplates, and a new rivet-like titanium clamp (CranioFix): Technical note. Neurosurgery 1999, 44, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Estin, D.; Troffkin, N.; Heilman, C.B. Bone flap fixation with titanium clamps: A new technique. Surg Neurol 2000, 53, 391–394; discussion 394–395. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, M.; Jeray, K.J.; Petrisor, B.A.; et al. FLOW Investigators. A trial of wound irrigation in the initial management of open fracture wounds. N Engl J Med 2015, 373, 2629–2641. [Google Scholar] [PubMed]
© 2016 by the author. The Author(s) 2016.