Comprehensive Effect of Carbon Tetrachloride and Reversal of Gandankang Formula in Mice Liver: Involved in Oxidative Stress, Excessive Inflammation, and Intestinal Microflora
Abstract
:1. Introduction
2. Materials and Methods
2.1. LC-MS Conditions and Chemical Composition Analysis
2.2. Establishment of Chronic Liver Injury Models
2.3. Measurement of Cytokines
2.4. Hepatic Function Assays
2.5. Histopathology of Liver
2.6. Detection of Hepatic Fibrosis and Oxidation Parameters
2.7. Western Blot Analysis
2.8. RNA Extraction, Reverse Transcription, and Q-PCR
2.9. TUNEL Assay
2.10. Determination of Fecal Flora Diversity
2.11. Statistical Analysis
3. Results
3.1. Chemical Composition Identification in GDK
3.2. GDK Improves Hepatic Function and Structure in Chronic Liver Injury
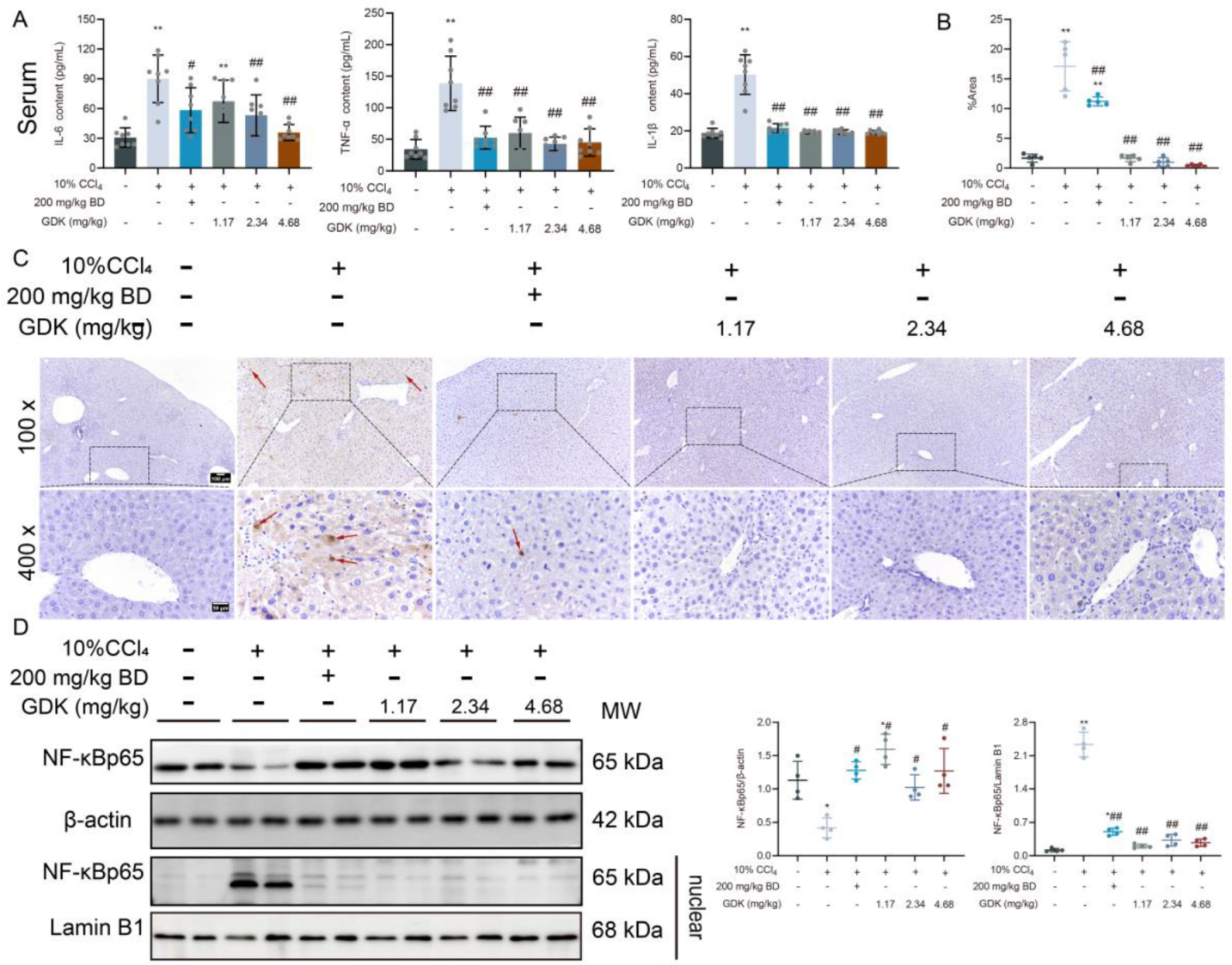
3.3. GDK Reduces Inflammation in CCl4-Induced Chronic Liver Injury
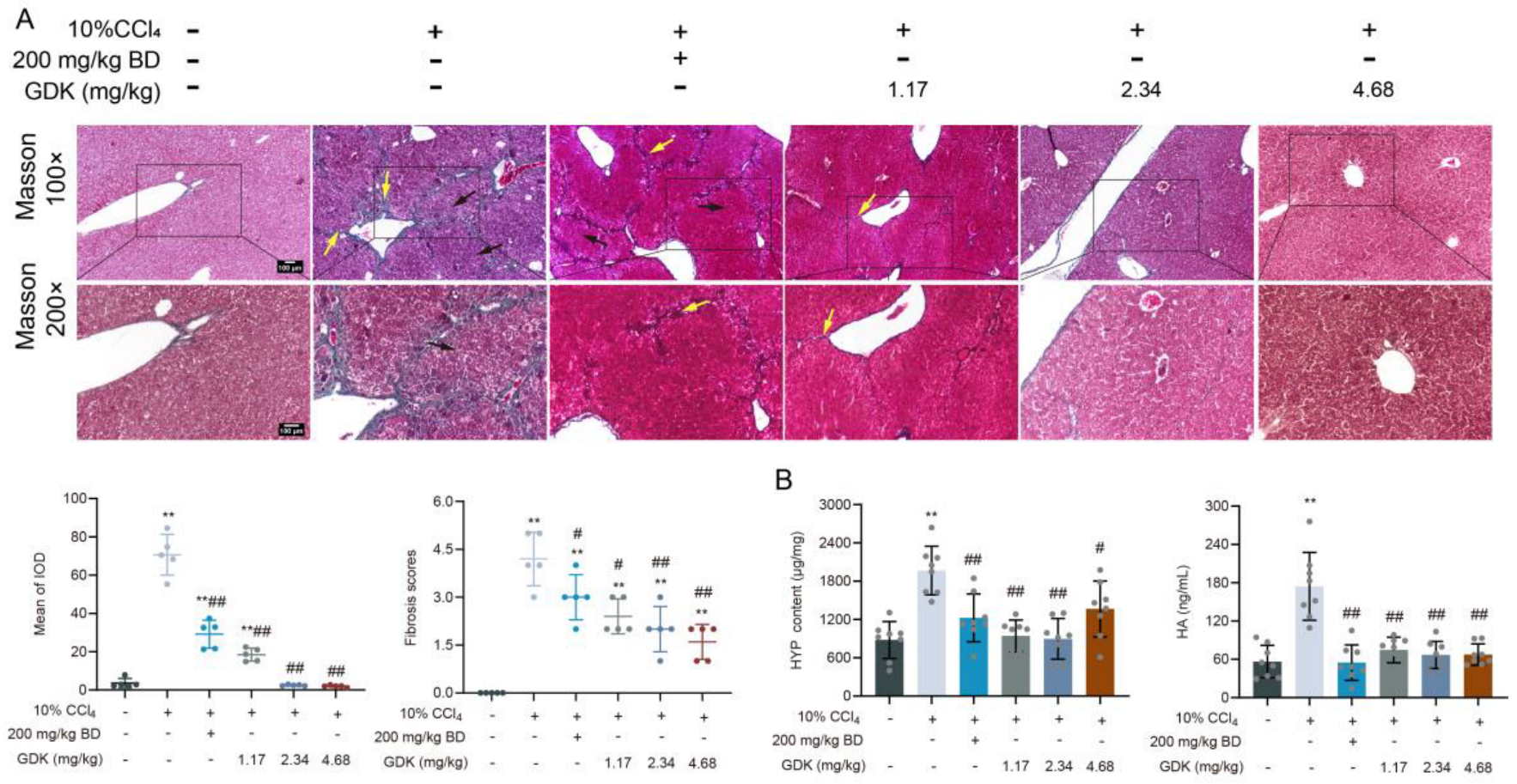
3.4. GDK Suppresses Fibrosis in CCl4-Induced Chronic Liver Injury
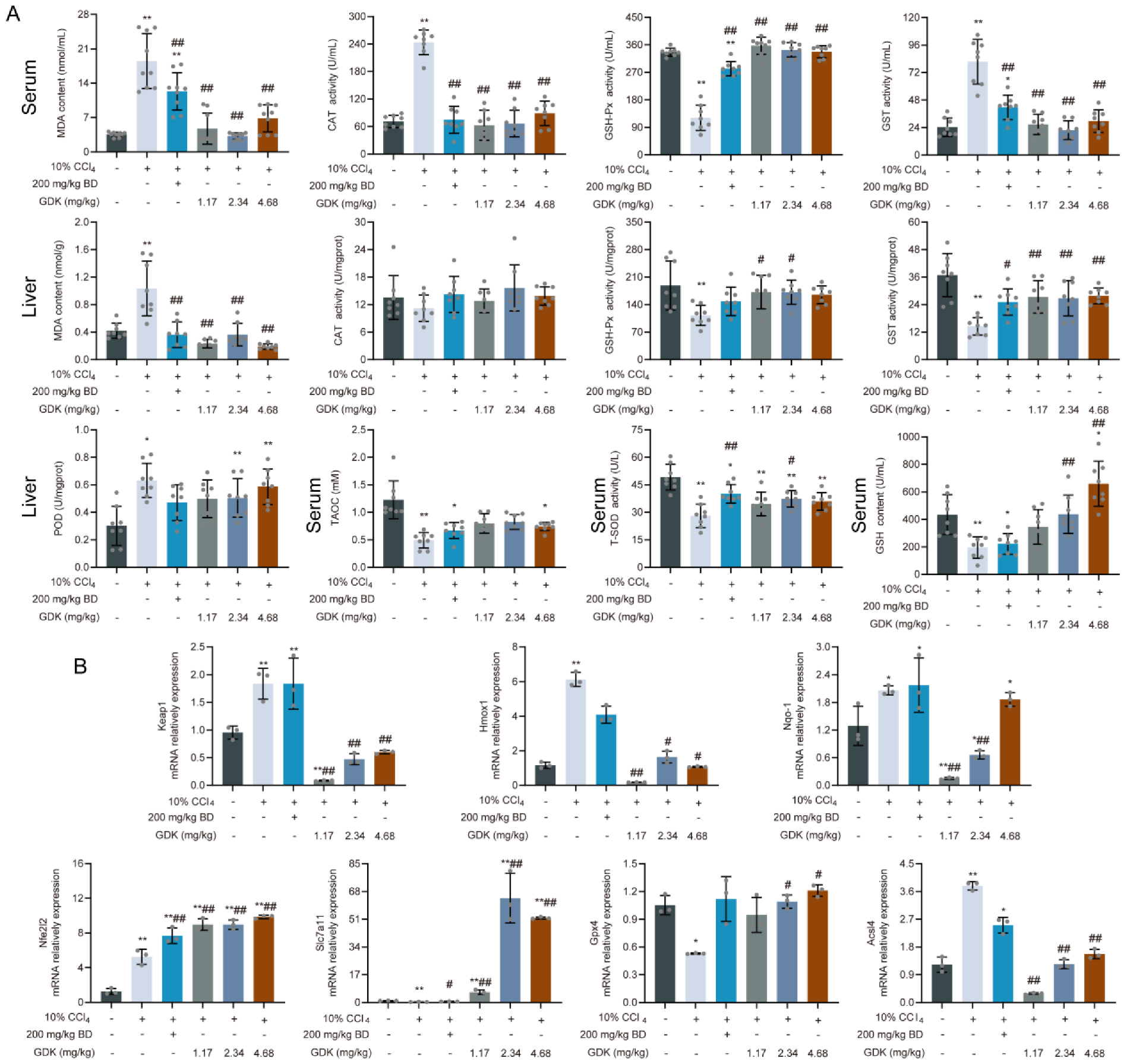
3.5. GDK Weakens Ferroptosis through the Antioxidant System in CCl4-Induced Chronic Liver Injury
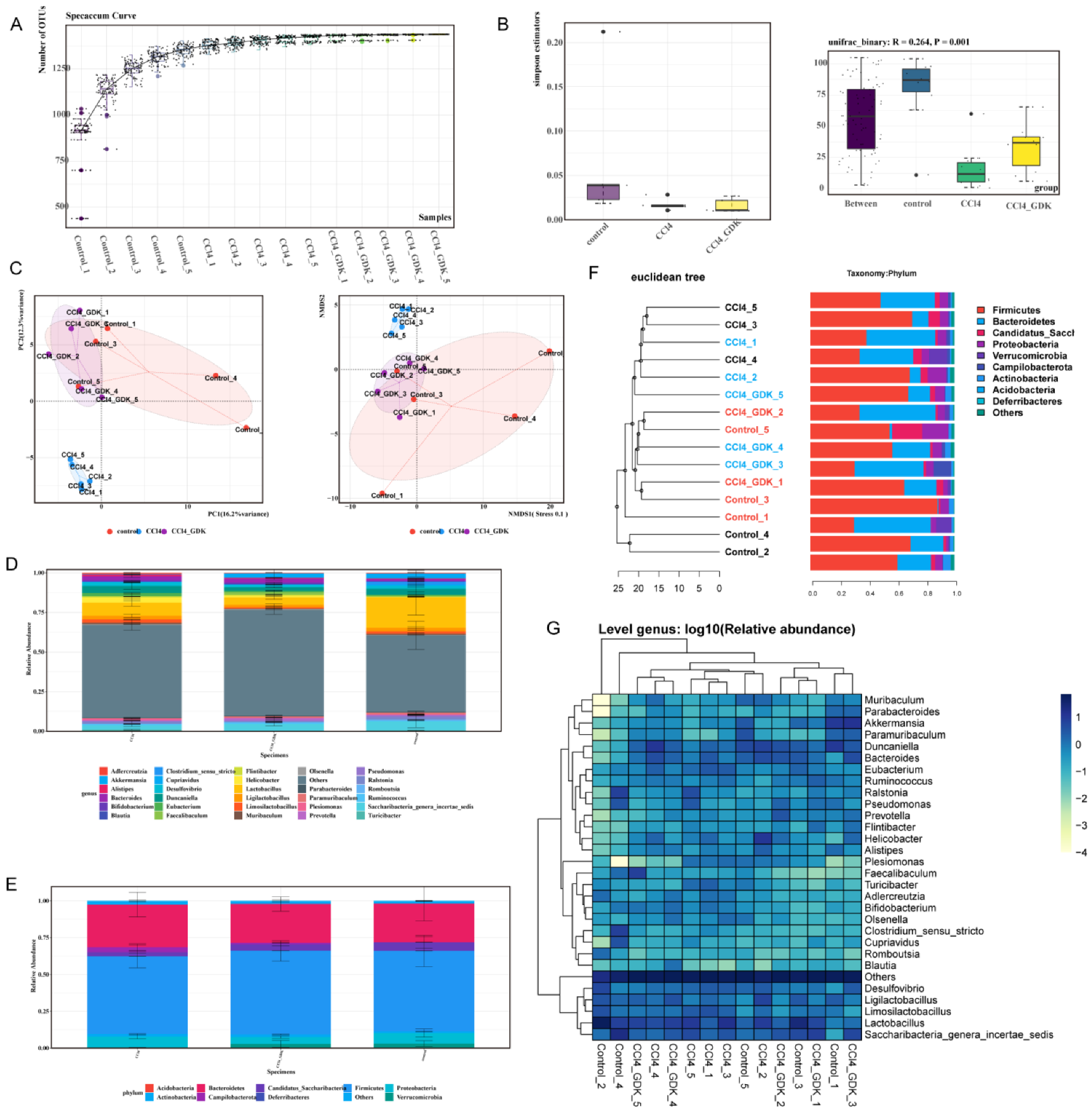
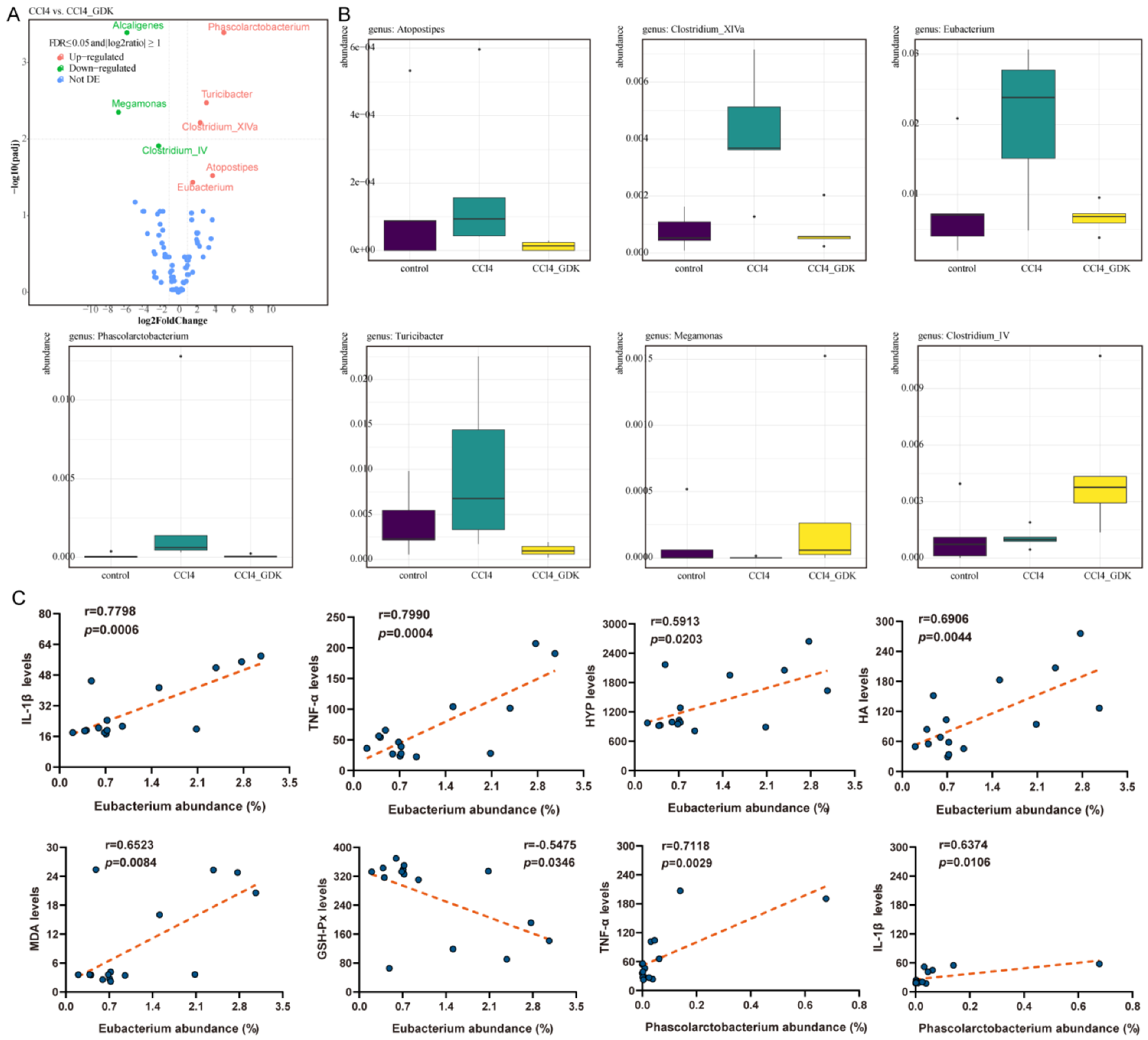
3.6. GDK Restores Intestinal Flora Diversity to Curb Chronic Liver Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marsh, K.; Tayler, R.; Pollock, L.; Roy, K.; Lakha, F.; Ho, A.; Henderson, D.; Divala, T.; Currie, S.; Yirrell, D.; et al. Investigation into Cases of Hepatitis of Unknown Aetiology among Young Children, Scotland, 1 January 2022 to 12 April 2022. Eurosurveillance 2022, 27, 2200318. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Evolving Challenges in Hepatic Fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Pinzani, M. Liver Fibrosis: Pathophysiology, Pathogenetic Targets and Clinical Issues. Mol. Asp. Med. 2019, 65, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Zou, A.; Magee, N.; Deng, F.; Lehn, S.; Zhong, C.; Zhang, Y. Hepatocyte Nuclear Receptor Shp Suppresses Inflammation and Fibrosis in a Mouse Model of Nonalcoholic Steatohepatitis. J. Biol. Chem. 2018, 293, 8656–8671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Dong, M.Q.; Liu, Z.M.; Xu, M.; Huang, Z.H.; Liu, H.J.; Gao, Y.; Zhou, W.J. A Strategy of Vascular-Targeted Therapy for Liver Fibrosis. Hepatology 2022, 76, 660–675. [Google Scholar] [CrossRef]
- Sanchez-Valle, V.; Chavez-Tapia, N.C.; Uribe, M.; Mendez-Sanchez, N. Role of Oxidative Stress and Molecular Changes in Liver Fibrosis: A Review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef]
- Ke, B.; Shen, X.D.; Zhang, Y.; Ji, H.; Gao, F.; Yue, S.; Kamo, N.; Zhai, Y.; Yamamoto, M.; Busuttil, R.W.; et al. Keap1-Nrf2 Complex in Ischemia-Induced Hepatocellular Damage of Mouse Liver Transplants. J. Hepatol. 2013, 59, 1200–1207. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, N.; Slocum, S.L.; Skoko, J.J.; Shin, S.; Kensler, T.W. When Nrf2 Talks, Who’s Listening? Antioxid. Redox Signal. 2010, 13, 1649–1663. [Google Scholar] [CrossRef] [Green Version]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Keap1-Dependent Proteasomal Degradation of Transcription Factor Nrf2 Contributes to the Negative Regulation of Antioxidant Response Element-Driven Gene Expression. J. Biol. Chem. 2003, 278, 21592–21600. [Google Scholar] [CrossRef] [Green Version]
- Nam, E.; Derrick, J.S.; Lee, S.; Kang, J.; Han, J.; Lee, S.J.C.; Chung, S.W.; Lim, M.H. Regulatory Activities of Dopamine and Its Derivatives toward Metal-Free and Metal-Induced Amyloid-Β Aggregation, Oxidative Stress, and Inflammation in Alzheimer’s Disease. ACS Chem. Neurosci. 2018, 9, 2655–2666. [Google Scholar] [CrossRef]
- Fan, J.; Chen, Q.; Wei, L.; Zhou, X.; Wang, R.; Zhang, H. Asiatic Acid Ameliorates Ccl4-Induced Liver Fibrosis in Rats: Involvement of Nrf2/Are, Nf-Kappab/Ikappabalpha, and Jak1/Stat3 Signaling Pathways. Drug Des. Dev. Ther. 2018, 12, 3595–3605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordillo-Bastidas, D.; Oceguera-Contreras, E.; Salazar-Montes, A.; Gonzalez-Cuevas, J.; Hernandez-Ortega, L.D.; Armendariz-Borunda, J. Nrf2 and Snail-1 in the Prevention of Experimental Liver Fibrosis by Caffeine. World J. Gastroenterol. 2013, 19, 9020–9033. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Huang, Z.; Bai, Q.; Sheng, Y.; Hao, Z.; Wang, Z.; Ji, L. Natural Product Andrographolide Alleviated Apap-Induced Liver Fibrosis by Activating Nrf2 Antioxidant Pathway. Toxicology 2018, 396–397, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Husain, H.; Latief, U.; Ahmad, R. Pomegranate Action in Curbing the Incidence of Liver Injury Triggered by Diethylnitrosamine by Declining Oxidative Stress Via Nrf2 and Nfkappab Regulation. Sci. Rep. 2018, 8, 8606. [Google Scholar] [CrossRef] [PubMed]
- Orru, C.; Szydlowska, M.; Taguchi, K.; Zavattari, P.; Perra, A.; Yamamoto, M.; Columbano, A. Genetic Inactivation of Nrf2 Prevents Clonal Expansion of Initiated Cells in a Nutritional Model of Rat Hepatocarcinogenesis. J. Hepatol. 2018, 69, 635–643. [Google Scholar] [CrossRef]
- Yu, M.; Wang, D.; Xu, M.; Liu, Y.; Wang, X.; Liu, J.; Yang, X.; Yao, P.; Yan, H.; Liu, L. Quinocetone-Induced Nrf2/Ho-1 Pathway Suppression Aggravates Hepatocyte Damage of Sprague-Dawley Rats. Food Chem. Toxicol. 2014, 69, 210–219. [Google Scholar] [CrossRef]
- Xu, W.; Hellerbrand, C.; Kohler, U.A.; Bugnon, P.; Kan, Y.W.; Werner, S.; Beyer, T.A. The Nrf2 Transcription Factor Protects from Toxin-Induced Liver Injury and Fibrosis. Lab Investig. 2008, 88, 1068–1078. [Google Scholar] [CrossRef] [Green Version]
- Duarte, T.L.; Caldas, C.; Santos, A.G.; Silva-Gomes, S.; Santos-Gonçalves, A.; Martins, M.J.; Porto, G.; Lopes, J.M. Genetic Disruption of Nrf2 Promotes the Development of Necroinflammation and Liver Fibrosis in a Mouse Model of Hfe-Hereditary Hemochromatosis. Redox Biol. 2017, 11, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Koyama, Y.; Brenner, D.A. Liver Inflammation and Fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- Brenner, D.A. Molecular Pathogenesis of Liver Fibrosis. Trans. Am. Clin. Climatol. Assoc. 2009, 120, 361–368. [Google Scholar]
- Elsharkawy, A.M.; Mann, D.A. Nuclear Factor-Kappab and the Hepatic Inflammation-Fibrosis-Cancer Axis. Hepatology 2007, 46, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Muriel, P. Nf-Kappab in Liver Diseases: A Target for Drug Therapy. J. Appl. Toxicol. 2009, 29, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.G.; Thomas, B.; Koff, J. Tgf-Beta: Master Regulator of Inflammation and Fibrosis. Respirology 2018, 23, 1096–1097. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ke, Y.; Zhan, R.; Liu, C.; Zhao, M.; Zeng, A.; Shi, X.; Ji, L.; Cheng, S.; Pan, B.; et al. Trimethylamine-N-Oxide Promotes Brain Aging and Cognitive Impairment in Mice. Aging Cell 2018, 17, e12768. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Wang, X.; Ji, F.; Ronco, C.; Tian, J.; Yin, Y. Gut-Liver Crosstalk in Sepsis-Induced Liver Injury. Crit. Care 2020, 24, 614. [Google Scholar] [CrossRef]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-Invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2019, 30, 607. [Google Scholar] [CrossRef] [Green Version]
- Saeedi, B.J.; Liu, K.H.; Owens, J.A.; Hunter-Chang, S.; Camacho, M.C.; Eboka, R.U.; Chandrasekharan, B.; Baker, N.F.; Darby, T.M.; Robinson, B.S.; et al. Gut-Resident Lactobacilli Activate Hepatic Nrf2 and Protect against Oxidative Liver Injury. Cell Metab. 2020, 31, 956–968.e5. [Google Scholar] [CrossRef]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef] [Green Version]
- Cai, F.F.; Wu, R.; Song, Y.N.; Xiong, A.Z.; Chen, X.L.; Yang, M.D.; Yang, L.; Hu, Y.; Sun, M.Y.; Su, S.B. Yinchenhao Decoction Alleviates Liver Fibrosis by Regulating Bile Acid Metabolism and Tgf-Beta/Smad/Erk Signalling Pathway. Sci. Rep. 2018, 8, 15367. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.L.; Hu, X.D.; Liu, P. Immunomodulation and Liver Protection of Yinchenhao Decoction against Concanavalin a-Induced Chronic Liver Injury in Mice. J. Integr. Med. 2015, 13, 262–268. [Google Scholar] [CrossRef]
- Xu, L.; Xie, T.; Shen, T.; Jian, S. Yinchenhao Decoction for Chronic Hepatitis B: Protocol for a Systematic Review and Meta-Analysis. Medicine 2019, 98, e14648. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Li, Z.L.; Zhang, X.B.; Liu, H. Yinchenhao Decoction Attenuates Obstructive Jaundice-Induced Liver Injury and Hepatocyte Apoptosis by Suppressing Protein Kinase Rna-Like Endoplasmic Reticulum Kinase-Induced Pathway. World J. Gastroenterol. 2019, 25, 6205–6221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Hu, H.; Zhao, G.; Liu, P.; Wang, Y.; Zhang, H. Effect and Related Mechanism of Yinchenhao Decoction on Mice with Lithogenic Diet-Induced Cholelithiasis. Exp. Ther. Med. 2021, 21, 316. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.F.; Bian, Y.Q.; Wu, R.; Sun, Y.; Chen, X.L.; Yang, M.D.; Zhang, Q.R.; Hu, Y.; Sun, M.Y.; Su, S.B. Yinchenhao Decoction Suppresses Rat Liver Fibrosis Involved in an Apoptosis Regulation Mechanism Based on Network Pharmacology and Transcriptomic Analysis. Biomed. Pharmacother. 2019, 114, 108863. [Google Scholar] [CrossRef] [PubMed]
- Xing, P.; Niu, Y.; Mu, R.; Wang, Z.; Xie, D.; Li, H.; Dong, L.; Wang, C. A Pocket-Escaping Design to Prevent the Common Interference with near-Infrared Fluorescent Probes in Vivo. Nat. Commun. 2020, 11, 1573. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Hu, R.; Li, J.; Xing, X.; Chen, J.; Zhou, Q.; Sun, J. Alpinetin Exerts Anti-Inflammatory, Anti-Oxidative and Anti-Angiogenic Effects through Activating the Nrf2 Pathway and Inhibiting Nlrp3 Pathway in Carbon Tetrachloride-Induced Liver Fibrosis. Int. Immunopharmacol. 2021, 96, 107660. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.Y.; Zhang, Y.N.; Wang, H.; Ga, Y.; Fan, Y.; Wang, Q.; Gu, J.H.; Zhang, X.Y.; Gong, X.H.; Hao, Z.H. Mori Fructus Aqueous Extracts Attenuate Carbon Tetrachloride-Induced Renal Injury Via the Nrf2 Pathway and Intestinal Flora. Ecotoxicol. Environ. Saf. 2022, 245, 114118. [Google Scholar] [CrossRef]
- Arroyo, V.; Moreau, R.; Jalan, R. Acute-on-Chronic Liver Failure. N. Engl. J. Med. 2020, 382, 2137–2145. [Google Scholar] [CrossRef]
- Capelletti, M.M.; Manceau, H.; Puy, H.; Peoc’h, K. Ferroptosis in Liver Diseases: An Overview. Int. J. Mol. Sci. 2020, 21, 4908. [Google Scholar] [CrossRef]
- Yuan, H.; Li, X.; Zhang, X.; Kang, R.; Tang, D. Identification of Acsl4 as a Biomarker and Contributor of Ferroptosis. Biochem. Biophys. Res. Commun. 2016, 478, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and Function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and Regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Jiang, X.; Gu, W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 2020, 30, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Hadian, K.; Stockwell, B.R. Snapshot: Ferroptosis. Cell 2020, 181, 1188–1188.e1. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Kong, X.Y.; Yao, Y.; Wang, X.A.; Yang, W.; Wu, H.; Li, S.; Ding, J.W.; Yang, J. The Critical Role and Molecular Mechanisms of Ferroptosis in Antioxidant Systems: A Narrative Review. Ann. Transl. Med. 2022, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Y.; Fan, Y.M.; Yuan, Y.Y.; Ga, Y.; Zhang, Y.N.; Han, J.C.; Hao, Z.H. Protective Effect of Aqueous Extract of Yinshanlian on Acute Liver Injury Induced by Carbon Tetrachloride in Mice. Acta Vet. Zootech. Sin. 2022, 53, 2333–2342. [Google Scholar]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. Nrf2 Plays a Critical Role in Mitigating Lipid Peroxidation and Ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Mohs, A.; Otto, T.; Schneider, K.M.; Peltzer, M.; Boekschoten, M.; Holland, C.H.; Hudert, C.A.; Kalveram, L.; Wiegand, S.; Saez-Rodriguez, J.; et al. Hepatocyte-Specific Nrf2 Activation Controls Fibrogenesis and Carcinogenesis in Steatohepatitis. J. Hepatol. 2021, 74, 638–648. [Google Scholar] [CrossRef]
- Roh, J.L.; Kim, E.H.; Jang, H.; Shin, D. Nrf2 Inhibition Reverses the Resistance of Cisplatin-Resistant Head and Neck Cancer Cells to Artesunate-Induced Ferroptosis. Redox Biol. 2017, 11, 254–262. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the P62-Keap1-Nrf2 Pathway Protects against Ferroptosis in Hepatocellular Carcinoma Cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a P53-Mediated Activity During Tumour Suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Frissen, M.; Liao, L.; Schneider, K.M.; Djudjaj, S.; Haybaeck, J.; Wree, A.; Rolle-Kampczyk, U.; von Bergen, M.; Latz, E.; Boor, P.; et al. Bidirectional Role of Nlrp3 During Acute and Chronic Cholestatic Liver Injury. Hepatology 2021, 73, 1836–1854. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, D.; Zhu, B.; Wang, S.; Xu, Y.; Zhang, C.; Yang, H.; Wang, S.; Liu, P.; Qin, L.; et al. Rubus chingii Hu. Unripe Fruits Extract Ameliorates Carbon Tetrachloride-Induced Liver Fibrosis and Improves the Associated Gut Microbiota Imbalance. Chin. Med. 2022, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Huang, Z.R.; Jia, R.B.; Lv, X.C.; Zhao, C.; Liu, B. Spirulina Platensis Polysaccharides Attenuate Lipid and Carbohydrate Metabolism Disorder in High-Sucrose and High-Fat Diet-Fed Rats in Association with Intestinal Microbiota. Food Res. Int. 2021, 147, 110530. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, M.; Kessoku, T.; Ozaki, A.; Kasai, Y.; Kobayashi, T.; Nogami, A.; Honda, Y.; Ogawa, Y.; Imajo, K.; Yoneda, M.; et al. Gut Microbiota Composition Associated with Hepatic Fibrosis in Non-Obese Patients with Non-Alcoholic Fatty Liver Disease. J. Gastroenterol. Hepatol. 2021, 36, 2275–2284. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut Microbes from the Phylogenetically Diverse Genus Eubacterium and Their Various Contributions to Gut Health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Shih, Y.Y.; Yeh, Y.T.; Huang, C.H.; Liao, C.A.; Hu, C.Y.; Nagabhushanam, K.; Ho, C.T.; Chen, Y.K. Pterostilbene and Its Derivative 3′-Hydroxypterostilbene Ameliorated Nonalcoholic Fatty Liver Disease through Synergistic Modulation of the Gut Microbiota and Sirt1/Ampk Signaling Pathway. J. Agric. Food Chem. 2022, 70, 4966–4980. [Google Scholar] [CrossRef]
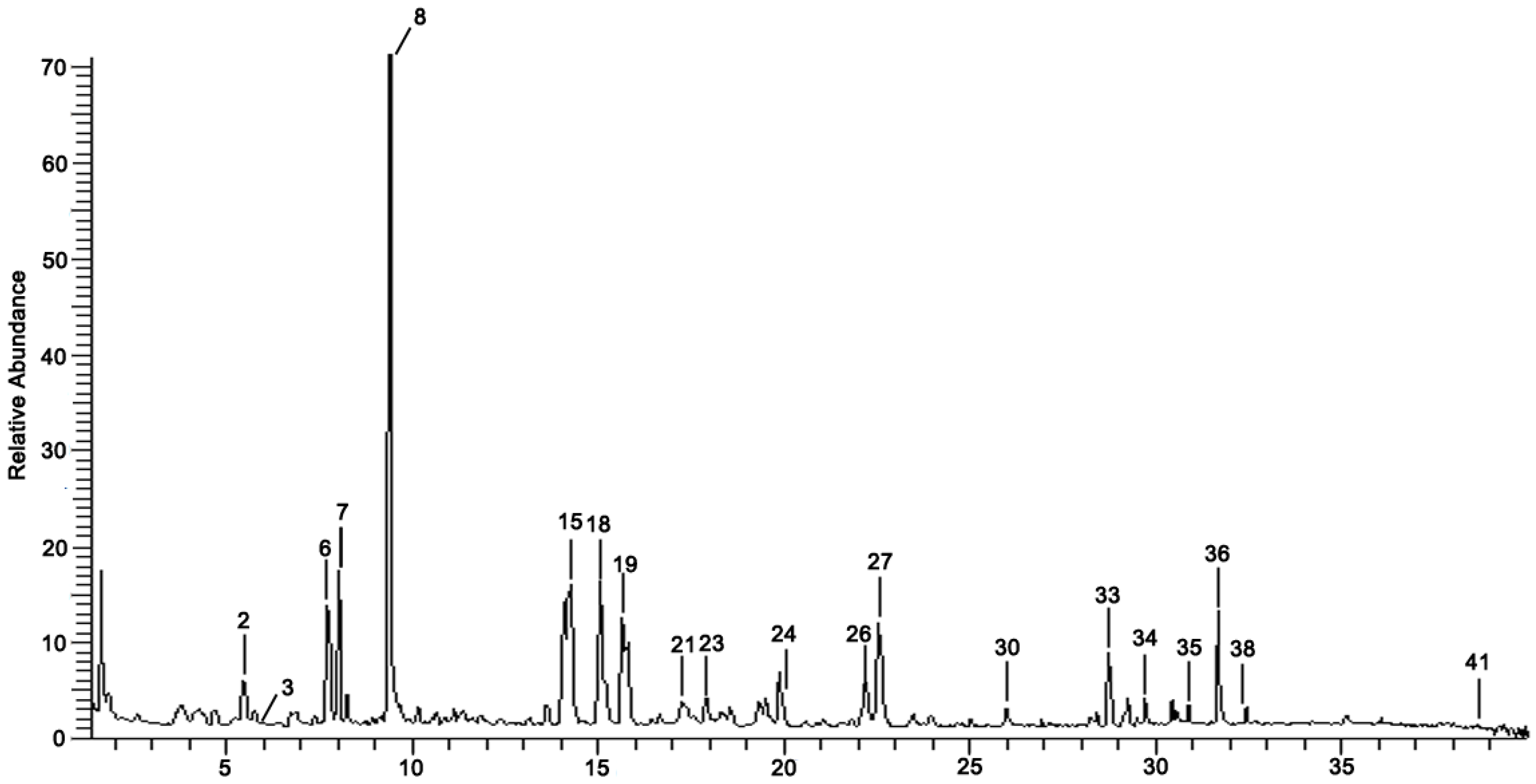

| No | Rt (min) | Formula | Measured [M-H]− | MS/MS (m/z) | Tentative Identification | Structural Formula |
|---|---|---|---|---|---|---|
| 1 | 2.69 | C17H24O9 | 371.13366 | 191.05609 | Eleutheroside B | 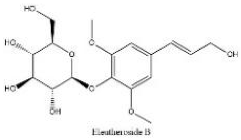 |
| 2 | 5.89 | C12H14O8 | 285.0395 | 152.01164; 153.01944; 108.02183; 285.06177 | Uralenneoside | 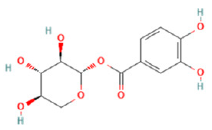 |
| 3 | 5.92 | C10H18O4 | 201.11214 | 110.97005; 201.80287 | Sebacic Acid | 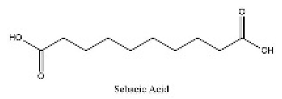 |
| 4 | 6.9 | C20H20O5 | 339.0036 | 265.00217; 238.98785 | Licocoumarone | 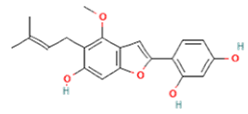 |
| 5 | 7.46 | C20H20O4 | 323.1349 | 323.13495; 186.85599 | licoagrochalcone A | 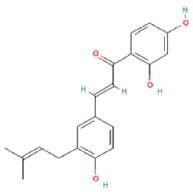 |
| 6 | 7.73 | C25H28O5 | 407.0068 | 248.98572; 317.01288 | 6,8-Diprenylnaringenin | 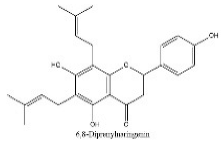 |
| 7 | 8.01 | C27H30O15 | 593.1725 | 593.17535 | Nicotiflorin | 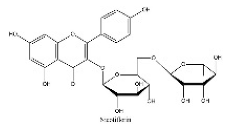 |
| 8 | 9.46 | C20H18O6 | 353.0879 | 191.05623; 161.02438; 93.03436 | Gancaonin C | 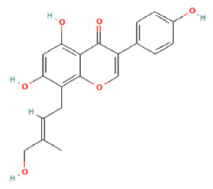 |
| 9 | 9.77 | C26H28O14 | 563.2354 | 101.02442; 563.16235; 149.04539 | Schaftoside | 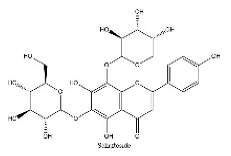 |
| 10 | 10.16 | C27H30O15 | 593.1309 | 353.06696; 383.07755; 473.10950 | Vicenin-2 | 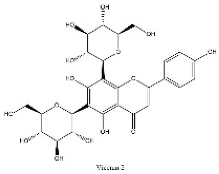 |
| 11 | 10.33 | C26H32O5 | 423.188 | 221.06671; 179.05582 | Licoricidin | 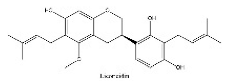 |
| 12 | 10.99 | C21H20O6 | 367.1038 | 191.05617; 173.04538; 193.05045 | Curcumin |  |
| 13 | 10.99 | C21H20O6 | 367.1038 | 191.05617; 149.06084 | Gancaonin B | 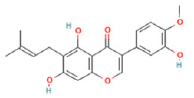 |
| 14 | 13.71 | C15H12O2 | 223.0614 | 223.06140; 69.80861 | Dibenzoylmethane | 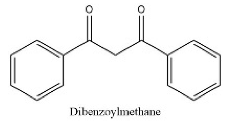 |
| 15 | 14.06 | C21H22O9 | 417.1402 | 255.06669; 135.00889; 119.05022 | neoliquiritin | 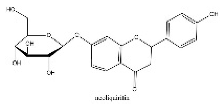 |
| 16 | 14.1 | C21H20O12 | 463.0887 | 301.03516; 243.06598 | Isoquercetin | 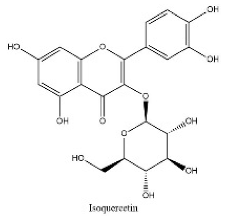 |
| 17 | 14.13 | C15H12O4 | 255.0512 | 119.05028; 135.00887; 255.06685 | Liquiritigenin | 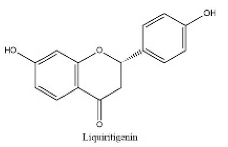 |
| 18 | 14.24 | C27H30O16 | 609.1467 | 300.02774; 301.03549; 609.14716 | Rutin | 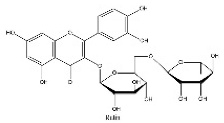 |
| 19 | 15.79 | C21H20O10 | 431.09727 | 269.04592; 431.09910 | Isovitexin | 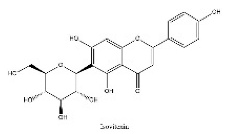 |
| 20 | 16.60 | C27H32O14 | 579.17083 | 271.06131; 151.00371 | naringin | 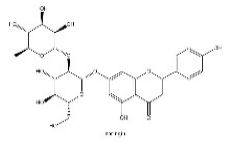 |
| 21 | 17.37 | C20H18O6 | 353.0875 | 191.05620; 353.08804; 192.90018 | Gancaonin O |  |
| 22 | 17.4 | C28H32O16 | 623.1624 | 315.05094; 623.19692; 299.01936 | Narcissoside | 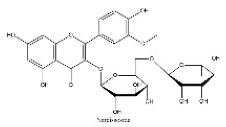 |
| 23 | 17.56 | C15H10O7 | 301.0355 | 301.03574; 272.84845; 255.03066 | Quercetin | 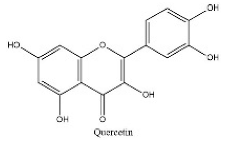 |
| 24 | 20.71 | C24H3004 | 381.1325 | 337.20242; 381.19220 | Gancaonin R | 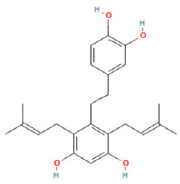 |
| 25 | 21.79 | C16H12O4 | 267.0663 | 252.04292; 267.06641 | Formononetin | 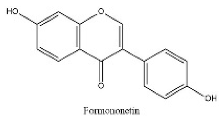 |
| 26 | 22.2 | C15H10O6 | 285.0395 | 285.04072; 154.89865; 284.03243 | Kaempferol | 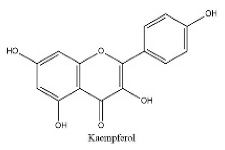 |
| 27 | 22.54 | C15H10O4 | 253.0356 | 107.05025; 94.02987 | Chrysophanol | 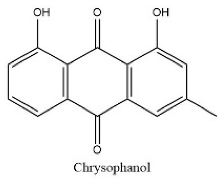 |
| 28 | 23.45 | C16H14O5 | 285.0395 | 285.04056; 241.05064; 165.01947 | isosakuranetin | 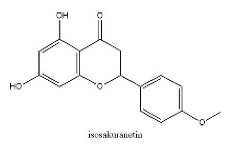 |
| 29 | 23.97 | C16H32O2 | 255.0512 | 255.06648 | palmitic acid |  |
| 30 | 26.09 | C16H12O7 | 315.0514 | 300.02780; 315.05176; 162.83954 | Isorhamnetin | 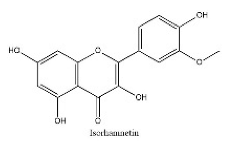 |
| 31 | 28.21 | C15H12O5 | 271.0614 | 151.00372; 271.06125; 119.05020; 107.01391 | naringenin | 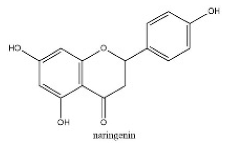 |
| 32 | 28.45 | C25H28O4 | 391.1403 | 391.14026; 202.92906 | (S)-4′,7-Dihydroxy-3′,8-diprenylflavanone |  |
| 33 | 28.83 | C16H14O4 | 269.0446 | 269.04580; 241.05042 | Medicarpin |  |
| 34 | 29.72 | C42H62O17 | 837.3926 | 837.39126; 193.03549; 661.36050 | Licorice saponin G2 | 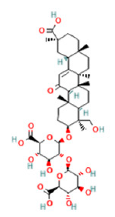 |
| 35 | 30.79 | C15H12O4 | 255.0665 | 119.05023; 255.06645; 153.01938; 135.00890 | Isoliquiritigenin | 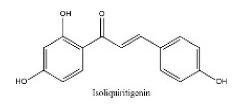 |
| 36 | 31.68 | C42H62O16 | 821.3978 | 821.39753 | Glycyrrhizic Acid |  |
| 37 | 31.83 | C42H62O16 | 821.3978 | 351.05722; 821.39856 | Glycyrrhizin |  |
| 38 | 32.24 | C42H64O15 | 807.4183 | 351.05713; 807.41803; 193.03543 | Licorice saponin B2 |  |
| 39 | 32.93 | C42H64O16 | 823.4135 | 351.05748; 823.41446; 193.03565 | Licorice saponin J2 |  |
| 40 | 37.78 | C17H14O5 | 297.1531 | 297.15314; 166.92424 | 3-Hydroxy-3′,4′-Dimethoxyflavone |  |
| 41 | 38.55 | C16H22O4 | 277.1446 | 121.02955; 134.03772; | Di isobutyl phthalate |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Wang, H.; Zhang, Y.; Gu, J.; Zhang, X.; Gong, X.; Hao, Z. Comprehensive Effect of Carbon Tetrachloride and Reversal of Gandankang Formula in Mice Liver: Involved in Oxidative Stress, Excessive Inflammation, and Intestinal Microflora. Antioxidants 2022, 11, 2234. https://doi.org/10.3390/antiox11112234
Wei Y, Wang H, Zhang Y, Gu J, Zhang X, Gong X, Hao Z. Comprehensive Effect of Carbon Tetrachloride and Reversal of Gandankang Formula in Mice Liver: Involved in Oxidative Stress, Excessive Inflammation, and Intestinal Microflora. Antioxidants. 2022; 11(11):2234. https://doi.org/10.3390/antiox11112234
Chicago/Turabian StyleWei, Yuanyuan, Huiru Wang, Yannan Zhang, Jinhua Gu, Xiuying Zhang, Xuhao Gong, and Zhihui Hao. 2022. "Comprehensive Effect of Carbon Tetrachloride and Reversal of Gandankang Formula in Mice Liver: Involved in Oxidative Stress, Excessive Inflammation, and Intestinal Microflora" Antioxidants 11, no. 11: 2234. https://doi.org/10.3390/antiox11112234
APA StyleWei, Y., Wang, H., Zhang, Y., Gu, J., Zhang, X., Gong, X., & Hao, Z. (2022). Comprehensive Effect of Carbon Tetrachloride and Reversal of Gandankang Formula in Mice Liver: Involved in Oxidative Stress, Excessive Inflammation, and Intestinal Microflora. Antioxidants, 11(11), 2234. https://doi.org/10.3390/antiox11112234





