Application of Phanerochaete chrysopsorium-Based Carbon Paste Electrode as an Electrochemical Sensor for Voltammetric Detection of Hg (II) in Chlor-Alkali Industrial Effluent
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Solutions
2.2. Microorganism’s Culture Conditions
2.3. Preparation of Modified Carbon Paste Electrode
2.4. Apparatus and Voltammetric Procedure
2.5. Surface Characterization of Modified Carbon Paste Electrode
2.6. Collection of Mercury Samples from Chlor-Alkali Industrial Effluent
2.7. Statistical Comparison of New and Reference Method
3. Results and Discussion
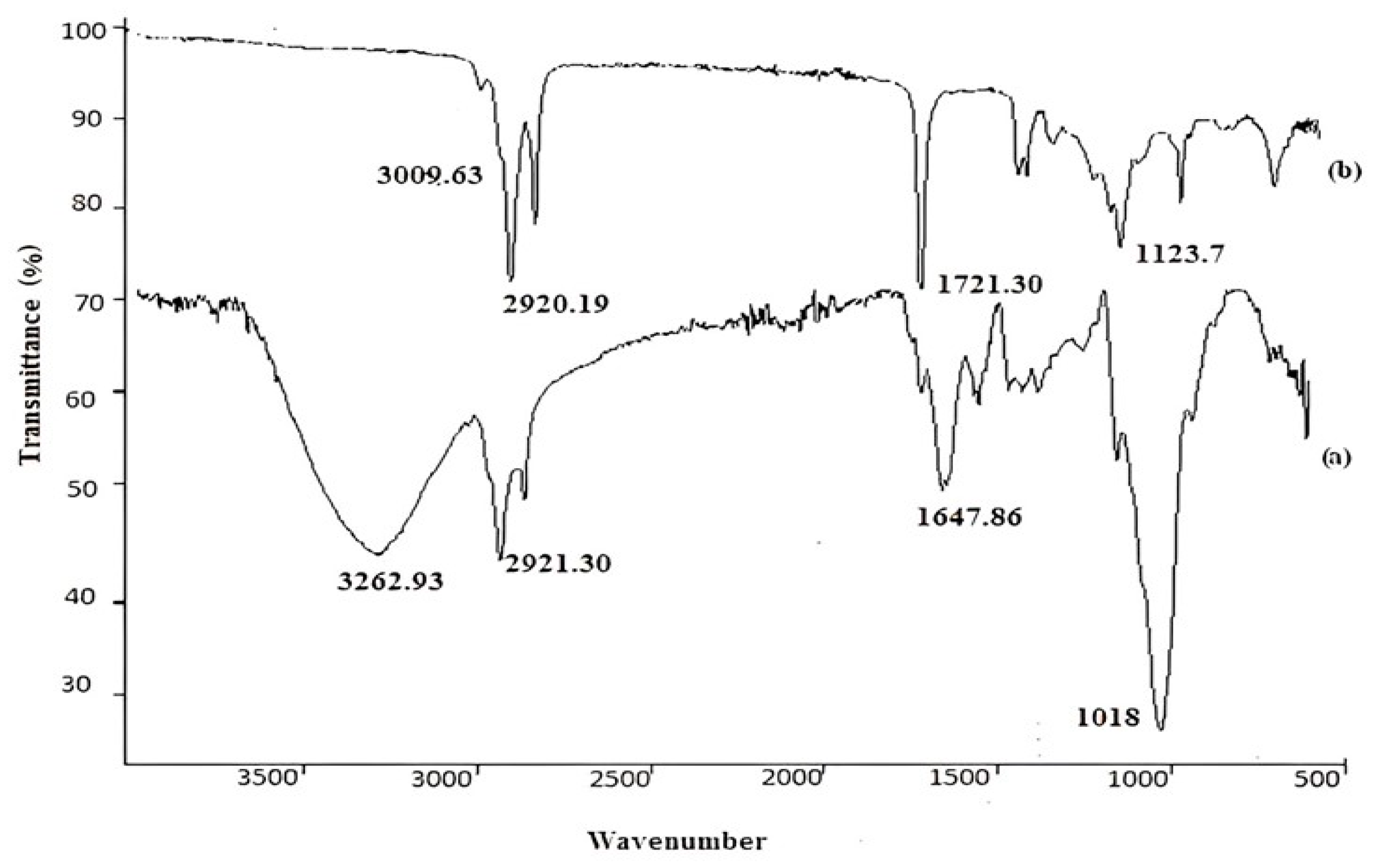
3.1. Surface Characterization of Unloaded and Loaded Carbon PASTE Electrode
3.2. Electrochemical Characterization of Modified Carbon Paste Electrode
3.3. Stripping Procedure and Effect of Stripping Electrolyte
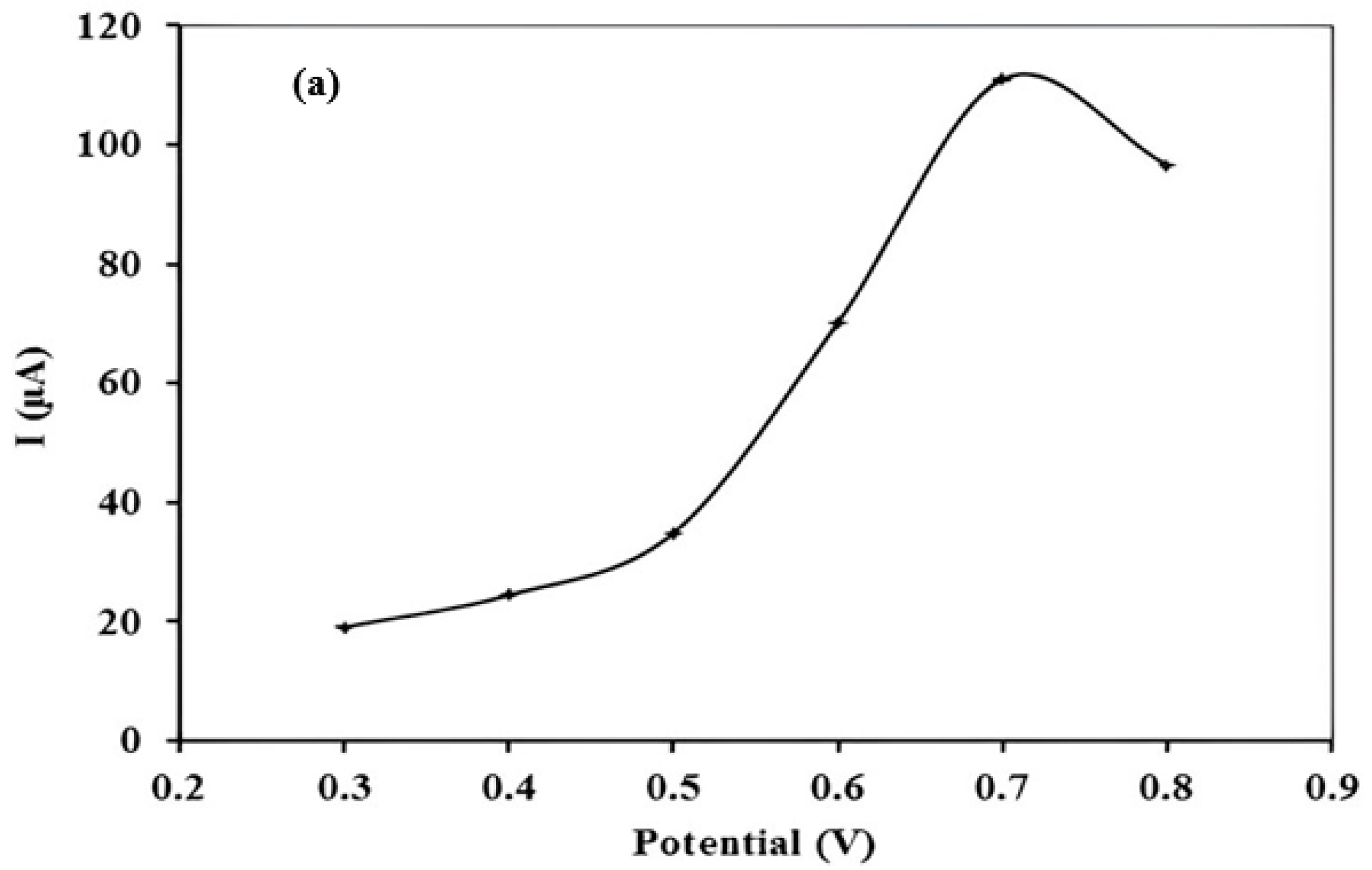
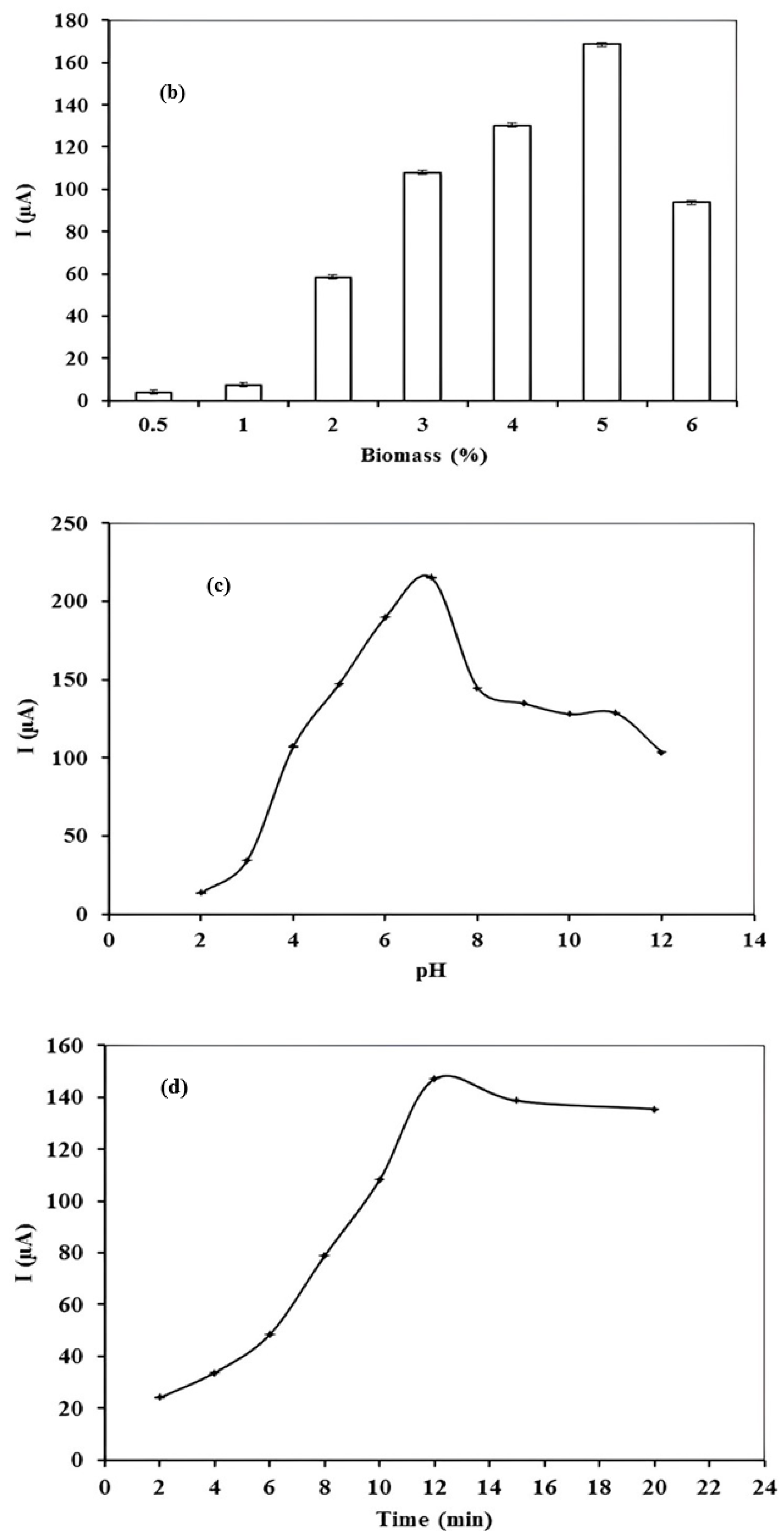
3.4. Optimization of Stripping Parameters
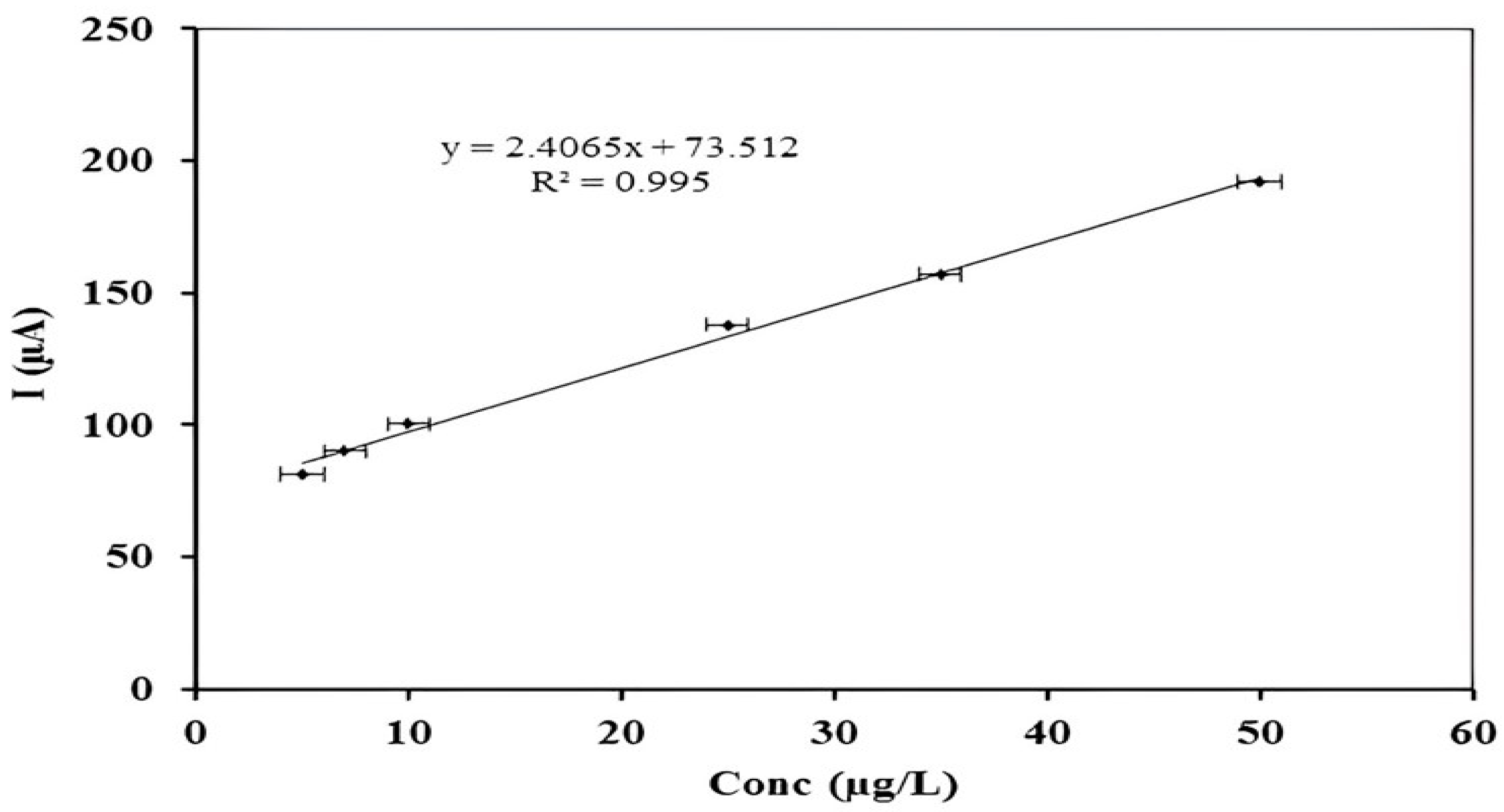
3.5. Analytical Performance
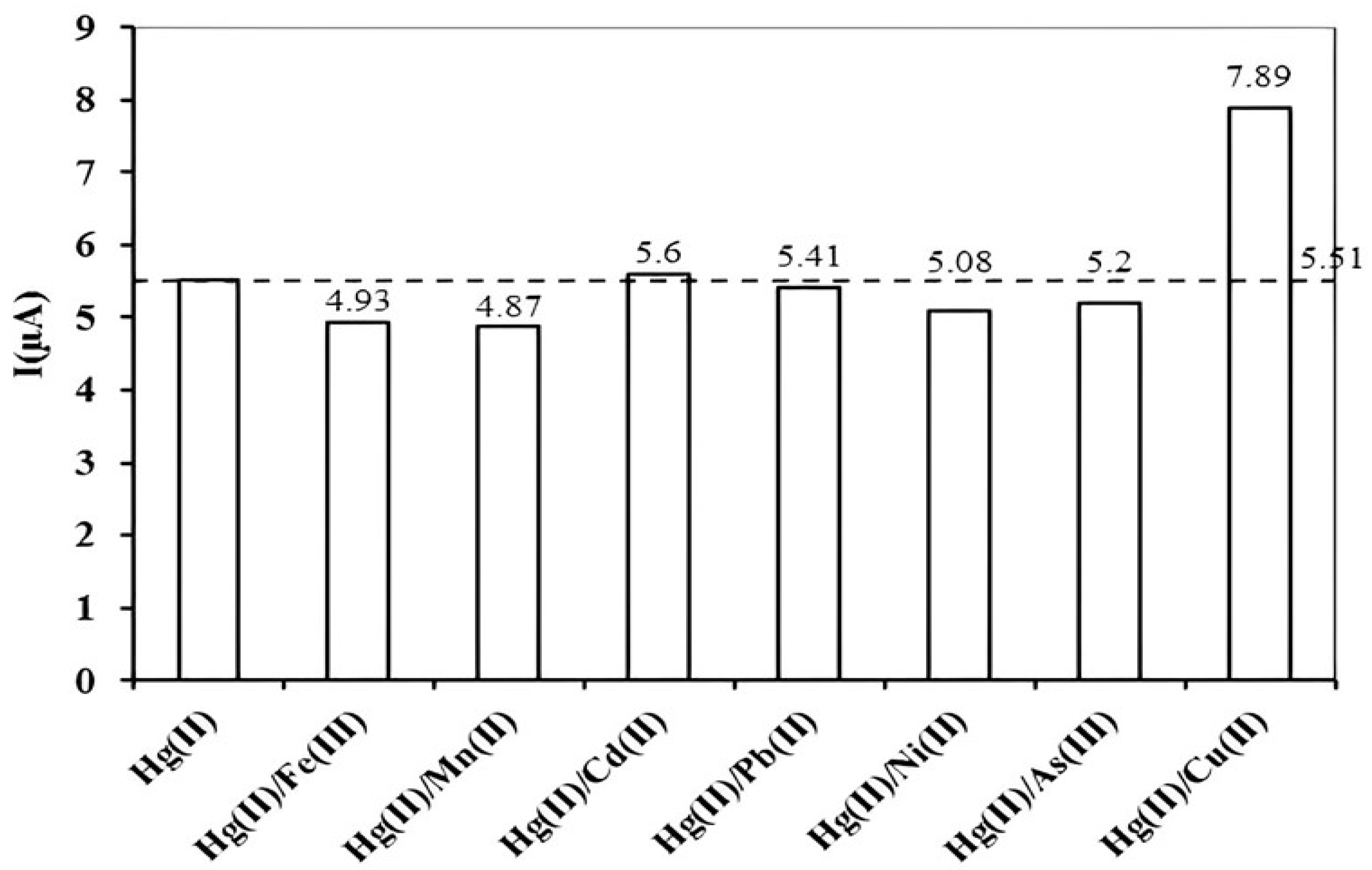
3.6. Interference Studies
3.7. Statistical Comparison of New and Reference Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fathinezhad, M.; Tarighat, A.M.; Dastan, D. Chemometrics heavy metal content clusters using electrochemical data of modified carbon paste electrode. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100307. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, W.; Pi, S.; Cheng, Y.; Xie, Q. Anodic stripping voltammetry analysis of mercury(II) on a pyridine-Au/pyridine/glassy carbon electrode. Sens. Actuators B Chem. 2020, 317, 128202. [Google Scholar] [CrossRef]
- Fadillah, G.; Inayatussholeha, N.E.; Mukarom, A.N.; Rattyananda, S.B.; Wicaksono, P.W.; Fatimah, I.; Saleh, A.T. Ion imprinted-carbon paste electrode as electrochemical sensor for ultra-trace recognizing speciation of mercury. Results Chem. 2022, 4, 100489. [Google Scholar] [CrossRef]
- Azizullah, A.; Khattak, K.N.M.; Richter, P.; Häder, P.D. Water pollution in Pakistan and its impact on public health—A review. Environ. Int. 2011, 37, 479. [Google Scholar] [CrossRef]
- Nordberg, F.G.; Fowler, A.B.; Nordberg, M. Handbook on the Toxicology of Metals; Elsevier: London, UK, 2014. [Google Scholar]
- Hua, K.; Xu, L.X.; Luo, P.Z.; Fang, D.; Bao, R.; Yi, H.J. Effective removal of mercury ions in aqueous solutions: A review. Curr. Nanosci. 2020, 16, 363. [Google Scholar] [CrossRef]
- Zaib, M.; Athar, M.M.; Saeed, A.; Farooq, U. Electrochemical determination of inorganic mercury and arsenic—A review. Biosens. Bioelectron. 2015, 74, 895. [Google Scholar] [CrossRef]
- Tian, J.; Zhu, Y. Rapid determination of mercuryions in environmental water based on an N-rich ccvalent organic framework potential sensor. Int. J. Chem. Eng. 2022, 2022, 3112316. [Google Scholar] [CrossRef]
- Gao, C.; Huang, J.X. Voltammetric determination of mercury(II). TrAC Trends Anal. Chem. 2013, 51, 1. [Google Scholar] [CrossRef]
- Kiliç, D.H.; Deveci, S.; Dönmez, B.K.; Çetinkaya, E.; Karadağ, S.; Doğu, M. Application of stripping voltammetry method for the analysis of available copper, zinc and manganese contents in soil samples. Inter. J. Environ. Anal. Chem. 2018, 98, 308. [Google Scholar] [CrossRef]
- Yerga, M.D.; González-García, B.M.; Costa-García, A. Electrochemical determination of mercury: A review. Talanta 2013, 116, 1091. [Google Scholar] [CrossRef]
- Barek, J. How to improve the performance of electrochemical sensors via minimization of electrode passivation. Chemosensors 2021, 9, 12. [Google Scholar] [CrossRef]
- Yuan, S.; Peng, D.; Song, D.; Gong, J. Layered titanate nanosheets as an enhanced sensing platform for ultrasensitive stripping voltammetric detection of mercury(II). Sens. Actuators B Chem. 2013, 181, 432. [Google Scholar] [CrossRef]
- Michalkiewicz, S.; Agata Skorupa, A.; Magdalena Jakubczyk, M. Carbon materials in electroanalysis of preservatives: A review. Materials 2021, 14, 7630. [Google Scholar] [CrossRef] [PubMed]
- Yüce, M.; Nazır, H.; Dönmez, G. Using of Rhizopus arrhizus as a sensor modifying component for determination of Pb(II) in aqueous media by voltammetry. Biores. Technol. 2010, 101, 7551. [Google Scholar] [CrossRef]
- D’souza, F.S. Microbial Biosensors. Biosens. Bioelectron. 2001, 16, 337. [Google Scholar] [CrossRef]
- Baldrian, P. Interactions of heavy metals with white-rot fungi. Enzyme Micro. Technol. 2003, 32, 78. [Google Scholar] [CrossRef]
- Saglam, A.; Yalcinkaya, Y.; Denizli, A.; Arica, M.; Genc, O.; Bektas, S. Biosorption of mercury by carboxymethylcellulose and immobilized Phanerochaete chrysosporium. Microchem. J. 2002, 71, 73. [Google Scholar] [CrossRef]
- Sağlam, N.; Say, R.; Denizli, S.; Patır, M.; Arıca, Y. Biosorption of inorganic mercury and alkylmercury species on to Phanerochaete chrysosporium mycelium. Process Biochem. 1999, 34, 725. [Google Scholar] [CrossRef]
- Sheiner, B.L.; Beal, L.S. Some suggestions for measuring predictive performance. J. Pharmacokinet. Biopharm. 1981, 9, 503. [Google Scholar] [CrossRef]
- Linnet, K. Necessary sample size for method comparison studies based on regression analysis. Clinical Chem. 1999, 45, 882. [Google Scholar] [CrossRef]
- Lynch, M.J.; Barbano, M.D. Kjeldahl nitrogen analysis as a reference method for protein determination in dairy products. J. AOAC Int. 1999, 82, 1389. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A. Biosorption of reactive dye by loofa sponge-immobilized fungal biomass of Phanerochaete chrysosporium. Process Biochem. 2007, 42, 1160. [Google Scholar] [CrossRef]
- Zaib, M.; Saeed, A.; Hussain, I.; Athar, M.M.; Iqbal, M. Voltammetric detection of As(III) with Porphyridium cruentum based modified carbon paste electrode biosensor. Biosens. Bioelectron. 2014, 62, 242. [Google Scholar] [CrossRef] [PubMed]
- Bashardoost, R.; Vahabzadeh, F.; Shokrollahzadeh, S.; Monazzami, R.A. Sorption performance of live and heat inactivated loofa immobilized Phanerochaete chrysosporium in mercury removal from aqueous solution. Iran. J. Chem. Chem. Eng. 2010, 29, 79. [Google Scholar]
- Zaib, M.; Athar, M. Electrochemical evaluation of Phanerocheaete chrysosporium based carbon paste electrode with potassium ferricyanide redox system. Int. J. Electrochem. Sci. 2015, 10, 6690. [Google Scholar]
- Cesarino, I.; Marino, G.; Matos, R.J.; Cavalheiro, G.T.E. Evaluation of a carbon paste electrode modified with organofunctionalised SBA-15 nanostructured silica in the simultaneous determination of divalent lead, copper and mercury ions. Talanta 2008, 75, 15. [Google Scholar] [CrossRef]
- Bernalte, E.; Sánchez, M.C.; Gil, P.E. Determination of mercury in ambient water samples by anodic stripping voltammetry on screen-printed gold electrodes. Anal. Chim. Acta 2011, 689, 60. [Google Scholar] [CrossRef]
- Giacomino, A.; Abollino, O.; Malandrino, M.; Mentasti, E. Parameters affecting the determination of mercury by anodic stripping voltammetry using a gold electrode. Talanta 2008, 75, 266. [Google Scholar] [CrossRef]
- Yantasee, W.; Lin, Y.; Zemanian, S.T.; Fryxell, E.G. Voltammetric detection of lead(II) and mercury(II) using a carbon paste electrode modified with thiol self-assembled monolayer on mesoporous silica (SAMMS). Analyst 2003, 128, 467. [Google Scholar] [CrossRef]
- Popa, E.D.; Mureseanu, M.; Tanase, G.I. Organofunctionalized mesoporous silica carbon paste electrode for simultaneously determination of copper, lead and cadmium. Rev. Chim. 2012, 63, 507. [Google Scholar]
- Khani, H.; Rofouei, K.M.; Arab, P.; Gupta, K.V.; Vafaei, Z. Multi-walled carbon nanotubes-ionic liquid-carbon paste electrode as a super selectivity sensor: Application to potentiometric monitoring of mercury ion (II). J. Hazard. Mater. 2010, 183, 402. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Aroua, K.M.; Yusoff, R. Potentiometric determination of trace amounts of mercury (II) in water sample using a new modified palm shell activated carbon paste electrode based on Kryptofix 5. Am. J. Anal. Chem. 2012, 3, 859. [Google Scholar] [CrossRef]
- Alpat, S.; Alpat, K.S.; Çadırcı, H.B.; Yaşa, I.; Telefoncu, A. A novel microbial biosensor based on Circinella sp. modified carbon paste electrode and its voltammetric application. Sens. Actuators B Chem. 2008, 134, 175. [Google Scholar] [CrossRef]
- Al-Ghamdi, F.A.; Hefnawy, M.M.; Almaged, A.; Belal, F.F. Development of square-wave adsorptive stripping voltammetric method for determination of acebutolol in pharmaceutical formulations and biological fluids. Chem. Cent. J. 2012, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Junaid, M.; Aslam, M.W.; Ali, K.; Rasool, A.; Zhang, H. A review on the status of mercury pollution in Pakistan: Sources and impacts. Arch. Environ. Contam. Toxicol. 2019, 76, 519. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Shah, A.I.; Tulcan, S.X.R.; Rashid, W.; Sillanpaa, M. Contamination, exposure, and health risk assessment of Hg in Pakistan: A review. Environ. Pollut. 2022, 301, 118995. [Google Scholar] [CrossRef]
- Rajawat, S.D.; Srivastava, S.; Satsangee, P.S. Electrochemical determination of mercury at trace levels using Eichhornia crassipes modified carbon paste electrode. Int. J. Electrochem. Sci. 2012, 7, 11456. [Google Scholar]
- Devnani, H.; Satsangee, P.S. Voltammetric trace determination of mercury using plant refuse modified carbon paste electrodes. Environ. Monitor. Assess. 2013, 185, 9333. [Google Scholar] [CrossRef]
- Rotake, D.; Goswami, P.P.; Singh, G.S. Ultraselective, ultrasensitive, point-of-care electrochemical sensor for detection of Hg(II) ions with electrospun-InZnO nanofibers. J. Electroanal. Chem. 2022, 915, 116350. [Google Scholar] [CrossRef]
- Salimi, A.; Noorbakhash, A.; Karonian, S.F. Amperometric detection of nitrite, iodate and periodate on glassy carbon electrode modified with thionin and multi-wall carbon nanotubes. Int. J. Electrochem. Sci. 2006, 1, 435. [Google Scholar]
- Cheng, C.; Wang, J.; Yang, X.; Li, A.; Philippe, C. Adsorption of Ni(II) and Cd(II) from water by novel chelating sponge and the effect of alkali-earth metal ions on the adsorption. J. Hazard. Mater. 2014, 264, 332. [Google Scholar] [CrossRef] [PubMed]
- Herrero, R.; Lodeiro, P.; Rey-Castro, C.; Vilariño, T.; De Vicente, S.E.M. Removal of inorganic mercury from aqueous solutions by biomass of the marine macroalga Cystoseira baccata. Water Res. 2005, 39, 3199. [Google Scholar] [CrossRef] [PubMed]
- Rajawat, S.D.; Kumar, N.; Satsangee, P.S. Trace determination of cadmium in water using anodic stripping voltammetry at a carbon paste electrode modified with coconut shell powder. J. Anal. Sci. Technol. 2014, 5, 19. [Google Scholar] [CrossRef]
- Oftedal, T.O.; Eisert, R.; Barrell, K.G. Comparison of analytical and predictive methods for water, protein, fat, sugar, and gross energy in marine mammal milk. J. Dairy Sci. 2014, 97, 4713. [Google Scholar] [CrossRef]

| Stripping Electrolytes | Peak Current Values I (µA) |
|---|---|
| 0.1 M HCl | 123.5 |
| 0.1 M HNO3 | 87.72 |
| 0.1 M H2SO4 | 46.64 |
| 0.1 M KCl | 17.46 |
| Modifier | Deposition Time (min) | Linear Range (µgL−1) | Sample | LOD (µgL−1) | Reference |
|---|---|---|---|---|---|
| MWCNTs and 3H-spiro[isobenzofuran-1,6′-pyrrolo [2,3-d]pyrimidine]-2′,3,4′,5′-tetraones (3HSIPPT) | 0.05–1 | Seawater and sewage sample | 0.017 | [1] | |
| Pyridine and gold-based nanocomposite | 0–10 | River and spring | 0.012 | [2] | |
| Dipenylcarbazone (DPC) and ethylene glycol dimethacrylate (EGDMA)-based ion imprinted polymer | 0.1–40 | Water and urine | 0.07 | [3] | |
| Vermiculite | 15 | 20–1600 | Synthetic water sample | 11.4 | [7] |
| Montmorillonite | 15 | 10–35 | Saline and bottled water | 3.5 | [7] |
| Mesostructured silica NP b/5-mercapto-1-methyltetrazole | 10 | 20–200 | River and ground water | 20 | [7] |
| Carbon ionic liquid/Au c nanoparticles/amino acid | 10 | 2–4000 | Waste and tap water | 0.46 | [7] |
| TZT-HDTA-clay d | 5 | 10–2000 | River and sea water | 0.1 | [7] |
| MWCNTs e | 4 | 1.3–16.6 | Natural and industrial waste water | 0.48 | [7] |
| N-BDMP f | 3.5 | 10–2000 | Tap water, fish, human hair | 8.2 | [7] |
| MWCNTs paste electrode | 3.5 | 1–25 | Waste water | 0.42 | [7] |
| MWCNTs/Schiff base | 1.5 | 0.2–140 | Sea and waste water | 0.18 | [7] |
| Silica NP/Schiff base | 1 | 0.5–1000 | Tap and sea water, tobacco, fish and shrimps | 0.05 | [7] |
| α-cyclodextrin | 0.33 | 40–800 | Synthetic water sample | 10 | [7] |
| N-rich covalent organic framework | 10−9–10−4 | River, DI and polluted water sample | 4.5 × 10−12 | [8] | |
| Mesoporous silica/TH a | 20 | 20–1600 | Synthetic water sample | 3 | [30] |
| Water hyacinth leaves | 10 | 400–800 | - | 195 | [36] |
| Vegetable waste | 10 | 100–1000 | - | 57.75 | [37] |
| Doped zinc nano fibers coated with mercaptosuccinic acid and pyridine dicarboxylic acid | 3.13 × 10−15 | [40] | |||
| Phanerochaete chrysosporium | 12 | 5–50 | Chlor-alkali Industrial effluent | 4.4 | Present study |
| Target Ion | Conc. (µg L−1) | Current Intensity (µA) | Interfering Medium | Current Intensity (µA) | Decrease Efficiency (%) | RSD | |
|---|---|---|---|---|---|---|---|
| Cations | Conc. (mgL−1) | (%) | |||||
| Hg(II) | 10 | 7.36 | Na+ + K+ + Ca2+ + Mg2+ | 75 + 75 + 75 + 75 | 7.02 | 4.7 | 0.29 |
| Na+ + K+ + Ca2+ + Mg2+ | 150 + 150 + 150+ 150 | 5.5 | 25.41 | 0.18 | |||
| Na+ + K+ + Ca2+ + Mg2+ | 300 + 300 + 300 + 300 | 5.21 | 29.39 | 0.19 | |||
| Na+ + K+ + Ca2+ + Mg2+ | 600 + 600 + 600 + 600 | 4.92 | 33.38 | 0.11 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaib, M.; Farooq, U.; Athar, M.M. Application of Phanerochaete chrysopsorium-Based Carbon Paste Electrode as an Electrochemical Sensor for Voltammetric Detection of Hg (II) in Chlor-Alkali Industrial Effluent. Electrochem 2022, 3, 746-759. https://doi.org/10.3390/electrochem3040049
Zaib M, Farooq U, Athar MM. Application of Phanerochaete chrysopsorium-Based Carbon Paste Electrode as an Electrochemical Sensor for Voltammetric Detection of Hg (II) in Chlor-Alkali Industrial Effluent. Electrochem. 2022; 3(4):746-759. https://doi.org/10.3390/electrochem3040049
Chicago/Turabian StyleZaib, Maria, Umar Farooq, and Muhammad Makshoof Athar. 2022. "Application of Phanerochaete chrysopsorium-Based Carbon Paste Electrode as an Electrochemical Sensor for Voltammetric Detection of Hg (II) in Chlor-Alkali Industrial Effluent" Electrochem 3, no. 4: 746-759. https://doi.org/10.3390/electrochem3040049
APA StyleZaib, M., Farooq, U., & Athar, M. M. (2022). Application of Phanerochaete chrysopsorium-Based Carbon Paste Electrode as an Electrochemical Sensor for Voltammetric Detection of Hg (II) in Chlor-Alkali Industrial Effluent. Electrochem, 3(4), 746-759. https://doi.org/10.3390/electrochem3040049





