Effect of Zearalenone-Induced Ferroptosis on Mice Spermatogenesis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Processing
2.3. Animal Weight and Testicular Weight
2.4. Spermatozoa Motility Determined by a Computer-Assisted Sperm Analysis System
2.5. Hematoxylin and Eosin Staining
2.6. Measurement of MDA, T-GSH Contents
2.7. Evaluation of SOD Activity
2.8. Measurement of Fe3+ and Fe2+ Contents
2.9. Quantitative Real-Time PCR Assay
2.10. Western Blotting (WB)
2.11. Statistical Analyses
3. Results
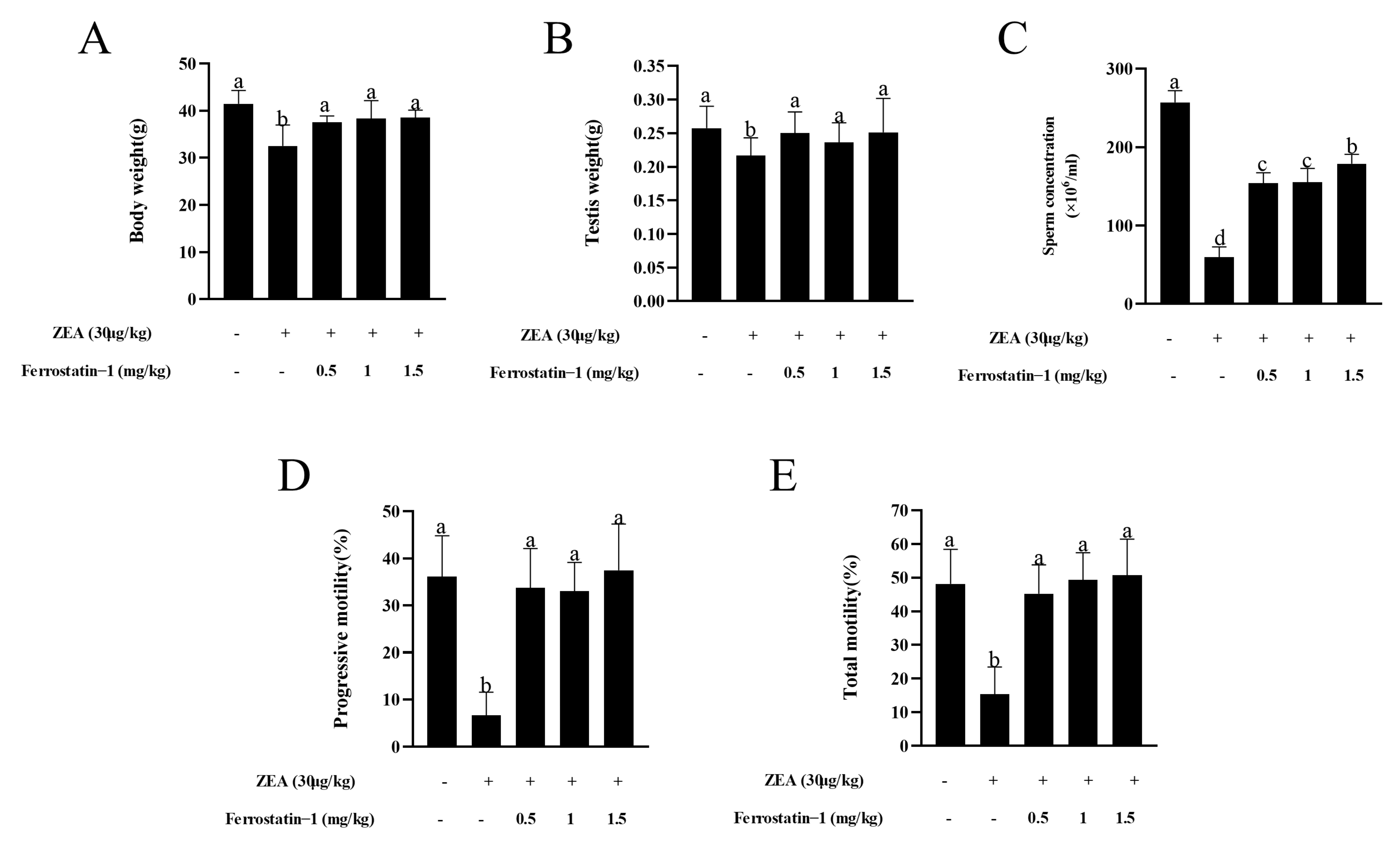
3.1. Fer-1 Protects against ZEA-Induced Reduction of Body Weight and Sperm Motility
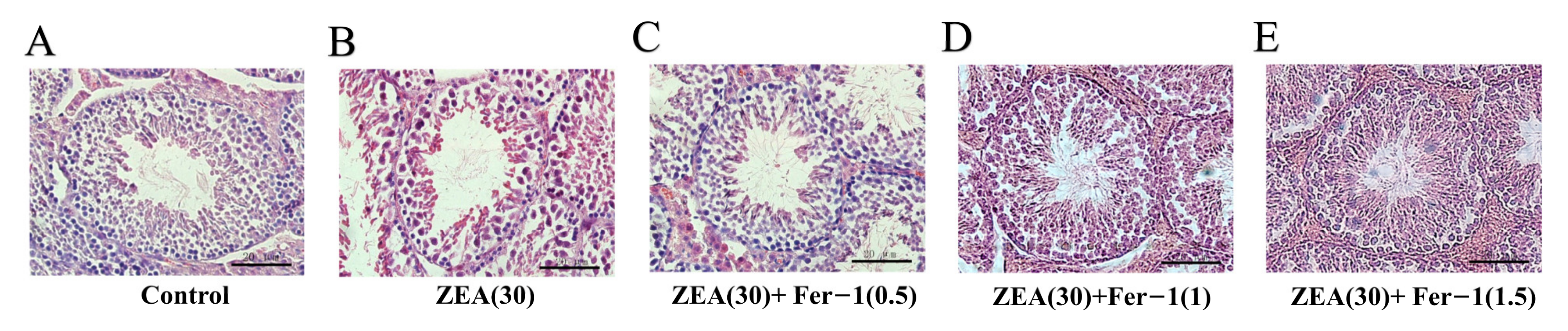
3.2. Fer-1 Can Alleviate ZEA-Induced Pathological Changes in Mouse Testis and Increases the Expression of Important Genes Involved in Spermatogenesis
3.3. Fer-1 Can Affect the Contents of MDA, T-GSH, SOD Activity, Fe3+ and Fe2+ in Mouse Testis
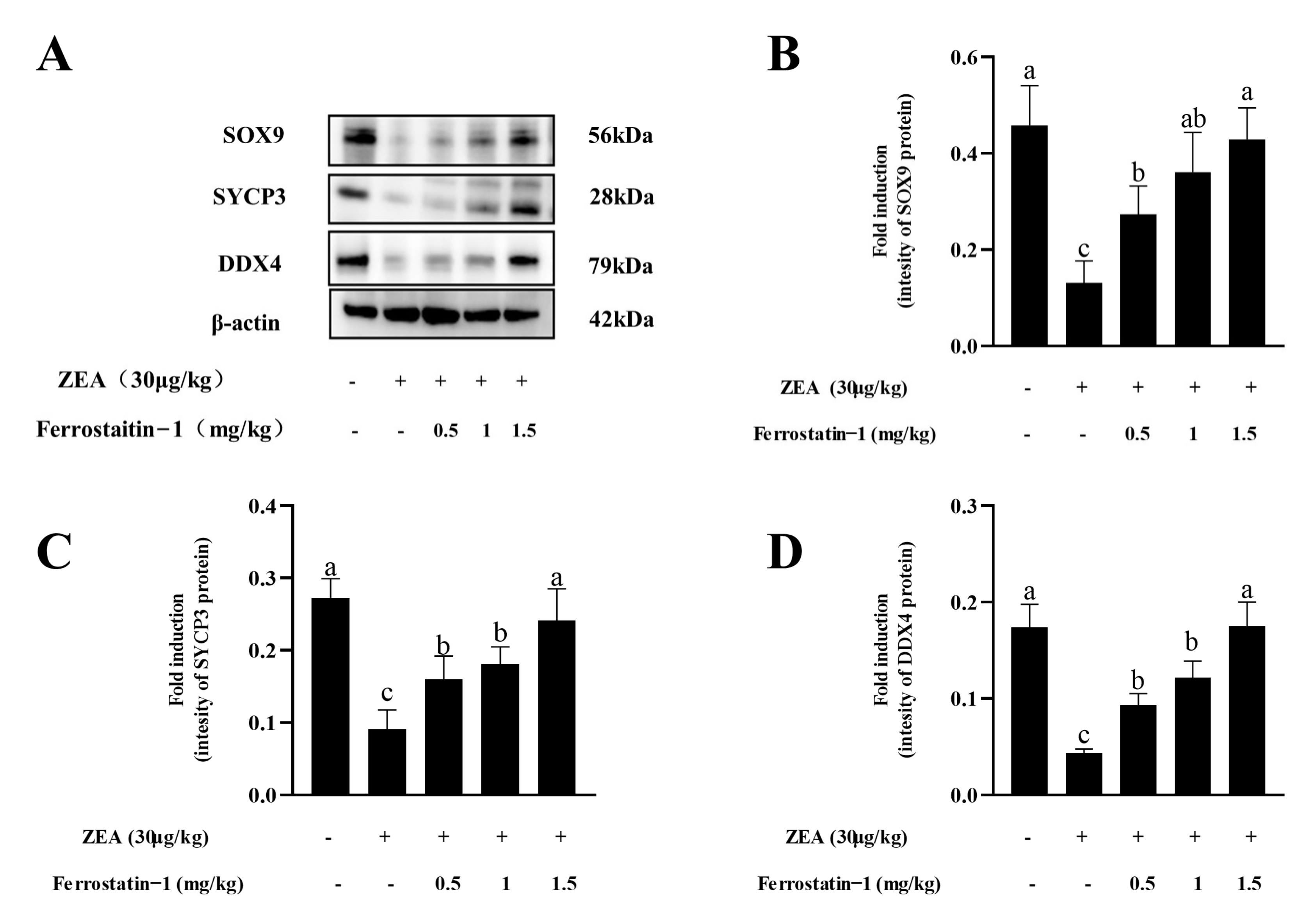
3.4. Fer-1 Can Alter ZEA-Induced Ferroptosis-Related Gene and Protein Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schollenberger, M.; Müller, H.-M.; Rüfle, M.; Terry-Jara, H.; Suchy, S.; Plank, S.; Drochner, W. Natural occurrence of Fusarium toxins in soy food marketed in Germany. Int. J. Food Microbiol. 2007, 113, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Domijan, A.M.; Peraica, M.; Cvjetković, B.; Turcin, S.; Jurjević, Z.; Ivić, D. Mould contamination and co-occurrence of mycotoxins in maize grain in Croatia. Acta Pharm. 2005, 55, 349–356. [Google Scholar] [PubMed]
- Chang, H.; Kim, W.; Park, J.-H.; Kim, D.; Kim, C.-R.; Chung, S.; Lee, C. The Occurrence of Zearalenone in South Korean Feedstuffs between 2009 and 2016. Toxins 2017, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Ropejko, K.; Twarużek, M. Zearalenone and Its Metabolites—General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Calado, T.; Abrunhosa, L.; Verde, S.C.; Alté, L.; Venâncio, A.; Fernández-Cruz, M.L. Effect of Gamma-Radiation on Zearalenone—Degradation, Cytotoxicity and Estrogenicity. Foods 2020, 9, 1687. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y.; Zhang, H.; Zhang, P.; Liu, J.; Feng, Y.; Men, Y.; Li, L.; Shen, W.; Sun, Z.; et al. Pubertal exposure to low doses of zearalenone disrupting spermatogenesis through ERα related genetic and epigenetic pathways. Toxicol. Lett. 2019, 315, 31–38. [Google Scholar] [CrossRef]
- Yin, S.; Meng, Q.; Zhang, B.; Shi, B.; Shan, A.; Li, Z. Alleviation of zearalenone toxicity by modified halloysite nanotubes in the immune response of swine. Food Addit. Contam. Part A 2015, 32, 87–99. [Google Scholar] [CrossRef]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Urbanek, K.A.; Domińska, K.; Sakowicz, A.; Piastowska-Ciesielska, A.W. Estrogen receptor β plays a protective role in zearalenone-induced oxidative stress in normal prostate epithelial cells. Ecotoxicol. Environ. Saf. 2019, 172, 504–513. [Google Scholar] [CrossRef]
- Lee, R.; Kim, D.-W.; Lee, W.-Y.; Park, H.-J. Zearalenone Induces Apoptosis and Autophagy in a Spermatogonia Cell Line. Toxins 2022, 14, 148. [Google Scholar] [CrossRef]
- She, J.; Feng, N.; Zheng, W.; Zheng, H.; Cai, P.; Zou, H.; Yuan, Y.; Gu, J.; Liu, Z.; Bian, J. Zearalenone Exposure Disrupts Blood–Testis Barrier Integrity through Excessive Ca2+-Mediated Autophagy. Toxins 2021, 13, 875. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Proneth, B.; Tyurina, Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, M.I.J.; Aichler, M.; Walch, M.A.A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Park, M.W.; Cha, H.W.; Kim, J.; Kim, J.H.; Yang, H.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Yoo, I.D.; Moon, J.-S. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer's diseases. Redox Biol. 2021, 41, 101947. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Ai, Y.; Sun, Q.; Ma, Y.; Cao, Y.; Wang, J.; Zhang, Z.; Wang, X. Membrane Damage during Ferroptosis Is Caused by Oxidation of Phospholipids Catalyzed by the Oxidoreductases POR and CYB5R1. Mol. Cell 2021, 81, 355–369.e10. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Porto Freitas, F.P.; Seibt, T.; et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 2018, 172, 409–422.e21. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Zhang, Y.; Swanda, R.V.; Nie, L.; Liu, X.; Wang, C.; Lee, H.; Lei, G.; Mao, C.; Koppula, P.; Cheng, W.; et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat. Commun. 2021, 12, 1589. [Google Scholar] [CrossRef]
- Bridges, R.J.; Natale, N.R.; Patel, S.A. System xc- cystine/glutamate antiporter: An update on molecular pharmacology and roles within the CNS. Br. J. Pharmacol. 2012, 165, 20–34. [Google Scholar] [CrossRef]
- Lewerenz, J.; Hewett, S.; Huang, Y.; Lambros, M.; Gout, P.W.; Kalivas, P.W.; Massie, A.; Smolders, I.; Methner, A.; Pergande, M.; et al. The Cystine/Glutamate Antiporter System xc− in Health and Disease: From Molecular Mechanisms to Novel Therapeutic Opportunities. Antioxid. Redox Signal. 2013, 18, 522–555. [Google Scholar] [CrossRef]
- Qiang, Z.; Dong, H.; Xia, Y.; Chai, D.; Hu, R.; Jiang, H. Nrf2 and STAT3 Alleviates Ferroptosis-Mediated IIR-ALI by Regulating SLC7A11. Oxidative Med. Cell. Longev. 2020, 2020, 5146982. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Hakkaku, N.; Iwamoto, R.; Suzuki, J.; Suzuki, T.; Tajima, Y.; Konishi, K.; Minami, S.; Ichinose, S.; Ishizaka, K.; et al. Depletion of Selenoprotein GPx4 in Spermatocytes Causes Male Infertility in Mice. J. Biol. Chem. 2009, 284, 32522–32532. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Mai, Z.; Zhou, Y.; Gao, X.; Yu, B. Low NRF2 mRNA Expression in Spermatozoa from Men with Low Sperm Motility. Tohoku J. Exp. Med. 2012, 228, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rodríguez, J.M.; Martín-Cano, F.E.; Gaitskell-Phillips, G.; Silva, A.; Tapia, J.A.; Gil, M.C.; Redondo, E.; Masot, J.; Ortega-Ferrusola, C.; Peña, F.J. The SLC7A11: Sperm mitochondrial function and non-canonical glutamate metabolism. Reproduction 2020, 160, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, W.-D.; Liu, X.-Q.; Zhang, P.-F.; Hao, Y.-N.; Li, L.; Chen, L.; Shen, W.; Tang, X.-F.; Min, L.-J.; et al. Hydrogen Sulfide and/or Ammonia Reduces Spermatozoa Motility through AMPK/AKT Related Pathways. Sci. Rep. 2016, 6, 37884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, Y.; Zhang, P.; Hao, Y.; Yu, S.; Min, L.; Li, L.; Ma, D.; Chen, L.; Yi, B.; et al. Decrease in male mouse fertility by hydrogen sulfide and/or ammonia can Be inheritable. Chemosphere 2018, 194, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Yu, S.; Feng, Y.; Zhang, H.; Kou, X.; Chu, M.; Cui, L.; Li, L.; Zhang, P.; et al. Regulation of steroid hormones and energy status with cysteamine and its effect on spermatogenesis. Toxicol. Appl. Pharmacol. 2016, 313, 149–158. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Kim, I.; Son, H.Y.; Cho, S.W.; Ha, C.S.; Kang, B.H. Zearalenone induces male germ cell apoptosis in rats. Toxicol. Lett. 2003, 138, 185–192. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Liu, N.; Cui, W.; Dong, W.; Xing, B.; Pan, C. Pig SOX9: Expression profiles of Sertoli cell (SCs) and a functional 18 bp indel affecting testis weight. Theriogenology 2019, 138, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Ma, X.; Wang, X.; Wang, F.; Dong, J.; Wu, Y.; Lv, C.; Liu, K.; Zhang, Y.; Zhang, Z.; et al. hnRNPU in Sertoli cells cooperates with WT1 and is essential for testicular development by modulating transcriptional factors Sox8/9. Theranostics 2021, 11, 10030–10046. [Google Scholar] [CrossRef]
- Ben Salah-Abbès, J.; Abbès, S.; Abdel-Wahhab, M.A.; Oueslati, R. Raphanus sativus extract protects against Zearalenone induced reproductive toxicity, oxidative stress and mutagenic alterations in male Balb/c mice. Toxicon 2009, 53, 525–533. [Google Scholar] [CrossRef]
- El Golli-Bennour, E.; Bacha, H. Hsp70 expression as biomarkers of oxidative stress: Mycotoxins’ exploration. Toxicology 2011, 287, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hassen, W.; Ayed-Boussema, I.; Oscoz, A.A.; Lopez, A.D.C.; Bacha, H. The role of oxidative stress in zearalenone-mediated toxicity in Hep G2 cells: Oxidative DNA damage, gluthatione depletion and stress proteins induction. Toxicology 2007, 232, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Hassannia, B.; Wiernicki, B.; Ingold, I.; Qu, F.; Van Herck, S.; Tyurina, Y.Y.; Bayır, H.; Abhari, B.A.; Angeli, J.P.F.; Choi, S.M.; et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J. Clin. Investig. 2018, 128, 3341–3355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Wang, D.-W.; Xu, S.-F.; Zhang, S.; Fan, Y.-G.; Yang, Y.-Y.; Guo, S.-Q.; Wang, S.; Guo, T.; Wang, Z.-Y.; et al. α-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biol. 2018, 14, 535–548. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Kang, R.; Kroemer, G.; Tang, D. Cellular degradation systems in ferroptosis. Cell Death Differ. 2021, 28, 1135–1148. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Song, X.; Long, D. Nrf2 and Ferroptosis: A New Research Direction for Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 267. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Wang, H.; Zhu, J.; Wang, T.; Nepovimova, E.; Long, M.; Li, P.; Kuca, K.; Wu, W. Procyanidins inhibit zearalenone-induced apoptosis and oxidative stress of porcine testis cells through activation of Nrf2 signaling pathway. Food Chem. Toxicol. 2022, 165, 113061. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, B.N.; Lawson, G.; Chan, J.Y.; Banuelos, J.; Cortés, M.M.; Hoang, Y.D.; Ortiz, L.; Rau, B.A.; Luderer, U. Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age-dependent manner. Free Radic. Biol. Med. 2010, 49, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wu, B.; Zhong, B.; Lin, L.; Ding, Y.; Jin, X.; Huang, Z.; Lin, M.; Wu, H.; Xu, D. Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2) /System xc-/ glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis. Bioengineered 2021, 12, 10924–10934. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, H.; Yamamoto, M.; Motohashi, H. Nrf2 Redirects Glucose and Glutamine into Anabolic Pathways in Metabolic Reprogramming. Cancer Cell 2012, 22, 66–79. [Google Scholar] [CrossRef]
- Sasaki, H.; Sato, H.; Kuriyama-Matsumura, K.; Sato, K.; Maebara, K.; Wang, H.; Tamba, M.; Itoh, K.; Yamamoto, M.; Bannai, S. Electrophile Response Element-mediated Induction of the Cystine/Glutamate Exchange Transporter Gene Expression. J. Biol. Chem. 2002, 277, 44765–44771. [Google Scholar] [CrossRef]
- Imai, H.; Suzuki, K.; Ishizaka, K.; Ichinose, S.; Oshima, H.; Okayasu, I.; Emoto, K.; Umeda, M.; Nakagawa, Y. Failure of the Expression of Phospholipid Hydroperoxide Glutathione Peroxidase in the Spermatozoa of Human Infertile Males1. Biol. Reprod. 2001, 64, 674–683. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress:4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef]
- Cohen, G.; Riahi, Y.; Sunda, V.; Deplano, S.; Chatgilialoglu, C.; Ferreri, C.; Kaiser, N.; Sasson, S. Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radic. Biol. Med. 2013, 65, 978–987. [Google Scholar] [CrossRef]
- Csala, M.; Kardon, T.; Legeza, B.; Lizák, B.; Mandl, J.; Margittai, É.; Puskás, F.; Száraz, P.; Szelényi, P.; Bánhegyi, G. On the role of 4-hydroxynonenal in health and disease. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2015, 1852, 826–838. [Google Scholar] [CrossRef]
- Mali, V.R.; Palaniyandi, S.S. Regulation and therapeutic strategies of 4-hydroxy-2-nonenal metabolism in heart disease. Free Radic. Res. 2014, 48, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Breitzig, M.; Bhimineni, C.; Lockey, R.; Kolliputi, N. 4-Hydroxy-2-nonenal: A critical target in oxidative stress? Am. J. Physiol. Cell Physiol. 2016, 311, C537–C543. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Primer Sequence |
|---|---|
| Nrf2 | F:5′-ATGACTCTGACTCTGGCATTTC-3′ |
| R:5′-GCACTATCTAGCTCCTCCATTTC-3′ | |
| SLC7A11 | F:5′-GTGGGAGGCTGGTAGTTAATG-3′ |
| R:5′-CTGCTGTACCGTGGTTATGT-3′ | |
| GPX4 | F:5′-GCAGGAGCCAGGAAGTAATC-3′ |
| R:5′-CCTTGGGCTGGACTTTCAT-3′ | |
| β-actin | F:5′-GAAGTGTGACGTTGACATCCG-3′ |
| R:5′-TGCTGATCCACATCTGCTGGA-3′ |
| Sperm Parameters | Control | ZEA 30 | ZEA 30 + Fer−1(0.5) | ZEA 30 + Fer−1(1) | ZEA 30 + Fer−1(1.5) |
|---|---|---|---|---|---|
| VSL (μm/s) | 40.89 ± 2.35 a | 17.56 ± 2.00 c | 26.99 ± 1.43 b | 24.80 ± 1.22 b | 25.66 ± 1.61 b |
| VCL (μm/s) | 90.90 ± 3.63 a | 45.17 ± 4.83 c | 70.60 ± 2.83 b | 70.68 ± 2.30 b | 70.99 ± 2.77 b |
| VAP (μm/s) | 47.75 ± 2.47 a | 23.11 ± 2.22 c | 35.79 ± 1.35 b | 32.92 ± 1.38 b | 35.21 ± 1.44 b |
| LIN (%) | 44.68 ± 2.21 a | 32.53 ± 2.51 c | 40.17 ± 1.35 b | 40.60 ± 1.05 b | 42.02 ± 1.74 b |
| STR (%) | 79.58 ± 1.16 a | 72.63 ± 2.56 b | 75.26 ± 1.64 a | 70.34 ± 1.19 b | 75.20 ± 0.85 a |
| WOB (%) | 57.15 ± 2.06 a | 50.47 ± 2.52 b | 51.40 ± 1.14 b | 48.23 ± 0.89 b | 51.28 ± 1.72 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhu, Z.; Cui, H.; Ding, K.; Zhao, Y.; Ma, X.; Adetunji, A.O.; Min, L. Effect of Zearalenone-Induced Ferroptosis on Mice Spermatogenesis. Animals 2022, 12, 3026. https://doi.org/10.3390/ani12213026
Li Y, Zhu Z, Cui H, Ding K, Zhao Y, Ma X, Adetunji AO, Min L. Effect of Zearalenone-Induced Ferroptosis on Mice Spermatogenesis. Animals. 2022; 12(21):3026. https://doi.org/10.3390/ani12213026
Chicago/Turabian StyleLi, Yajing, Zhendong Zhu, Haixiang Cui, Kexin Ding, Yong Zhao, Xiangping Ma, Adedeji Olufemi Adetunji, and Lingjiang Min. 2022. "Effect of Zearalenone-Induced Ferroptosis on Mice Spermatogenesis" Animals 12, no. 21: 3026. https://doi.org/10.3390/ani12213026
APA StyleLi, Y., Zhu, Z., Cui, H., Ding, K., Zhao, Y., Ma, X., Adetunji, A. O., & Min, L. (2022). Effect of Zearalenone-Induced Ferroptosis on Mice Spermatogenesis. Animals, 12(21), 3026. https://doi.org/10.3390/ani12213026





