Hybrid Carbon Supports Composed of Small Reduced Graphene Oxide and Carbon Nanotubes for Durable Oxygen Reduction Catalysts in Proton Exchange Membrane Fuel Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology and Crystal Structure
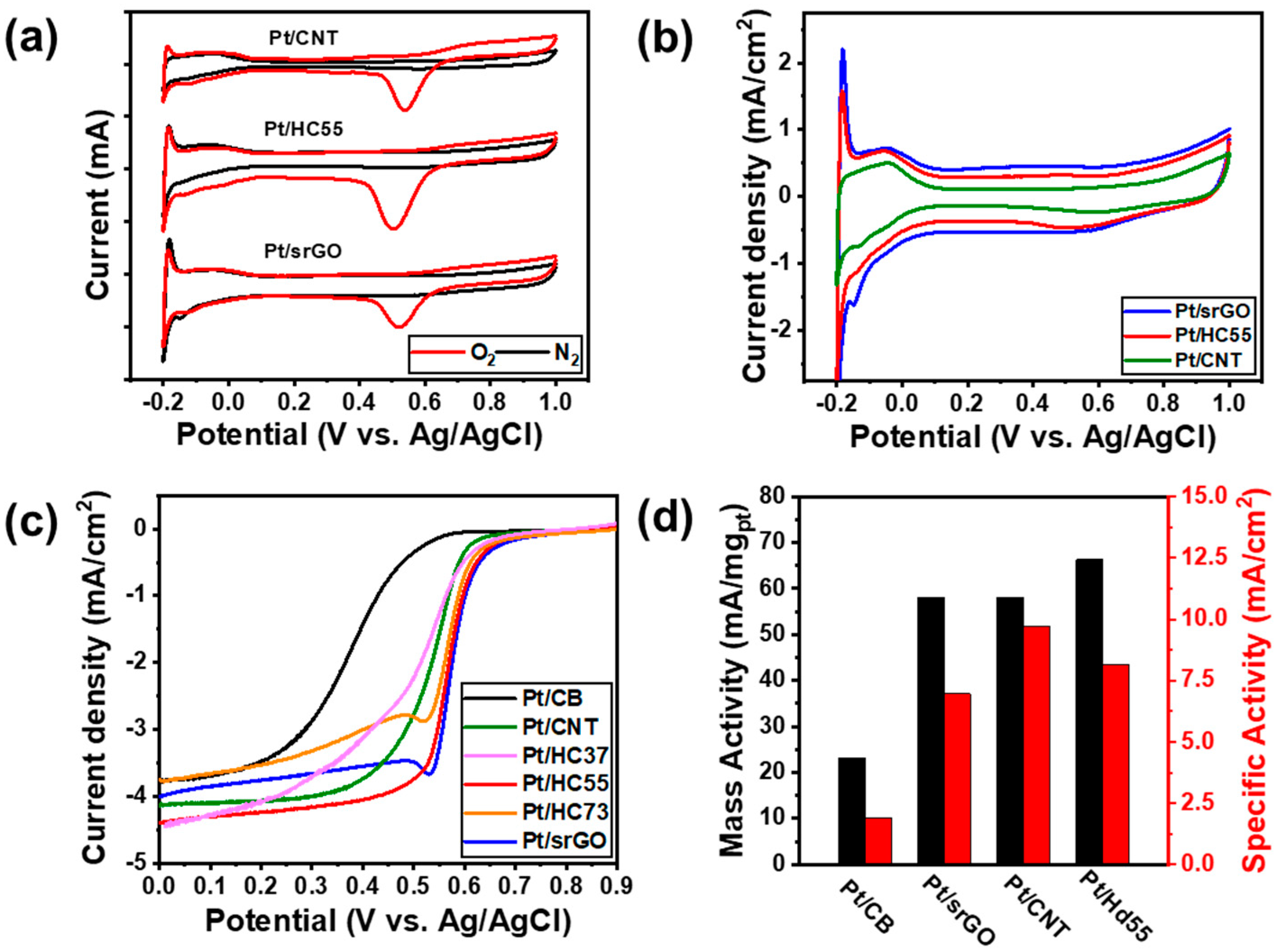
2.2. Electrocatalytic Performance
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirubakaran, A.; Jain, S.; Nema, R.K. A review on fuel cell technologies and power electronic interface. Renew. Sustain. Energy Rev. 2009, 13, 2430–2440. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Bak, S.J.; Kim, S.I.; Lim, S.Y.; Kim, T.; Kwon, S.H.; Lee, D.H. Small reduced graphene oxides for highly efficient oxygen reduction catalysts. Int. J. Mol. Sci. 2021, 22, 12300. [Google Scholar] [CrossRef] [PubMed]
- Chhina, H.; Campbell, S.; Kesler, O. High surface area synthesis, electrochemical activity, and stability of tungsten carbide supported Pt during oxygen reduction in proton exchange membrane fuel cells. J. Power Sources 2008, 179, 50–59. [Google Scholar] [CrossRef]
- Wu, B.; Kuang, Y.; Zhang, X.; Chen, J. Noble metal nanoparticles/carbon nanotubes nanohybrids: Synthesis and applications. Nano Today 2011, 6, 75–90. [Google Scholar] [CrossRef]
- Ye, B.; Kim, S.I.; Lee, M.; Ezazi, M.; Kim, H.D.; Kwon, G.; Lee, D.H. Synthesis of oxygen functionalized carbon nanotubes and their application for selective catalytic reduction of NOx with NH3. RSC Adv. 2020, 10, 16700–16708. [Google Scholar] [CrossRef]
- Wei, Q.; Tong, X.; Zhang, G.; Qiao, J.; Gong, Q.; Sun, S. Nitrogen-doped carbon nanotube and graphene materials for oxygen reduction reactions. Catalysts 2015, 5, 1574–1602. [Google Scholar] [CrossRef] [Green Version]
- Cha, B.-C.; Jun, S.; Jeong, B.; Ezazi, M.; Kwon, G.; Kim, D.; Lee, D.H. Carbon nanotubes as durable catalyst supports for oxygen reduction electrode of proton exchange membrane fuel cells. J. Power Sources 2018, 401, 296–302. [Google Scholar] [CrossRef]
- Li, W.; Liang, C.; Zhou, W.; Qiu, J.; Zhou, Z.; Sun, G.; Xin, Q. Preparation and characterization of multiwalled carbon nanotube-supported platinum for cathode catalysts of direct methanol fuel cells. J. Phys. Chem. B 2003, 107, 6292–6299. [Google Scholar] [CrossRef]
- Huang, H.; Wang, X. Recent progress on carbon-based support materials for electrocatalysts of direct methanol fuel cells. J. Mater. Chem. A 2014, 2, 6266–6291. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, S.P.; White, T.J.; Guo, J.; Wang, X. Electrocatalytic activity and interconnectivity of Pt nanoparticles on multiwalled carbon nanotubes for fuel cells. J. Phys. Chem. C 2009, 113, 18935–18945. [Google Scholar] [CrossRef]
- Singh, S.K.; Takeyasu, K.; Nakamura, J. Active sites and mechanism of oxygen reduction reaction electrocatalysis on nitrogen-doped carbon materials. Adv. Mater. 2019, 31, e1804297. [Google Scholar] [CrossRef] [PubMed]
- Matter, P.H.; Zhang, L.; Ozkan, U.S. The role of nanostructure in nitrogen-containing carbon catalysts for the oxygen reduction reaction. J. Cat. 2006, 239, 83–96. [Google Scholar] [CrossRef]
- He, W.; Jiang, H.; Zhou, Y.; Yang, S.; Xue, X.; Zou, Z.; Zhang, X.; Akins, D.L.; Yang, H. An efficient reduction route for the production of Pd–Pt nanoparticles anchored on graphene nanosheets for use as durable oxygen reduction electrocatalysts. Carbon 2012, 50, 265–274. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Q.; Zhang, H.; Tian, W.; Tan, Y.; Qian, W.; Liu, Z. Low content Pt nanoparticles anchored on N-doped reduced graphene oxide with high and stable electrocatalytic activity for oxygen reduction reaction. Sci. Rep. 2017, 7, 43352. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; He, K.; Zhang, Z.; Zhou, A.; Duan, H. Real-time electrochemical detection of hydrogen peroxide secretion in live cells by Pt nanoparticles decorated graphene–carbon nanotube hybrid paper electrode. Biosens. Bioelectron. 2015, 68, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.-I.; Jo, S.G.; Hong, N.E.; Ye, B.; Lee, S.; Dow, H.S.; Lee, D.H.; Lee, J.W. Enhanced activity and durability of Pt nanoparticles supported on reduced graphene oxide for oxygen reduction catalysts of proton exchange membrane fuel cells. Cat. Today 2020, 352, 10–17. [Google Scholar] [CrossRef]
- Lin, Y.; Cui, X.; Yen, C.H.; Wai, C.M. PtRu/carbon nanotube nanocomposite synthesized in supercritical fluid: A novel electrocatalyst for direct methanol fuel cells. Langmuir 2005, 21, 11474–11479. [Google Scholar] [CrossRef]
- Belin, T.; Epron, F. Characterization methods of carbon nanotubes: A review. Mater. Sci. Eng. B 2005, 119, 105–118. [Google Scholar] [CrossRef]
- Kuila, U.; Prasad, M. Specific surface area and pore-size distribution in clays and shales. Geophys. Prospect. 2013, 61, 341–362. [Google Scholar] [CrossRef]
- Alazmi, A.; El Tall, O.; Rasul, S.; Hedhili, M.N.; Patole, S.P.; Costa, P.M. A process to enhance the specific surface area and capacitance of hydrothermally reduced graphene oxide. Nanoscale 2016, 8, 17782–17787. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zeng, X.; Xie, H.; Hing, P. A phenomenological approach for the Id/Ig ratio and sp3 fraction of magnetron sputtered aC films. Surf. Coat. Technol. 2000, 123, 256–260. [Google Scholar] [CrossRef]
- Brown, S.D.M.; Jorio, A.; Dresselhaus, M.S.; Dresselhaus, G. Observations of the D-band feature in the Raman spectra of carbon nanotubes. Phys. Rev. B 2001, 64, 073403. [Google Scholar] [CrossRef]
- Nesselberger, M.; Ashton, S.; Meier, J.C.; Katsounaros, I.; Mayrhofer, K.J.; Arenz, M. The particle size effect on the oxygen reduction reaction activity of Pt catalysts: Influence of electrolyte and relation to single crystal models. J. Am. Chem. Soc. 2011, 133, 17428–17433. [Google Scholar] [CrossRef]
- Kinoshita, K. Particle size effects for oxygen reduction on highly dispersed platinum in acid electrolytes. J. Electrochem. Soc. 1990, 137, 845–848. [Google Scholar] [CrossRef]
- Nardecchia, S.; Carriazo, D.; Ferrer, M.L.; Gutiérrez, M.C.; del Monte, F. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: Synthesis and applications. Chem. Soc. Rev. 2013, 42, 794–830. [Google Scholar] [CrossRef]
- Quan, H.; Cheng, B.; Xiao, Y.; Lei, S. One-pot synthesis of α-Fe2O3 nanoplates-reduced graphene oxide composites for supercapacitor application. Chem. Eng. J. 2016, 286, 165–173. [Google Scholar] [CrossRef]
- Eckardt, M.; Gebauer, C.; Jusys, Z.; Wassner, M.; Hüsing, N.; Behm, R.J. Oxygen reduction reaction activity and long-term stability of platinum nanoparticles supported on titania and titania-carbon nanotube composites. J. Power Sources 2018, 400, 580–591. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Kim, D.; Jeon, S. Covalently grafted platinum nanoparticles to multi walled carbon nanotubes for enhanced electrocatalytic oxygen reduction. Electro. Acta 2013, 92, 168–175. [Google Scholar] [CrossRef]
- Hasché, F.; Oezaslan, M.; Strasser, P. Activity, stability and degradation of multi walled carbon nanotube (MWCNT) supported Pt fuel cell electrocatalysts. Phys. Chem. Chem. Phys. 2010, 12, 15251–15258. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cheon, J.Y.; Shin, T.J.; Park, J.Y.; Joo, S.H. Effect of surface oxygen functionalization of carbon support on the activity and durability of Pt/C catalysts for the oxygen reduction reaction. Carbon 2016, 101, 449–457. [Google Scholar] [CrossRef]
- Liu, R.; Wu, D.; Feng, X.; Müllen, K. Nitrogen-doped ordered mesoporous graphitic arrays with high electrocatalytic activity for oxygen reduction. Angew. Chem. 2010, 122, 2619–2623. [Google Scholar] [CrossRef]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Lin, J.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, C.; Chen, G.; Fang, X.; Zheng, N.; Xie, Q. Facile synthesis of manganese-oxide-containing mesoporous nitrogen-doped carbon for efficient oxygen reduction. Adv. Funct. Mater. 2012, 22, 4584–4591. [Google Scholar] [CrossRef]
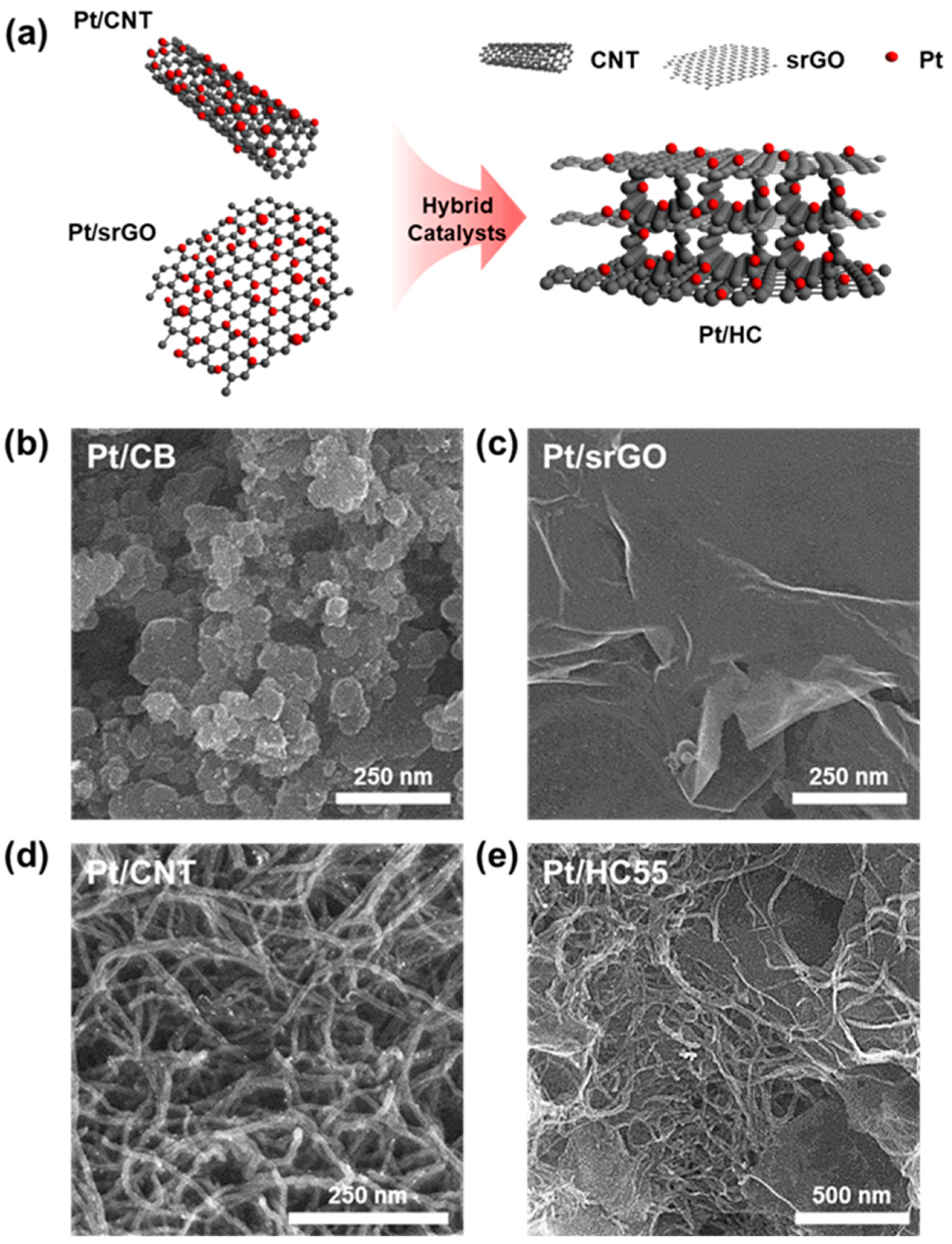

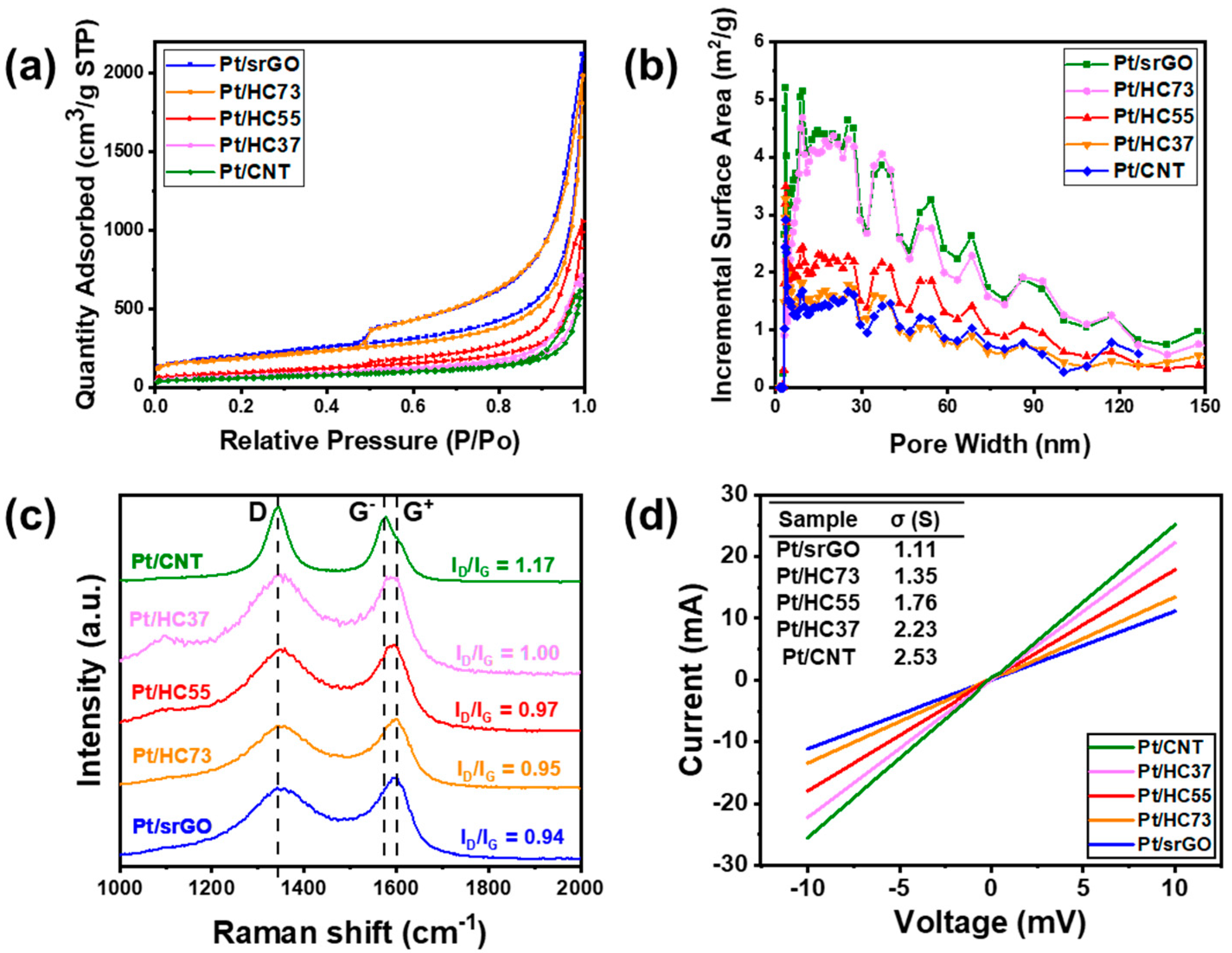

| Sample | SBET (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| Pt/srGO | 700.9 | 3.278 | 18.80 |
| Pt/HC73 | 664.3 | 3.074 | 18.60 |
| Pt/HC55 | 334.0 | 1.637 | 19.60 |
| Pt/HC37 | 245.4 | 1.105 | 18.01 |
| Pt/CNT | 210.2 | 0.950 | 18.08 |
| Sample | Eonset (mV) | Ehalf-wave (mV) | MA (mA/mgPt) | SA (mA/cm2) |
|---|---|---|---|---|
| Pt/CB | 489.6 | 381.05 | 23.2 | 1.90 |
| Pt/srGO | 612.6 | 568.8 | 57.9 | 6.95 |
| Pt/CNTs | 610.3 | 533.0 | 60.9 | 9.72 |
| Pt/HC55 | 614.3 | 580.2 | 66.2 | 8.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bak, S.-J.; Son, M.; Shin, J.; Kim, S.-I.; Lee, J.W.; Lee, D.H. Hybrid Carbon Supports Composed of Small Reduced Graphene Oxide and Carbon Nanotubes for Durable Oxygen Reduction Catalysts in Proton Exchange Membrane Fuel Cells. Int. J. Mol. Sci. 2022, 23, 13312. https://doi.org/10.3390/ijms232113312
Bak S-J, Son M, Shin J, Kim S-I, Lee JW, Lee DH. Hybrid Carbon Supports Composed of Small Reduced Graphene Oxide and Carbon Nanotubes for Durable Oxygen Reduction Catalysts in Proton Exchange Membrane Fuel Cells. International Journal of Molecular Sciences. 2022; 23(21):13312. https://doi.org/10.3390/ijms232113312
Chicago/Turabian StyleBak, Su-Jeong, Mingyu Son, Jeehoon Shin, Sun-I Kim, Jung Woo Lee, and Duck Hyun Lee. 2022. "Hybrid Carbon Supports Composed of Small Reduced Graphene Oxide and Carbon Nanotubes for Durable Oxygen Reduction Catalysts in Proton Exchange Membrane Fuel Cells" International Journal of Molecular Sciences 23, no. 21: 13312. https://doi.org/10.3390/ijms232113312
APA StyleBak, S.-J., Son, M., Shin, J., Kim, S.-I., Lee, J. W., & Lee, D. H. (2022). Hybrid Carbon Supports Composed of Small Reduced Graphene Oxide and Carbon Nanotubes for Durable Oxygen Reduction Catalysts in Proton Exchange Membrane Fuel Cells. International Journal of Molecular Sciences, 23(21), 13312. https://doi.org/10.3390/ijms232113312








