Natural Substances, Probiotics, and Synthetic Agents in the Treatment and Prevention of Honeybee Nosemosis
Abstract
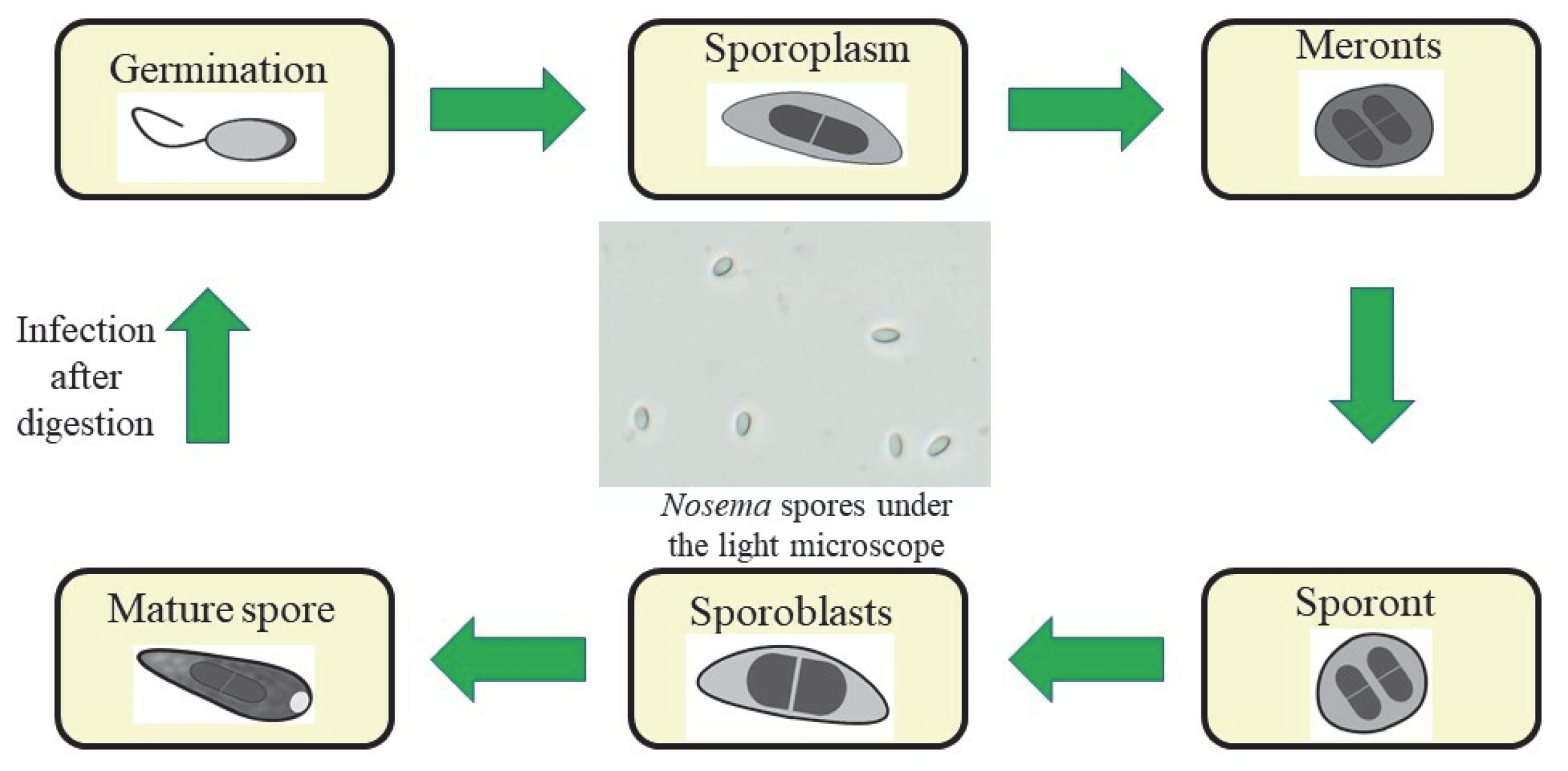
1. Nosemosis
2. Natural Substances
2.1. Essential Oils
2.2. Plant Extracts
2.3. Thymol
2.4. Natural Polysaccharide
2.5. Honeybee Product—Propolis
2.6. Probiotics
2.7. Fungal Extract
2.8. Other Natural Substances
3. Synthetic Substances
3.1. Phytochemicals
3.2. Organic Compounds
3.3. Alcohol
3.4. Commercial Preparations
3.5. Vitamins
3.6. Antibiotics
3.7. RNA Interference
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ptaszyńska, A.A. A Short Guide to the Sixth Mass Extinction—Is the Anthropocene an Extended Suicide? Rev. Educ. 2022, 395, 27–41. Available online: https://revistaeducacion.org/EDU/journals/published/1628853580_j47Wb.pdf (accessed on 10 October 2022).
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Szawarski, N.; Saez, A.; Dominguez, E.; Dickson, R.; de Matteis, A.; Eciolaza, C.; Justel, M.; Aliano, A.; Solar, P.; Bergara, I.; et al. Effect of Abscisic Acid (ABA) Combined with Two Different Beekeeping Nutritional Strategies to Confront Overwintering: Studies on Honeybees’ Population Dynamics and Nosemosis. Insects 2019, 10, 329. [Google Scholar] [CrossRef] [PubMed]
- Oleksa, A.; Burczyk, J. Markery DNA w hodowli zachowawczej rodzimych linii pszczoły miodnej. Wiad. Zootech. 2010, 1, 55–67. [Google Scholar]
- Alonso-Prados, E.; Muñoz, I.; de la Rúa, P.; Serrano, J.; Fernández-Alba, A.R.; García-Valcárcel, A.I.; Hernando, M.D.; Alonso, A.; Alonso-Prados, J.L.; Bartolomé, C.; et al. The toxic unit approach as a risk indicator in honeybees surveillance programmes: A case of study in Apis mellifera iberiensis. Sci. Total Environ. 2019, 698, 134208. [Google Scholar] [CrossRef]
- Alaux, C.; Brunet, J.L.; Dussaubat, C.; Mondet, F.; Tchamitchan, S.; Cousin, M.; Brillard, J.; Baldy, A.; Belzunces, L.P.; Le Conte, Y. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ. Microbiol. 2010, 12, 774–782. [Google Scholar] [CrossRef]
- Wittner, M.; Weiss, L.M. The Microsporidia and Microsporidiosis; ASM Press: Washington, DC, USA, 1999. [Google Scholar]
- Zander, E. Tierische Parasiten als Krankheitserreger bei der Biene. Münch. Bienenz. 1909, 31, 196–204. [Google Scholar]
- Fries, I.; Martín, R.; Meana, A.; García-Palencia, P.; Higes, M. Natural infections of Nosema ceranae in European honey bees. J. Apic. Res. 2006, 45, 230–233. [Google Scholar] [CrossRef]
- Chemurot, M.; Smet, L.; Brunain, M.; de Rycke, R.; de Graaf, D.C. Nosema neumanni n. sp. (Microsporidia, Nosematidae), a new microsporidian parasite of honeybees, Apis mellifera in Uganda. Eur. J. Protistol. 2017, 61, 13–19. [Google Scholar] [CrossRef]
- Soklič, M.; Gregorc, A. Comparison of the two microsporidia that infect honeybees—A review. Agricultura 2016, 13, 49–56. [Google Scholar] [CrossRef][Green Version]
- Fries, I.; Feng, F.; Da Silva, A.; Slemenda, S.B.; Pieniazek, N.J. Nosema ceranae n. sp. (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). Eur. J. Protistol. 1996, 32, 356–365. [Google Scholar] [CrossRef]
- Huang, W.F.; Jiang, J.H.; Chen, Y.W.; Wang, C.H. Nosema ceranae infection in Apis mellifera. In Proceedings of the 38th Annual Meeting of the Society for Invertebrate Pathology, Anchorage, AK, USA, 7–11 August 2005. [Google Scholar]
- Goblirsch, M. Nosema ceranae disease of the honeybee (Apis mellifera). Apidologie 2017, 49, 131–150. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Borsuk, G.; Mułenko, W.; Demetraki-Paleolog, J. Differentiation of Nosema apis and Nosema ceranae spores under Scanning Electron Microscopy (SEM). J. Apic. Res. 2014, 53, 537–544. [Google Scholar] [CrossRef]
- Tauber, J.; Nguyen, V.; Lopez, D.L.; Evans, J. Effects of a Resident Yeast from the Honeybee Gut on Immunity, Microbiota, and Nosema Disease. Insects 2019, 10, 296. [Google Scholar] [CrossRef]
- Solter, L.; Becnel, J.; Oi, D. Microsporidian Entomopathogens. In Insect Pathology; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 88, pp. 221–263. [Google Scholar] [CrossRef]
- Delbac, F.; Polonais, V. The microsporidian polar tube and its role in invasion. Subcell. Biochem. 2008, 47, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Fries, I.; Granados, R.R.; Morse, R.A. Intracellular germination of spores of Nosema apis Z. Apidologie 1992, 23, 61–71. [Google Scholar] [CrossRef]
- Bravo, J.; Carbonell, V.; Sepúlveda, B.; Delporte, C.; Valdovinos, C.E.; Martín-Hernández, R.; Higes, M. Antifungal activity of the essential oil obtained from Cryptocarya alba against infection in honeybees by Nosema ceranae. J. Invertebr. Pathol. 2017, 149, 141–147. [Google Scholar] [CrossRef]
- Hanson, F.R.; Eble, T.E. An antiphage agent isolated from Aspergillus sp. J. Bacteriol. 1949, 58, 527–529. [Google Scholar] [CrossRef]
- Katznelson, H.; Jamieson, C.A. Control of nosema disease of honeybees with fumagillin. Science 1952, 115, 70–71. [Google Scholar] [CrossRef]
- Bailey, L. The treatment of nosema disease with fumagillin. Bee World 1953, 34, 136–137. [Google Scholar] [CrossRef]
- Van den Heever, J.P.; Thompson, T.S.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. Fumagillin: An overview of recent scientific advances and their significance for apiculture. J. Agric. Food Chem. 2014, 62, 2728–2737. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Sampson, M.A.; Shutler, D.; Rogers, R.E. Does fumagillin control the recently detected invasive parasite Nosema ceranae in western honey bees (Apis mellifera)? J. Invertebr. Pathol. 2008, 99, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Sarlo, E.G.; Medici, S.K.; Porrini, M.P.; Garrido, M.; Floris, I.; Euguaras, M.J. Comparison between different fumagillin dosage and evaluation method in the apiary control of nosemosis type C. Redia 2011, 94, 39–44. [Google Scholar]
- Huang, W.; Solter, L.; Yau, P.; Imai, B. Nosema ceranae escapes fumagillin control in honeybees. PLoS Pathog. 2013, 9, e1003185. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation No 470/2009 of the European Parliament and of the Council of 6 May 2009 laying down Community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin, repealing Council Regulation (EEC) No. 2377/90 and amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No. 726/2004 of the European Parliament and of the Council. Off. J. Eur. Union 2009, L152, 11–22. [Google Scholar]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Dumitru, A.; Chioveanu, G.; Ionita, M.; Dobre, G.; Mitrea, I.L. “In vitro” Studies on using natural essential oils in treatment of nosemosis in honeybees: Determination of the therapeutic dose. Sci. Works Ser. C Vet. Med. 2017, 63, 165–170. [Google Scholar]
- Porrini, M.P.; Garrido, P.M.; Gende, L.B.; Rossini, C.; Hermida, L.; Marcángeli, J.A.; Eguaras, M.J. Oral administration of essential oils and main components: Study on honeybee survival and Nosema ceranae development. J. Apic. Res. 2017, 56, 616–624. [Google Scholar] [CrossRef]
- Froiio, F.; Ginot, L.; Paolino, D.; Lebaz, N.; Bentaher, A.; Fessi, H.; Elaissari, A. Essential Oils-Loaded Polymer Particles: Preparation, Characterization and Antimicrobial Property. Polymers 2019, 11, 1017. [Google Scholar] [CrossRef]
- Ahmed, M. Acute Toxicity (Lethal Dose 50 Calculation) of Herbal Drug Somina in Rats and Mice. Pharmacol. Pharm. 2015, 6, 185–189. [Google Scholar] [CrossRef]
- Arismendi, N.; Vargas, M.; López, M.D.; Barría, Y.; Zapata, N. Promising antimicrobial activity against the honeybee parasite Nosema ceranae by methanolic extracts from Chilean native plants and propolis. J. Apic. Res. 2018, 57, 522–535. [Google Scholar] [CrossRef]
- Özkırım, A.; Küçüközmen, B. Application of Herbal Essential Oil Extract Mixture for Honey Bees (Apis mellifera L.) against Nosema ceranae and Nosema apis. J. Apic. Sci. 2021, 65, 163–175. [Google Scholar] [CrossRef]
- Maistrello, L.; Lodesani, M.; Costa, C.; Leonardi, F.; Marani, G.; Caldon, M.; Mutinelli, F.; Granato, A. Screening of natural compounds for the control of nosema disease in honeybees (Apis mellifera). Apidologie 2008, 39, 436–445. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Załuski, D. Extracts from Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Roots: A New Hope against Honeybee Death Caused by Nosemosis. Molecules 2020, 25, 4452. [Google Scholar] [CrossRef]
- Porrini, M.P.; Fernández, N.J.; Garrido, P.M.; Gende, L.B.; Medici, S.K.; Eguaras, M.J. In vivo evaluation of antiparasitic activity of plant extracts on Nosema ceranae (Microsporidia). Apidologie 2011, 42, 700–707. [Google Scholar] [CrossRef]
- Damiani, N.; Fernández, N.J.; Porrini, M.P.; Gende, L.B.; Álvarez, E.; Buffa, F.; Brasesco, C.; Maggi, M.D.; Marcangeli, J.A.; Eguaras, M.J. Laurel leaf extracts for honeybee pest and disease management: Antimicrobial, microsporicidal, and acaricidal activity. Parasitol. Res. 2014, 113, 701–709. [Google Scholar] [CrossRef]
- Pohorecka, K. Laboratory studies on the effect of standardized Artemisia absinthium L. extract on Nosema apis infection in the worker Apis mellifera. J. Apic. Sci. 2004, 48, 131–136. [Google Scholar]
- Kim, J.H.; Park, J.K.; Lee, J.K. Evaluation of antimicrosporidian activity of plant extracts on Nosema ceranae. J. Apic. Sci. 2016, 60, 167–178. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Xu, Y.; Gong, H.; Wu, Y.; Chen, Y.; Zheng, H. Protective potential of Chinese herbal extracts against microsporidian Nosema ceranae, an emergent pathogen of western honeybees, Apis mellifera L. J. Asia-Pac. Entomol. 2019, 24, 502–512. [Google Scholar] [CrossRef]
- Radoi, I.; Sapcaliu, A.; Mateescu, C.; Pop, A.; Savu, V. In vitro screening of hydroalcoholic plant extracts to control Nosema apis infection. J. Biotechnol. 2014, 185, S46. [Google Scholar] [CrossRef]
- Song, H.; Kim, H.; Kim, K. Anti-Parasitic Activity of Lespedeza cuneata Extract on Causative Agent of Nosemosis Type C, Nosema ceranae. J. Apic. 2019, 34, 137–140. [Google Scholar] [CrossRef]
- Braglia, C.; Alberoni, D.; Porrini, M.P.; Garrido, P.M.; Baffoni, L.; Di Gioia, D. Screening of Dietary Ingredients against the Honey Bee Parasite Nosema ceranae. Pathogens 2021, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Yucel, B.; Dogaroglu, M. The Impact of Nosema apis Z. Infestation of Honey Bee (Apis mellifera L.) Colonies after Using Different Treatment Methods and their Effects on the Population Levels of Workers and Honey Production on Consecutive Years. Pak. J. Biol. Sci. 2005, 8, 1142–1145. [Google Scholar] [CrossRef][Green Version]
- Borges, D.; Guzman-Novoa, E.; Goodwin, P.H. Control of the microsporidian parasite Nosema ceranae in honeybees (Apis mellifera) using nutraceutical and immuno-stimulatory compounds. PLoS ONE 2020, 15, e0227484. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Lodesani, M.; Maistrello, L. Effect of thymol and resveratrol administered with candy or syrup on the development of Nosema ceranae and on the longevity of honeybees (Apis mellifera L.) in laboratory conditions. Apidologie 2010, 41, 141–150. [Google Scholar] [CrossRef]
- Vargas-Valero, A.; Barrientos-Medina, R.C.; Medina Medina, L.A. Efficacy of thymol in control of the fungus Nosema ceranae in Africanized Apis mellifera. Rev. Mex. Cienc. Pecu. 2021, 12, 633–643. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Saltykova, E.S.; Gaifullina, L.R.; Kaskinova, M.D.; Gataullin, A.R.; Matniyazov, R.T.; Poskryakov, A.V.; Nikolenko, A.G. Effect of chitosan on development of Nosema apis microsporidia in honeybees. Microbiology 2018, 87, 738–743. [Google Scholar] [CrossRef]
- Formato, G.; Rivera-Gomis, J.; Bubnic, J.; Martin-Hernandez, R.; Milito, M.; Croppi, S.; Higes, M. Nosemosis prevention and control. Appl. Sci. 2022, 12, 783. [Google Scholar] [CrossRef]
- Suwannapong, G.; Maksong, S.; Benbow, M.E. Stingless bee propolis effects on experimental infection of Apis florea with Nosema ceranae. J. Agric. Sci. Technol. A 2011, 1, 818–825. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Spivak, M. Propolis and bee health: The natural history and significance of resin use by honeybees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Borba, R.S.; Wilson, M.; Spivak, M. Propolis counteracts some threats to honeybee health. Insects 2017, 8, 46. [Google Scholar] [CrossRef]
- Mura, A.; Pusceddu, M.; Theodorou, P.; Angioni, A.; Floris, I.; Paxton, R.J.; Satta, A. Propolis Consumption Reduces Nosema ceranae Infection of European Honeybees (Apis mellifera). Insects 2020, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Borba, R.S.; Klyczek, K.K.; Mogen, K.L.; Spivak, M. Seasonal benefits of a natural propolis envelope to honeybee immunity and colony health. J. Exp. Biol. 2015, 218, 3689–3699. [Google Scholar] [CrossRef]
- Yemor, T.; Phiancharoen, M.; Benbow, M.E.; Suwannapong, G. Effects of stingless bee propolis on Nosema ceranae infected Asian honeybees, Apis cerana. J. Apic. Res. 2015, 54, 468–473. [Google Scholar] [CrossRef]
- Baffoni, L.; Gaggìa, F.; Alberoni, D.; Cabbri, R.; Nanetti, A.; Biavati, B.; Di Gioia, D. Effect of dietary supplementation of Bifidobacterium and Lactobacillus strains in Apis mellifera L. against Nosema ceranae. Benef. Microbes 2016, 7, 45–51. [Google Scholar] [CrossRef]
- Fiorella, G.; Maggi, M.; Pellegrini, M.C.; Cugnata, N.M.; Szawarski, N.; Buffa, F.; Negri, P.; Fuselli, S.R.; Audisio, C.M.; Ruffinengo, S.R. Effects of Lactobacillus johnsonii AJ5 metabolites on nutrition, Nosema ceranae development and performance of Apis mellifera L. J. Apic. Sci. 2017, 61, 93–104. [Google Scholar] [CrossRef][Green Version]
- Arredondo, D.; Castelli, L.; Porrini, M.P.; Garrido, P.M.; Eguaras, M.J.; Zunino, P.; Antúnez, K. Lactobacillus kunkeei strains decreased the infection by honeybee pathogens Paenibacillus larvae and Nosema ceranae. Benef. Microbes 2018, 9, 279–290. [Google Scholar] [CrossRef]
- Audisio, M.C.; Sabaté, D.C.; Benítez-Ahrendts, M.R. Effect of Lactobacillus johnsonii CRL1647 on different parameters of honeybee colonies and bacterial populations of the bee gut. Benef. Microbes 2015, 6, 687–695. [Google Scholar] [CrossRef]
- Maggi, M.; Negri, P.; Plischuk, S.; Szawarski, N.; de Piano, F.; de Feudis, L.; Eguaras, M.; Audisio, C. Effects of the organic acids produced by a lactic acid bacterium in Apis mellifera colony development, Nosema ceranae control and fumagillin efficiency. Vet. Microbiol. 2013, 167, 474–483. [Google Scholar] [CrossRef]
- Porrini, M.P.; Audisio, M.C.; Sabaté, D.C.; Ibarguren, C.; Medici, S.K.; Sarlo, E.G.; Garrido, P.M.; Eguaras, M.J. Effect of bacterial metabolites on microsporidian Nosema ceranae and on its host Apis mellifera. Parasitol. Res. 2010, 107, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Ptaszyńska, A.A.; Borsuk, G.; Zdybicka-Barabas, A.; Cytryńska, M.; Małek, W. Are commercial probiotics and prebiotics effective in the treatment and prevention of honeybee nosemosis C? Parasitol. Res. 2016, 115, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Ptaszyńska, A.A.; Paleolog, J.; Borsuk, G. Nosema ceranae Infection Promotes Proliferation of Yeasts in Honey Bee Intestines. PLoS ONE 2016, 11, e0164477. [Google Scholar] [CrossRef] [PubMed]
- Ptaszyńska, A.A.; Latoch, P.; Hurd, P.J.; Polaszek, A.; Michalska-Madej, J.; Grochowalski, Ł.; Strapagiel, D.; Gnat, S.; Załuski, D.; Gancarz, M.; et al. Amplicon Sequencing of Variable 16S rRNA from Bacteria and ITS2 Regions from Fungi and Plants, Reveals Honeybee Susceptibility to Diseases Results from Their Forage Availability under Anthropogenic Landscapes. Pathogens 2021, 10, 381. [Google Scholar] [CrossRef]
- Gancarz, M.; Hurd, P.J.; Latoch, P.; Polaszek, A.; Michalska-Madej, J.; Grochowalski, Ł.; Strapagiel, D.; Gnat, S.; Załuski, D.; Rusinek, R.; et al. Dataset of the next-generation sequencing of variable 16S rRNA from bacteria and ITS2 regions from fungi and plants derived from honeybees kept under anthropogenic landscapes. Data Brief 2021, 36, 107019. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, X.; Huang, S.; Zhang, L.; Su, S.; Huang, W.F. Nosema ceranae infection enhances Bifidobacterium spp. abundances in the honeybee hindgut. Apidologie 2019, 50, 353–362. [Google Scholar] [CrossRef]
- Ishii, P.L.; Prado, C.K.; de Oliveira Mauro, M.; Carreira, C.M.; Mantovani, M.S.; Ribeiro, L.R.; Dichi, J.B.; Oliveira, R.J. Evaluation of Agaricus blazei in vivo for antigenotoxic, anticarcinogenic, phagocytic and immunomodulatory activities. Regul. Toxicol. Pharm. 2011, 59, 412–422. [Google Scholar] [CrossRef]
- Glavinic, U.; Rajkovic, M.; Vunduk, J.; Vejnovic, B.; Stevanovic, J.; Milenkovic, I.; Stanimirovic, Z. Effects of Agaricus bisporus Mushroom Extract on Honey Bees Infected with Nosema ceranae. Insects 2021, 7, 915. [Google Scholar] [CrossRef]
- Kunat, M.; Wagner, G.K.; Staniec, B.; Jaszek, M.; Matuszewska, A.; Stefaniuk, D.; Ptaszyńska, A.A. Aqueous extracts of jet-black ant Lasius fuliginosus nests for controlling nosemosis, a disease of honeybees caused by fungi of the genus Nosema. Eur. Zool. J. 2019, 87, 770–780. [Google Scholar] [CrossRef]
- Erler, S.; Moritz, R. Pharmacophagy and pharmacophory: Mechanisms of self-medication and disease prevention in the honeybee colony (Apis mellifera). Apidologie 2016, 47, 389–411. [Google Scholar] [CrossRef]
- Bernklau, E.; Bjostad, L.; Hogeboom, A.; Carlisle, A.; Arathi, H.S. Dietary Phytochemicals, Honeybee Longevity and Pathogen Tolerance. Insects 2018, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Nakayama, F. Caffeine. In Biotechnology of Natural Products; Springer: Cham, Switzerland, 2018; pp. 131–143. [Google Scholar]
- Strachecka, A.; Krauze, M.; Olszewski, K.; Borsuk, G.; Paleolog, J.; Merska, M.; Chobotow, J.; Bajda, M.; Grzywnowicz, K. Unexpectedly strong effect of caffeine on the vitality of western honeybees (Apis mellifera). Biochemistry 2014, 79, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Strachecka, A.; Olszewski, K.; Paleolog, J. Curcumin stimulates biochemical mechanisms of Apis mellifera resistance and extends the apian lifespan. J. Apic. Sci. 2015, 59, 129–141. [Google Scholar] [CrossRef]
- Schulz, M.; Łoś, A.; Grzybek, M.; Ścibior, R.; Strachecka, A. Piperine as a new natural supplement with beneficial effects on the life-span and defence system of honeybees. J. Agric. Sci. 2019, 157, 140–149. [Google Scholar] [CrossRef]
- Hendriksma, H.P.; Bain, J.A.; Nguyen, N.; Nieh, J.C. Nicotine does not reduce Nosema ceranae infection in honeybees. Insectes Soc. 2020, 67, 249–259. [Google Scholar] [CrossRef]
- Papežíková, I.; Palíková, M.; Navrátil, S.; Heumannová, R.; Fronc, M. The effect of oxalic acid applied by sublimation on honeybee colony fitness: A comparison with amitraz. Acta Vet. 2016, 85, 255–260. [Google Scholar] [CrossRef]
- Burnham, A.J. Scientific Advances in Controlling Nosema ceranae (Microsporidia) Infections in Honeybees (Apis mellifera). Front. Vet. Sci. 2019, 6, 79. [Google Scholar] [CrossRef]
- Nanetti, A.; Rodriguez-García, C.; Meana, A.; Martín-Hernández, R.; Higes, M. Effect of oxalic acid on Nosema ceranae infection. Res. Vet. Sci. 2015, 102, 167–172. [Google Scholar] [CrossRef]
- Underwood, R.M.; Currie, R.W. Indoor winter fumigation with formic acid for control of Acarapis woodi (Acari: Tarsonemidae) and Nosema sp. disease. J. Econ. Entomol. 2009, 102, 1729–1736. [Google Scholar] [CrossRef]
- Forsgren, E.; Fries, I. Acidic-Benzoic Feed and Nosema Disease. J. Apic. Sci. 2005, 49, 81–88. [Google Scholar]
- Ptaszyńska, A.A.; Trytek, M.; Borsuk, G.; Buczek, K.; Rybicka-Jasińska, K.; Gryko, D. Porphyrins inactivate Nosema spp. microsporidia. Sci. Rep. 2018, 8, 5523. [Google Scholar] [CrossRef] [PubMed]
- Farrell, D.J.; Robbins, M.; Rhys-Williams, W.; Love, W.G. In vitro activity of XF-73, a novel antibacterial agent, against antibiotic-sensitive and -resistant Gram-positive and Gram-negative bacterial species. Int. J. Antimicrob. Agents 2010, 35, 531–536. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ptaszyńska, A.A.; Borsuk, G.; Mułenko, W.; Olszewski, K. Impact of ethanol on Nosema spp. infected bees. Med. Wet. 2013, 69, 736–740. [Google Scholar]
- Tsagkarakis, A.; Rokkas, C.; Katsimpoulas, I. Experimental Treatment with the Natural Water Acidifier Provigoro® for Nosema spp. Control: Preliminary Results. Adv. Entomol. 2015, 3, 83–85. [Google Scholar] [CrossRef][Green Version]
- Dumitru, A.; Chioveanu, G.; Ionita, M.; Dobre, G.; Mitrea, I.L. In vitro trial on using amprolium clorhidrat to control Nosema infection in honeybees. Sci. Works Ser. C Vet. Med. 2018, 64, 111–116. [Google Scholar]
- Chandra, V.; Singh, K.S.; Singh, S.; Kumar, A.; Tiwari, D.K.; Sahay, R.; Maurya, R.C.; Singh, A. Management of Colony Collapse Disorder in Honeybee (Apis mellifera): A Farmer’s Friendly Approach Running Head: Management of Colony Collapse Disorder in Honeybee. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2557–2568. [Google Scholar] [CrossRef]
- Gisder, S.; Genersch, E. Identification of candidate agents active against N. ceranae infection in honeybees: Establishment of a medium throughput screening assay based on N. ceranae infected cultured cells. PLoS ONE 2015, 6, e0117200. [Google Scholar] [CrossRef]
- Brutscher, L.M.; Flenniken, M.L. RNAi and antiviral defense in the honey bee. J. Immunol. Res. 2015, 2015, 941897. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Evans, J.D.; Li, W.; Branchiccela, B.; Li, J.H.; Heerman, M.C.; Banmeke, O.; Zhao, Y.; Hamilton, M.; Higes, M.; et al. Nosemosis control in European honey bees Apis mellifera by silencing the gene encoding Nosema ceranae polar tube protein 3. J. Exp. Biol. 2018, 2018, 184606. [Google Scholar] [CrossRef]
- Chaimanee, V.; Chantawannakul, P.; Chen, Y.; Evans, J.D.; Pettis, J.S. Differential expression of immune genes of adult honey bee (Apis mellifera) after inoculated by Nosema ceranae. J. Insect Physiol. 2012, 58, 1090–1095. [Google Scholar] [CrossRef]
- Panek, J.; Paris, L.; Roriz, D.; Mone, A.; Dubuffet, A.; Delbac, F.; Diogon, M.; El Alaoui, H. Impact of the microsporidian Nosema ceranae on the gut epithelium renewal of the honeybee, Apis mellifera. J. Invertebr. Pathol. 2018, 159, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Paris, L.; El Alaoui, H.; Delbac, F.; Diogon, M. Effects of the gut parasite Nosema ceranae on honey bee physiology and behavior. Curr. Opin. Insect Sci. 2018, 26, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ptaszyńska, A.A.; Gancarz, M.; Hurd, P.J.; Borsuk, G.; Wiącek, D.; Nawrocka, A.; Strachecka, A.; Załuski, D.; Paleolog, J. Changes in the bioelement content of summer and winter western honeybees (Apis mellifera) induced by Nosema ceranae infection. PLoS ONE 2018, 13, e0200410. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunat-Budzyńska, M.; Budzyński, M.; Schulz, M.; Strachecka, A.; Gancarz, M.; Rusinek, R.; Ptaszyńska, A.A. Natural Substances, Probiotics, and Synthetic Agents in the Treatment and Prevention of Honeybee Nosemosis. Pathogens 2022, 11, 1269. https://doi.org/10.3390/pathogens11111269
Kunat-Budzyńska M, Budzyński M, Schulz M, Strachecka A, Gancarz M, Rusinek R, Ptaszyńska AA. Natural Substances, Probiotics, and Synthetic Agents in the Treatment and Prevention of Honeybee Nosemosis. Pathogens. 2022; 11(11):1269. https://doi.org/10.3390/pathogens11111269
Chicago/Turabian StyleKunat-Budzyńska, Magdalena, Michał Budzyński, Michał Schulz, Aneta Strachecka, Marek Gancarz, Robert Rusinek, and Aneta A. Ptaszyńska. 2022. "Natural Substances, Probiotics, and Synthetic Agents in the Treatment and Prevention of Honeybee Nosemosis" Pathogens 11, no. 11: 1269. https://doi.org/10.3390/pathogens11111269
APA StyleKunat-Budzyńska, M., Budzyński, M., Schulz, M., Strachecka, A., Gancarz, M., Rusinek, R., & Ptaszyńska, A. A. (2022). Natural Substances, Probiotics, and Synthetic Agents in the Treatment and Prevention of Honeybee Nosemosis. Pathogens, 11(11), 1269. https://doi.org/10.3390/pathogens11111269









