Properties of Humic Substances in Composts Comprised of Different Organic Source Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Composts
2.2. Chemical Analyses
2.3. Extraction of Humic and Fulvic Acids
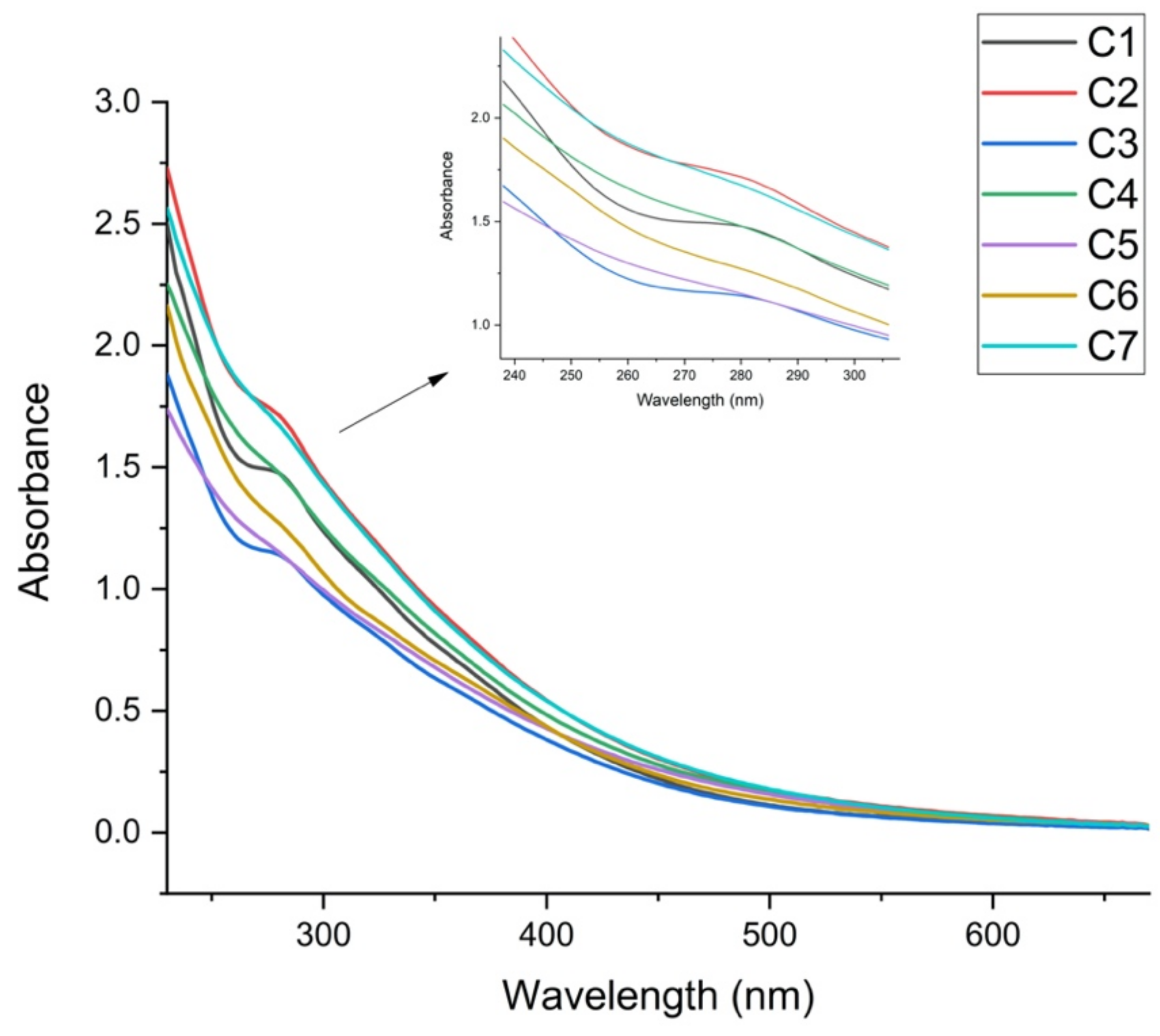
2.4. UV–Vis Absorption Spectra of HA
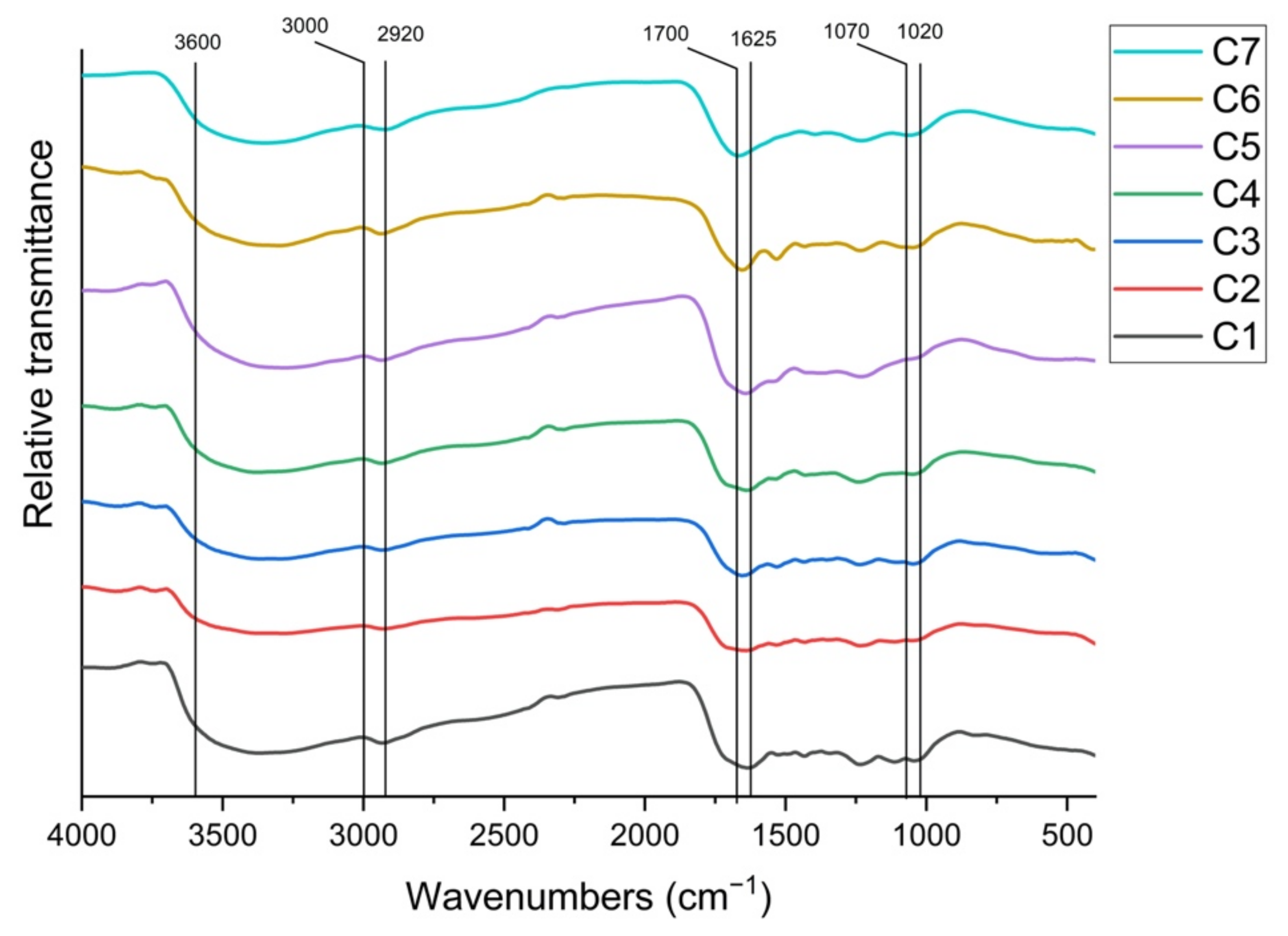
2.5. Fourier-Transform Infrared Absorption Spectra (FTIR) of HA
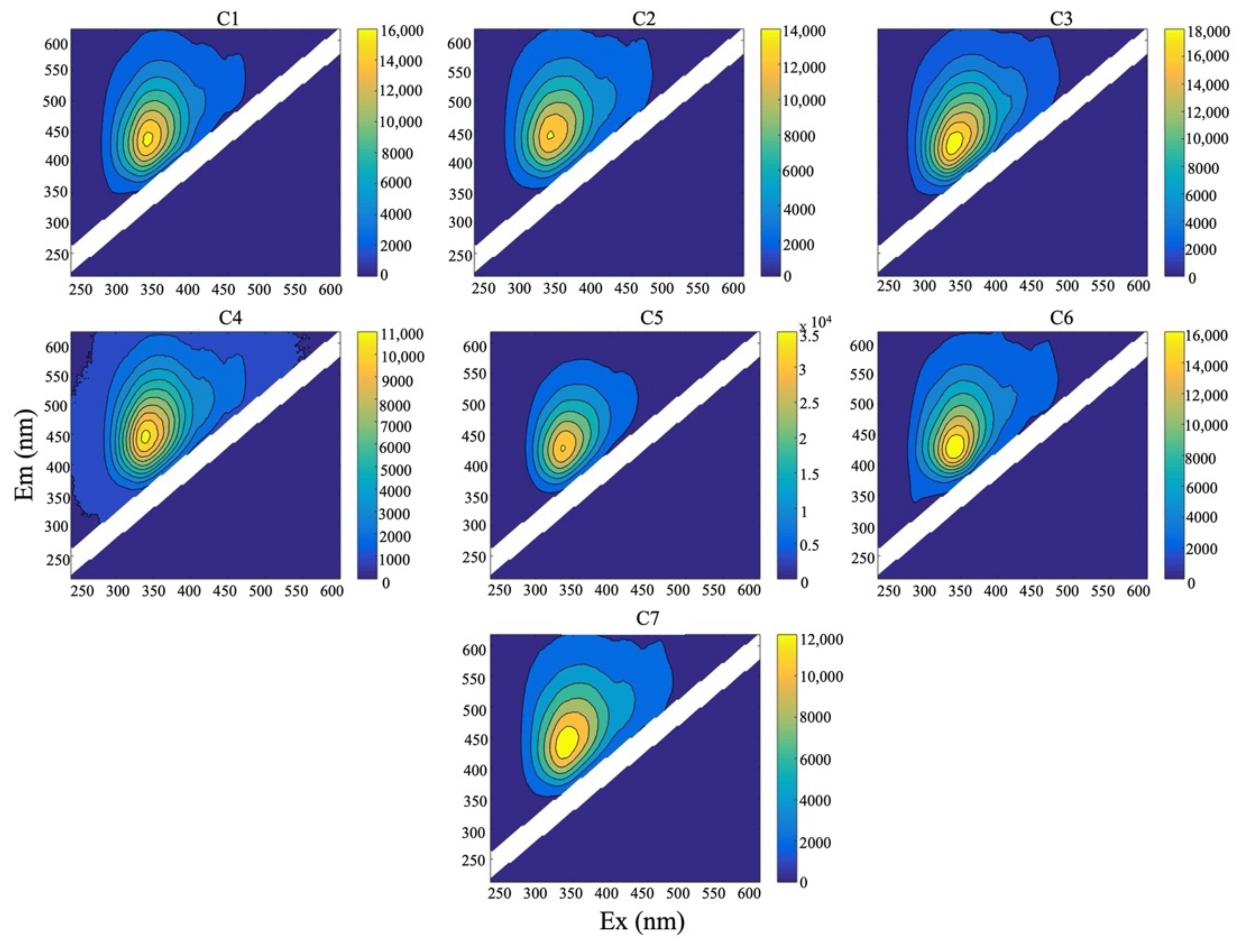
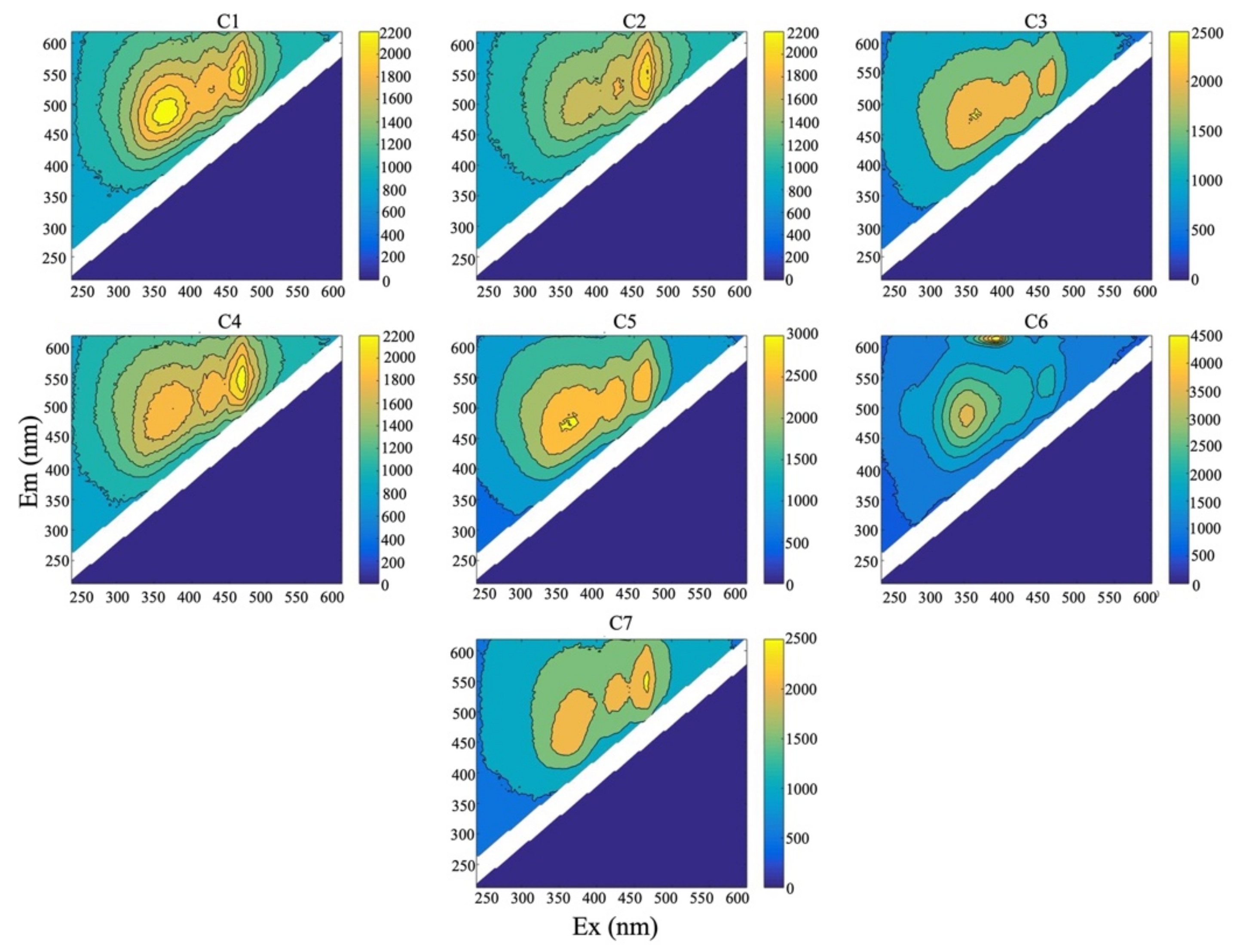
2.6. Excitation Emission Matrix (EEM) Spectroscopy of HA and FA
2.7. Statistical Analysis
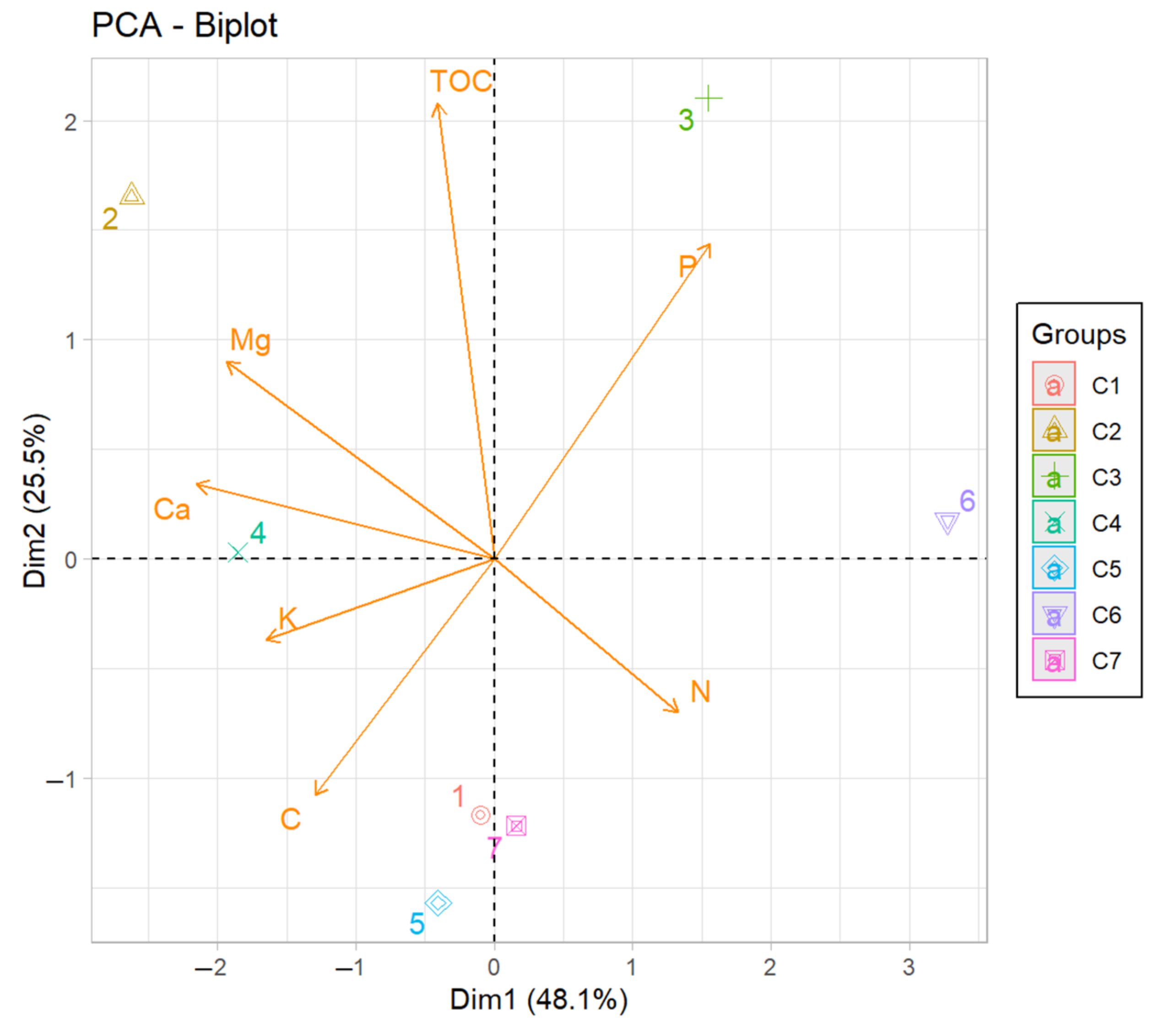
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Indicator | Parameter | Measurement Unit | Assessment Result |
|---|---|---|---|
| Improvement of soil qualities | Organic substance (OM) | [% in dry matter] | ≥15% presentation |
| Fertilising qualities | Total nitrogen (N) | [% in dry matter] | presentation |
| Total phosphorus (P) | [% in dry matter] | presentation | |
| Total potassium (K) | [% in dry matter] | presentation | |
| Material qualities | Maximum size of particles | [mm] | presentation |
| Overall density | [g/L] | presentation | |
| Water content | [g/L] | ||
| Salinity/conductivity | [mS/m] | presentation | |
| pH value | presentation |
References
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Washington, DC, USA, 2018. [Google Scholar]
- Interreg Europe. The Biowaste Management Challenge. A Policy Brief from the Policy Learning Platform on Environment and Resource Efficiency 2021; Interreg Europe: Lille, France, 2021. [Google Scholar]
- Ministry of the Environment Estonian National Waste Management Plan. Riigi Jäätmekava 2014–2020 (Pikendatud Kuni 2022 Lõpuni); Ministry of the Environment Estonian: Tallinn, Estonia, 2014; p. 94. [Google Scholar]
- Nanda, S.; Berruti, F. Municipal Solid Waste Management and Landfilling Technologies: A Review. Environ. Chem. Lett. 2021, 19, 1433–1456. [Google Scholar] [CrossRef]
- European Parliament Directive (EU). 2018/850 of the European Parliament and of the Council of 30 May 2018 amending Directive 1999/31/EC on the Landfill of Waste (Text with EEA Relevance) 2018; European Parliament Directive (EU): Strasbourg, France, 2018. [Google Scholar]
- European Parliament Directive (EU). 2018/851 of the European Parliament and of the Council of 30 May 2018 amending Directive 2008/98/EC on Waste (Text with EEA Relevance) 2018; European Parliament Directive (EU): Strasbourg, France, 2018. [Google Scholar]
- Smol, M. Transition to Circular Economy in the Fertilizer Sector—Analysis of Recommended Directions and End-Users’ Perception of Waste-Based Products in Poland. Energies 2021, 14, 4312. [Google Scholar] [CrossRef]
- The European Green Deal. European Comission Communication from the Commission to the European Parliament, the European Council, the Council, The European Economic and Social Committee and the Committee of the Regions; The European Green Deal: Brussels, Belgium, 2019. [Google Scholar]
- European Parliament Regulation (EU). 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003 2019; European Parliament Directive (EU): Strasbourg, France, 2019. [Google Scholar]
- Lanno, M.; Kriipsalu, M.; Shanskiy, M.; Silm, M.; Kisand, A. Distribution of Phosphorus Forms Depends on Compost Source Material. Resources 2021, 10, 102. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Y.; Xi, B.; Wei, Z.; Li, X.; Cao, Z. Changes in Phosphorus Fractions during Organic Wastes Composting from Different Sources. Bioresour. Technol. 2015, 189, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Siebert, S. Quality Requirements and Quality Assurance of Digestion Residuals in Germany; Bundesgütegemeinschaft Kompost t e.V.: Nuremberg, Germany, 2008. [Google Scholar]
- Siebert, S.; Auweele, W.V. ECN-Qas European Quality Assurance Scheme for Compost and Digestate; Quality Manual; European Compost Network ECN e.V.: Bochum, Germany, 2014; ISBN 978-3-00-047599-3. [Google Scholar]
- Bhattacharya, S.S.; Kim, K.-H.; Das, S.; Uchimiya, M.; Jeon, B.H.; Kwon, E.; Szulejko, J.E. A Review on the Role of Organic Inputs in Maintaining the Soil Carbon Pool of the Terrestrial Ecosystem. J. Environ. Manage. 2016, 167, 214–227. [Google Scholar] [CrossRef]
- Ben Mbarek, H.; Ben Mahmoud, I.; Chaker, R.; Rigane, H.; Maktouf, S.; Arous, A.; Soua, N.; Khlifi, M.; Gargouri, K. Change of Soil Quality Based on Humic Acid with Date Palm Compost Incorporation. Int. J. Recycl. Org. Waste Agric. 2019, 8, 317–324. [Google Scholar] [CrossRef] [Green Version]
- European Commission; European Environment Agency. The State of Soil in Europe: A Contribution of the JRC to the European Environment Agency’s Environment State and Outlook Report—SOER 2010; Publications Office of the European Union: Luxembourg, 2012. [Google Scholar]
- Gao, Y.; Gao, X.; Zhang, X. The 2 °C Global Temperature Target and the Evolution of the Long-Term Goal of Addressing Climate Change—From the United Nations Framework Convention on Climate Change to the Paris Agreement. Engineering 2017, 3, 272–278. [Google Scholar] [CrossRef]
- Bot, A.; Benites, J. The Importance of Soil Organic Matter: Key to Drought-Resistant Soil and Sustained Food Production; Food & Agriculture Org.: Rome, Italy, 2005; Volume 80. [Google Scholar]
- Lakhdar, A.; Rabhi, M.; Ghnaya, T.; Montemurro, F.; Jedidi, N.; Abdelly, C. Effectiveness of Compost Use in Salt-Affected Soil. J. Hazard. Mater. 2009, 171, 29–37. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Shindo, H. Quantitative and Qualitative Changes of Humus in Whole Soils and Their Particle Size Fractions as Influenced by Different Levels of Compost Application. Agric. Sci. 2011, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dou, S.; Shan, J.; Song, X.; Cao, R.; Wu, M.; Li, C.; Guan, S. Are Humic Substances Soil Microbial Residues or Unique Synthesized Compounds? A Perspective on Their Distinctiveness. Pedosphere 2020, 30, 159–167. [Google Scholar] [CrossRef]
- Von Lützow, M.; Kögel-Knabner, I.; Ekschmitt, K.; Flessa, H.; Guggenberger, G.; Matzner, E.; Marschner, B. SOM Fractionation Methods: Relevance to Functional Pools and to Stabilization Mechanisms. Soil Biol. Biochem. 2007, 39, 2183–2207. [Google Scholar] [CrossRef]
- Harrison, R.B. Composting and Formation of Humic Substances; Elsevier Science, Encyclopedia of Ecology: Amsterdam, Netherlands, 2008; pp. 713–719. [Google Scholar]
- Gerke, J. Concepts and Misconceptions of Humic Substances as the Stable Part of Soil Organic Matter: A Review. Agronomy 2018, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Liu, H.; Wu, S. Humic Substances Developed during Organic Waste Composting: Formation Mechanisms, Structural Properties, and Agronomic Functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef]
- Zhou, Y.; Selvam, A.; Wong, J.W.C. Evaluation of Humic Substances during Co-Composting of Food Waste, Sawdust and Chinese Medicinal Herbal Residues. Bioresour. Technol. 2014, 168, 229–234. [Google Scholar] [CrossRef]
- Zingaretti, D.; Lombardi, F.; Baciocchi, R. Soluble Organic Substances Extracted from Compost as Amendments for Fenton-like Oxidation of Contaminated Sites. Sci. Total Environ. 2018, 619–620, 1366–1374. [Google Scholar] [CrossRef]
- Minister of the Environment Regulation of the Republic of Estonia. “Requirements for Production of Compost from Biodegradable Waste” RT I, 18.12.2020, 23 2021; Environment Regulation of the Republic of Estionia: Tallinn, Estonia, 2021. [Google Scholar]
- Minister of the Environment Regulation of the Republic of Estonia. Haljastuses, Rekultiveerimisel Ja Põllumajanduses Kasutatava Reoveesette Kvaliteedi Piirväärtused Ning Kasutamise Nõuded. RT I, 06.08.2019, 7 2019; Environment Regulation of the Republic of Estionia: Tallinn, Estonia, 2019. [Google Scholar]
- Lanno, M.; Silm, M.; Shanskiy, M.; Kisand, A.; Orupõld, K.; Kriipsalu, M. Open Windrow Composting of Fish Waste in Estonia. EMU DSpace 2020, 18, 428. [Google Scholar] [CrossRef]
- Khan, M.B.; Cui, X.; Jilani, G.; Tang, L.; Lu, M.; Cao, X.; Sahito, Z.A.; Hamid, Y.; Hussain, B.; Yang, X.; et al. New Insight into the Impact of Biochar during Vermi-Stabilization of Divergent Biowastes: Literature Synthesis and Research Pursuits. Chemosphere 2020, 238, 124679. [Google Scholar] [CrossRef]
- Escuer, O.; Karp, K.; Escuer-Gatius, J.; Raave, H.; Teppand, T.; Shanskiy, M. Hardwood Biochar as an Alternative to Reduce Peat Use for Seed Germination and Growth of Tagetes patula. Acta Agric. Scand. Sect. B Soil Plant Sci. 2021, 71, 408–421. [Google Scholar] [CrossRef]
- Malińska, K.; Zabochnicka-Świątek, M.; Cáceres, R.; Marfà, O. The Effect of Precomposted Sewage Sludge Mixture Amended with Biochar on the Growth and Reproduction of Eisenia Fetida during Laboratory Vermicomposting. Ecol. Eng. 2016, 90, 35–41. [Google Scholar] [CrossRef]
- Egner, H.; Riehm, H.; Domingo, W.R. Investigations on the Chemical Soil Analysis as a Basis for Assessing the Soil Nutrient Status. II: Chemical Extraction Methods for Phosphorus and Potassium Determination. K. Lantbrukshügskolans Ann. 1960, 26, 199–215. [Google Scholar]
- Tan, K.H. Soil Sampling, Preparation, and Analysis; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Klavins, M.; Purmalis, O. Properties and Structure of Raised Bog Peat Humic Acids. J. Mol. Struct. 2013, 1050, 103–113. [Google Scholar] [CrossRef]
- Chen, Y.; Senesi, N.; Schnitzer, M. Information Provided on Humic Substances by E4/E6 Ratios. Soil Sci. Soc. Am. J. 1977, 41, 352–358. [Google Scholar] [CrossRef]
- Peuravuori, J.; Pihjala, K. Molecular Size Distribution and Spectroscopic Properties of Aquatic Humic Substances. Anal. Chim. Acta 1997, 337, 133–149. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence Spectroscopy and Multi-Way Techniques. PARAFAC Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Mendiburu, F.D. Agricolae: Statistical Procedures for Agricultural Research, R Package Version 1.2-3. 2015. Available online: http://cran.r-project.org/package=agricolae (accessed on 3 October 2022).
- Minero, C.; Lauri, V.; Falletti, G.; Maurino, V.; Pelizzetti, E.; Vione, D. Spectrophotometric Characterisation of Surface Lakewater Samples: Implications for the Quantification of Nitrate and the Properties of Dissolved Organic Matter. Ann. Chim. 2007, 97, 1107–1116. [Google Scholar] [CrossRef]
- Verillo, M.; Salzano, M.; Cozzolino, V.; Spaccini, R.; Piccolo, A. Bioactivity and Antimicrobial Properties of Chemically Characterized Compost Teas from Different Green Composts. Waste Manag. 2021, 120, 98–107. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, X.; Zhu, C.; Xi, B.; Zhao, Y.; Yu, X. Assessment of Humification Degree of Dissolved Organic Matter from Different Composts Using Fluorescence Spectroscopy Technology. Chemosphere 2014, 95, 261–267. [Google Scholar] [CrossRef]
- Valencia, S.; Marin, J.M.; Restrepo, G.; Frimmel, F.H. Application of Excitation–Emission Fluorescence Matrices and UV/Vis Absorption to Monitoring the Photocatalytic Degradation of Commercial Humic Acid. Sci. Total Environ. 2013, 442, 207–214. [Google Scholar] [CrossRef]
- Da Silva, L.S.; Constantino, I.C.; Bento, L.R.; Tadini, A.M.; Bisinoti, M.C.; Boscolo, M.; Ferreira, O.P. Humic Extracts from Hydrochar and Amazonian Anthrosol: Molecular Features and Metal Binding Properties Using EEM-PARAFAC and 2D FTIR Correlation Analyses. Chemosphere 2020, 256, 127110. [Google Scholar] [CrossRef]
- Richard, C.; Guyot, G.; Trubetskaya, O.; Grigatti, M.; Cavani, L. Fluorescence Analysis of Humic-Like Substances Extracted from Composts: Influence of Composting Time and Fractionation. Environ. Chem. Lett. 2009, 7, 61–65. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, C.; Cheng, Y.; Lei, T.; Wu, N.; Wang, X.; Xu, X. Dissolved Organic Matter Features in Black Soldier Fly Larvae Frass Derived From Two Types of Meat and Bone Meals with Straw Amendments. Waste Biomass Valorization 2022, 1–12. [Google Scholar] [CrossRef]
- Aguiar, N.O.; Olivares, F.L.; Novotny, E.H.; Dobbss, L.B.; Balmori, D.M.; Santos-Júnior, L.G.; Chagas, J.G.; Façanha, A.R.; Canellas, L.P. Bioactivity of Humic Acids Isolated from Vermicomposts at Different Maturation Stages. Plant Soil 2013, 362, 161–174. [Google Scholar] [CrossRef]
- Dores-Silva, P.R.; Landgraf, M.D.; Rezende, M.O.O. Humification Process in Different Kinds of Organic Residue by Composting and Vermicomposting: Have Microbioreactors Really Accelerated the Process? Environ. Sci. Pollut. Res. 2018, 25, 17490–17498. [Google Scholar] [CrossRef]
- Mosa, A.; Taha, A.; Elsaeid, M. Agro-Environmental Applications of Humic Substances: A Critical Review. Egypt. J. Soil Sci. 2020, 60, 211–229. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Y.; Qi, H.; Zhao, X.; Yang, T.; Du, Y.; Zhang, H.; Wei, Z. Identifying the Key Factors that Affect the Formation of Humic Substance during Different Materials Composting. Bioresour. Technol. 2017, 244, 1193–1196. [Google Scholar] [CrossRef]
- Raj, D.; Antil, R.S. Evaluation of Maturity and Stability Parameters of Composts Prepared from Agro-Industrial Wastes. Bioresour. Technol. 2011, 102, 2868–2873. [Google Scholar] [CrossRef]
- Silva, M.E.; Lemos, L.T.; Cunha-Queda, A.C.; Nunes, O.C. Co-Composting of Poultry Manure with Low Quantities of Carbon-Rich Materials. Waste Manag. Res. J. Sustain. Circ. Econ. 2009, 27, 119–128. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: New Jersey, NJ, USA, 1994. [Google Scholar]
- Zilong, Z.; Yu, X. Changes in the Spectral Characteristics of Hydrophilic Fraction in Compost Organic Matter. J. Phys. Conf. Ser. 2021, 2011, 012069. [Google Scholar] [CrossRef]
- Sutton, R.; Sposito, G. Molecular Structure in Soil Humic Substances: The New View. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef]
- Jurado, M.M.; Suárez-Estrella, F.; López, M.J.; Vargas-García, M.C.; López-González, J.A.; Moreno, J. Enhanced Turnover of Organic Matter Fractions by Microbial Stimulation during Lignocellulosic Waste Composting. Bioresour. Technol. 2015, 186, 15–24. [Google Scholar] [CrossRef]
- Kulikowska, D.; Sindrewicz, S. Effect of Barley Straw and Coniferous Bark on Humification Process during Sewage Sludge Composting. Waste Manag. 2018, 79, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Lopes, I.G.; Yong, J.W.; Lalander, C. Frass Derived from Black Soldier Fly Larvae Treatment of Biodegradable Wastes. A Critical Review and Future Perspectives. Waste Manag. 2022, 142, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ee, A.W.L.; Tan, J.K.N.; Cheong, J.C.; Chiam, Z.; Arora, S.; Lam, W.N.; Tan, H.T.W. Upcycling Food Waste Using Black Soldier Fly Larvae: Effects of Further Composting on Frass Quality, Fertilising Effect and Its Global Warming Potential. J. Clean. Prod. 2021, 288, 125664. [Google Scholar] [CrossRef]
- Liu, T.; Awasthi, M.K.; Awasthi, S.K.; Zhang, Y.; Zhang, Z. Impact of the Addition of Black Soldier Fly Larvae on Humification and Speciation of Trace Elements during Manure Composting. Ind. Crops Prod. 2020, 154, 112657. [Google Scholar] [CrossRef]
- Phooi, C.L.; Azman, E.A.; Ismail, R. Do it Yourself: Humic Acid. Pertanika J. Trop. Agric. Sci. 2022, 45, 547–564. [Google Scholar] [CrossRef]
- Campitelli, P.; Ceppi, S. Effects of Composting Technologies on the Chemical and Physicochemical Properties of Humic Acids. Geoderma 2008, 144, 325–333. [Google Scholar] [CrossRef]
- Iqbal, J.; Sarwar, G.; Shah, S.H.; Sabah, N.-U.; Tahir, M.A.; Muhammad, S.; Zeeshan Manzoor, M.; Zafar, A.; Shehzad, I. Evaluating the Combined Effect of Compost and Mineral Fertilizers on Soil Health, Growth and Mineral Acquisition in Maize (Zea mays L.). Pak. J. Bot. 2022, 54, 1793–1801. [Google Scholar] [CrossRef]
- Issoufa, B.B.; Ibrahim, A.; Abaidoo, R.C.; Ewusi-Mensah, N. Combined Use of Millet Glume-Derived Compost and Mineral Fertilizer Enhances Soil Microbial Biomass and Pearl Millet Yields in a Low-Input Millet Cropping System in Niger. Arch. Agron. Soil Sci. 2019, 65, 1107–1119. [Google Scholar] [CrossRef]
- Klavins, M.; Grandovska, S.; Obuka, V.; Ievinsh, G. Comparative Study of Biostimulant Properties of Industrially and Experimentally Produced Humic Substances. Agronomy 2021, 11, 1250. [Google Scholar] [CrossRef]
- Savy, D.; Brostaux, Y.; Cozzolino, V.; Delaplace, P.; Jardin, P.D.; Piccolo, A. Quantitative Structure-Activity Relationship of Humic-Like Biostimulants Derived from Agro-Industrial Byproducts and Energy Crops. Front. Plant Sci. 2020, 11, 581. [Google Scholar] [CrossRef]
- Hu, C.; Xia, X.; Chen, Y.; Han, X. Soil Carbon and Nitrogen Sequestration and Crop Growth as Influenced by Long-Term Application of Effective Microorganism Compost. Chil. J. Agric. Res. 2018, 78, 13–22. [Google Scholar] [CrossRef]
| Compost | Target Waste | Amendments | Scale | Certified Quality Regulations | Active Period (d) | Composting Site Location |
|---|---|---|---|---|---|---|
| C1 | Fish waste | Straw, peat, inoculum from previous composts | Industrial | No | 185 | 58°28′41.08″ N 24°49′28.66″ E |
| C2 * | Horse Manure | Straw | Industrial | Yes | 90 | 58°28′41.08″ N 24°49′28.66″ E |
| C3 * | Sewage sludge | Straw | Industrial | Yes | 120 | 58°13′37.89″ N 26°23′10.17″ E |
| C4 * | Green waste from municipal areas | None | Industrial | Yes | >180 | 59°28′14.63″ N 24°54′43.97″ E |
| C5 * | Kitchen Biowaste | Shredded wood | Industrial | Yes | 130 | 59°27′36.08″ N 25°5′17.18″ E |
| C6 | Cafeteria and grocery store biowaste | None | Pilot | No | 51 | 58°23′30.76″ N 26°41′40.43″ E |
| C7 | Kitchen biowaste | Straw, paper, green waste, biochar | Pilot | No | 365 | 58°23′30.76″ N 26°41′40.43″ E |
| Compost | N | C | C/N | Ca | K | Mg | P | pH |
|---|---|---|---|---|---|---|---|---|
| % | ratio | µg/g | ||||||
| C1 | 4.3 ± 0.1 | 65.3 ± 0.3 | 15.19 | 493 ± 8 | 1910 ± 25 | 80 ± 5 | 1469 ± 18 | 5.77 |
| C2 | 4.5 ± 0.1 | 62.7 ± 0.3 | 13.93 | 1472 ± 20 | 4700 ± 30 | 234 ± 5 | 1122 ± 20 | 7.40 |
| C3 | 5.7 ± 01. | 60.5 ± 0.3 | 10.61 | 546 ± 9 | 791 ± 12 | 91 ± 5 | 2782 ± 20 | 6.29 |
| C4 | 3.8 ± 0.1 | 63.2 ± 0.3 | 16.63 | 1224 ± 12 | 4164 ± 30 | 145 ± 5 | 796 ± 12 | 7.80 |
| C5 | 8.6 ± 0.1 | 61.9 ± 0.3 | 7.20 | 947 ± 10 | 6412 ± 35 | 58 ± 5 | 120 ± 8 | 6.85 |
| C6 | 7.9 ± 0.1 | 60.5 ± 0.3 | 7.66 | <32 | 114 ± 8 | <3 | 2432 ± 20 | 8.10 |
| C7 | 6.0 ± 0.1 | 62.8 ± 0.3 | 10.47 | 759 ± 8 | 578 ± 10 | 120 ± 5 | 839 ± 12 | 7.69 |
| Compost | TOC | HA/FA | E2/E3 | E4/E6 | Lipids |
|---|---|---|---|---|---|
| % | % | ||||
| C1 | 12.0 ± 0.2 a | 5.70 | 2.65 | 7.78 | 1.5 ± 0.1 b |
| C2 | 27.0 ± 0.2 c | 7.88 | 2.57 | 6.95 | 1.5 ± 0.1 b |
| C3 | 25.7 ± 0.2 c | 4.73 | 2.49 | 8.33 | 1.5 ± 0.1 b |
| C4 | 18.0 ± 0.2 ab | 7.82 | 2.56 | 7.34 | 1.7 ± 0.1 b |
| C5 | 15.3 ± 0.2 a | 1.42 | 2.38 | 6.88 | 4.2 ± 0.1 a |
| C6 | 17.1 ± 0.2 a | 1.46 | 2.66 | 6.40 | 2.1 ± 0.1 b |
| C7 | 8.7 ± 0.1 d | 1.60 | 2.61 | 8.59 | 1.4 ± 0.1 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanno, M.; Klavins, M.; Purmalis, O.; Shanskiy, M.; Kisand, A.; Kriipsalu, M. Properties of Humic Substances in Composts Comprised of Different Organic Source Material. Agriculture 2022, 12, 1797. https://doi.org/10.3390/agriculture12111797
Lanno M, Klavins M, Purmalis O, Shanskiy M, Kisand A, Kriipsalu M. Properties of Humic Substances in Composts Comprised of Different Organic Source Material. Agriculture. 2022; 12(11):1797. https://doi.org/10.3390/agriculture12111797
Chicago/Turabian StyleLanno, Marge, Maris Klavins, Oskars Purmalis, Merrit Shanskiy, Anu Kisand, and Mait Kriipsalu. 2022. "Properties of Humic Substances in Composts Comprised of Different Organic Source Material" Agriculture 12, no. 11: 1797. https://doi.org/10.3390/agriculture12111797






