A Look at the Importance of Chirality in Drug Activity: Some Significative Examples
Abstract
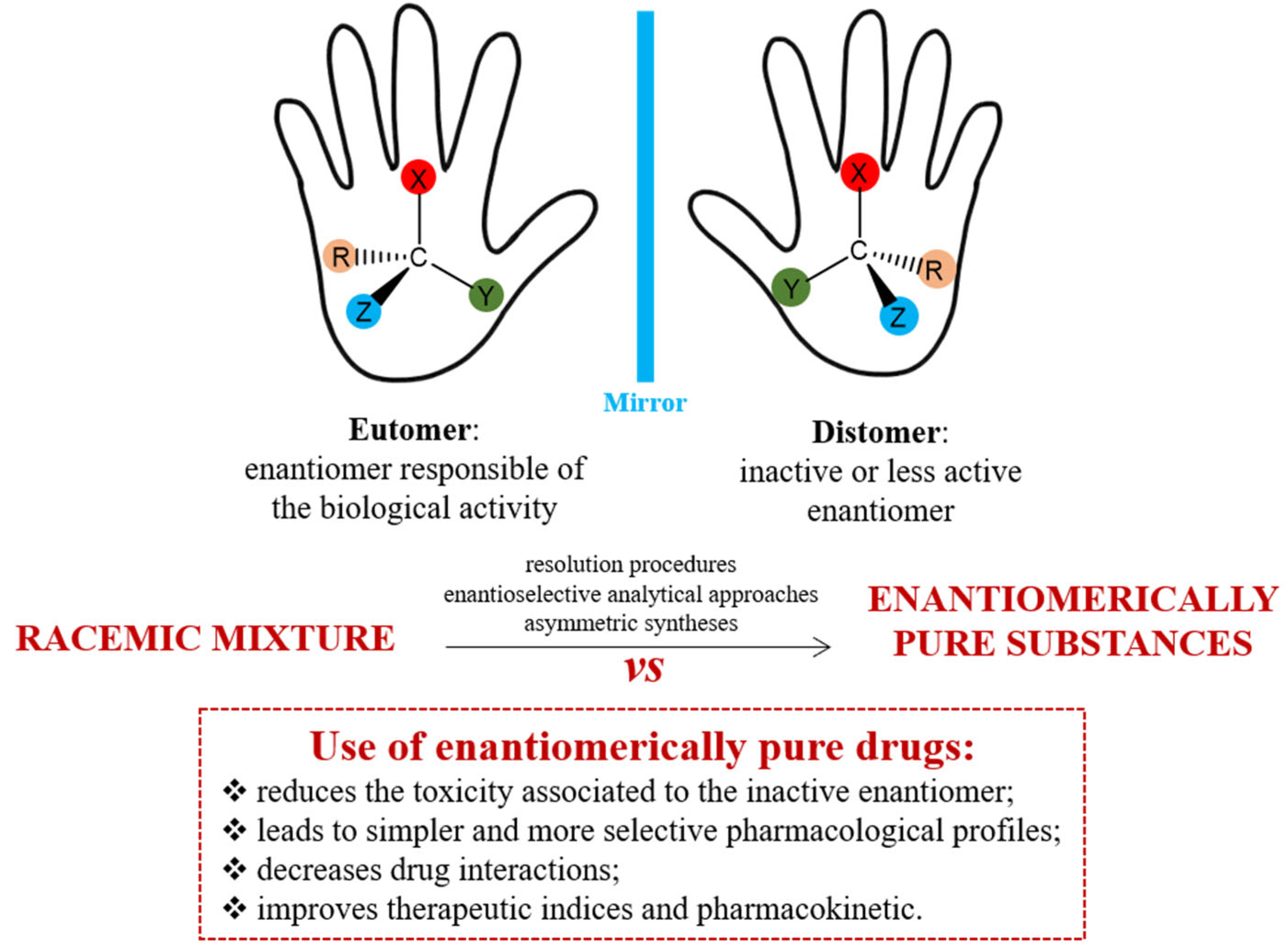
:1. Introduction
2. Drugs Acting on the Cardiovascular System
2.1. Antiarrhythmics
2.2. Antihyperthensive Drugs
2.2.1. Angiotensin-Converting Enzyme Inhibitors
2.2.2. Sartans
2.2.3. Neprilysin Inhibitors
3. Beta-Adrenergic Drugs
4. Drugs Acting on Central Nervous System (CNS)
5. Local Anesthetics
6. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
7. Antimicrobials
7.1. Antibacterials
7.2. Antimalarial and Antivirals
8. Proton Pump Inhibitors (PPIs)
9. Anticancer Drugs
10. Anti-Histamine Drugs (H1 Receptor Antagonists)
11. Selective Serotonin Reuptake Inhibitors (SSRIs)
12. Serine Protease Factor B Inhibitors
13. Repositioned Chiral Drugs
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eliel, E.L.; Wilen, S.H. Stereochemistry of Organic Compounds; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- De Camp, W.H. The FDA Perspective on the development of stereoisomers. Chirality 1989, 1, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Hutt, A.J. Drug chirality and its pharmacological consequences. In Smith and Williams’ Introduction to the Principles of Drug Design and Action; CRC Press: Boca Raton, FL, USA, 2019; pp. 97–166. [Google Scholar]
- Food and Drug Administration. FDA’s policy statement for the development of new stereoisomeric drugs. 57 Fed. Reg. 1992, 22, 249. [Google Scholar]
- De Camp, W.H. Chiral drugs: The FDA perspective on manufacturing and control. J. Pharm. Biomed. Anal. 1993, 11, 1167–1172. [Google Scholar] [CrossRef]
- Singh, M.; Sethi, S.; Bhushan, R. Liquid chromatographic methods for separation, determination, and bioassay of enantiomers of etodolac: A review. J. Sep. Sci. 2019, 43, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, L.M. FDA gives its nod to 53 new drugs in 2020. Chem. Eng. News 2021, 99. Available online: https://cen.acs.org/pharmaceuticals/drug-development/FDA-gives-nod-53-new/99/i2 (accessed on 5 August 2022).
- Coelho, M.M.; Fernandes, C.; Remião, F.; Tiritan, M.E. Enantioselectivity in drug pharmacokinetics and toxicity: Pharmacological relevance and analytical methods. Molecules 2021, 26, 3113. [Google Scholar] [CrossRef]
- Davies, N.M.; Teng, X.W. Importance of chirality in drug therapy and pharmacy practice: Implications for psychiatry. Adv. Pharm. 2003, 1, 242–252. [Google Scholar]
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The significance of chirality in drug design and development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef]
- Maier, N.M.; Franco, P.; Lindner, W. Separation of enantiomers: Needs, challenges, perspectives. J. Chromatog. A 2001, 906, 3–33. [Google Scholar] [CrossRef]
- Kumari Rayala, V.P.; Kandula, J.S. Advances and challenges in the pharmacokinetics and bioanalysis of chiral drugs. Chirality 2022, in press. [Google Scholar] [CrossRef]
- Hancu, G.; Papp, L.A.; Tóth, G.; Kelemen, H. The use of dual cyclodextrin chiral selector systems in the enantioseparation of pharmaceuticals by capillary electrophoresis: An overview. Molecules 2021, 26, 2261. [Google Scholar] [CrossRef] [PubMed]
- Cabedo, N.; Andreu, I.; Ramirez de Arellano, M.C.; Chagraoui, A.; Serrano, A.; Bermejo, A.; Protais, P.; Cortes, D. Enantioselective syntheses of dopaminergic (R)-and (S)-benzyltetrahydroisoquinolines. J. Med. Chem. 2001, 44, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, H.; Seidel-Morgenstern, A. Processes to separate enantiomers. Angew. Chem. Int. Ed. 2014, 53, 1218–1250. [Google Scholar] [CrossRef]
- Bredikhin, A.A.; Bredikhina, Z.A. Stereoselective crystallization as a basis for single-enantiomer drug production. Chem. Eng. Technol. 2017, 40, 1211–1220. [Google Scholar] [CrossRef]
- Betzenbichler, G.; Huber, L.; Kräh, S.; Morkos, M.L.; Siegle, A.; Trapp, O. Chiral stationary phases and applications in gas chromatography. Chirality 2022, 34, 732–759. [Google Scholar] [CrossRef]
- Chankvetadze, B. Application of enantioselective separation techniques to bioanalysis of chiral drugs and their metabolites. TrAC Trends Anal. Chem. 2021, 143, 116332. [Google Scholar] [CrossRef]
- Felletti, S.; Ismail, O.H.; De Luca, C.; Costa, V.; Gasparrini, F.; Pasti, L.; Marchetti, N.; Cavazzini, A.; Catani, M. Recent achievements and future challenges in supercritical fluid chromatography for the enantioselective separation of chiral pharmaceuticals. Chromatographia 2019, 82, 65–75. [Google Scholar] [CrossRef]
- Folprechtová, D.; Kalíková, K.; Kadkhodaei, K.; Reiterer, C.; Armstrong, D.W.; Tesařová, E.; Schmid, M.G. Enantioseparation performance of superficially porous particle vancomycin-based chiral stationary phases in supercritical fluid chromatography and high performance liquid chromatography; applicability for psychoactive substances. J. Chromatog. A 2021, 1637, 461846. [Google Scholar] [CrossRef]
- Woiwode, U.; Neubauer, S.; Lindner, W.; Buckenmaier, S.; Lämmerhofer, M. Enantioselective multiple heartcut two-dimensional ultra-high-performance liquid chromatography method with a coreshell chiral stationary phase in the second dimension for analysis of all proteinogenic amino acids in a single run. J. Chromatog. A 2018, 1562, 69–77. [Google Scholar] [CrossRef]
- Davankov, V.A. Resolution of racemates by ligand-exchange chromatography. In Advances in Chromatography; CRC Press: Boca Raton, FL, USA, 2021; pp. 139–195. [Google Scholar]
- Fanali, S.; Chankvetadze, B. History, advancement, bottlenecks, and future of chiral capillary electrochromatography. J. Chromatog. A 2021, 1637, 461832. [Google Scholar] [CrossRef]
- Pu, J.; Wang, H.; Huang, C.; Bo, C.; Gong, B.; Ou, J. Progress of molecular imprinting technique for enantioseparation of chiral drugs in recent ten years. J. Chromatog. A 2022, 1668, 462914. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Chen, Z.; Dong, J.; Liu, Y.; Cui, Y. Chiral metal-organic frameworks. Chem. Rev. 2022, 122, 9078–9144. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, R. ′Ab Ovo′ chiral phases and chiral reagents for liquid chromatographic separation and isolation of enantiomers. Chem. Rec. 2022, 22, e202100295. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jameson, C.J.; Murad, S. Modeling enantiomeric separations as an interfacial process using amylose Tris(3,5-dimethylphenyl carbamate) (ADMPC) polymers coated on amorphous silica. Langmuir 2020, 36, 1113–1124. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Huo, X.; Zhang, Z.; Yuan, Q.; Li, P.; Chen, J.; Zou, Y.; Wu, Z.; Zhang, W. Catalytic asymmetric synthesis of the anti-COVID-19 drug remdesivir. Angew. Chem. Int. Edit. 2020, 59, 20814–20819. [Google Scholar] [CrossRef]
- Catalano, A.; Carocci, A.; Fracchiolla, G.; Franchini, C.; Lentini, G.; Tortorella, V.; De Luca, A.; De Bellis, J.; Desaphy, J.-F.; Conte Camerino, D. Stereospecific synthesis of “para-hydroxymexiletine” and sodium channel blocking activity evaluation. Chirality 2004, 16, 72–78. [Google Scholar]
- Peepliwal, A.K.; Bagade, S.B.; Bonde, C.G. A review: Stereochemical consideration and eudismic ratio in chiral drug development. J. Biomed. Sci. Res. 2010, 2, 29–45. [Google Scholar]
- Mane, S. Racemic drug resolution: A comprehensive guide. Analyt. Met. 2016, 8, 7567–7586. [Google Scholar] [CrossRef]
- Bogaerts, J.; Aerts, R.; Vermeyen, T.; Johannessen, C.; Herrebout, W.; Batista, J.M. Tackling Stereochemistry in Drug Molecules with Vibrational Optical Activity. Pharmaceuticals 2021, 14, 877. [Google Scholar] [CrossRef]
- Silvestri, I.P.; Colbon, P.J. The growing importance of chirality in 3D chemical space exploration and modern drug discovery approaches for Hit-ID: Topical Innovations. ACS Med. Chem. Lett. 2021, 12, 1220–1229. [Google Scholar] [CrossRef]
- Calcaterra, A.; D’Acquarica, I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018, 147, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Hancu, G.; Modroiu, A. Chiral switch: Between therapeutical benefit and marketing strategy. Pharmaceuticals 2022, 15, 240. [Google Scholar] [CrossRef] [PubMed]
- Spalding, D. The importance of the physicochemical properties of drugs to drug metabolism. In A Handbook of Bioanalysis and Drug Metabolism; CRC Press: Boca Raton, FL, USA, 2021; pp. 8–31. ISBN 9780203642535. [Google Scholar]
- Kirchmair, J.; Howlett, A.; Peironcely, J.E.; Murrell, D.S.; Williamson, M.J.; Adams, S.E.; Hankemeier, T.; van Buren, L.; Duchateau, G.; Klaffke, W.; et al. How do metabolites differ from their parent molecules and how are they excreted? J. Chem. Inf. Mod. 2013, 53, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Amare, G.G.; Meharie, B.G.; Belayneh, Y.M. A drug repositioning success: The repositioned therapeutic applications and mechanisms of action of thalidomide. J. Oncol. Pharm. Pract. 2021, 27, 673–678. [Google Scholar] [CrossRef]
- Winter, W.; Frankus, E. Thalidomide enantiomers. Lancet 1992, 33, 365. [Google Scholar] [CrossRef]
- Vargesson, N. Thalidomide-induced teratogenesis: History and mechanisms. Birth Defects Res. Part A Clin. Mol. Teratol. 2015, 105, 140–156. [Google Scholar] [CrossRef] [Green Version]
- Schoetz, G.; Trapp, O.; Schurig, V. Determination of the enantiomerization barrier of thalidomide by dynamic capillary electrokinetic chromatography. Electrophoresis 2001, 22, 3185–3190. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Pellegrino, M.; Aquaro, S.; Franchini, C.; Sinicropi, M.S. Diarylureas: Repositioning from antitumor to antimicrobials or multi-target agents against new pandemics. Antibiotics 2021, 10, 92. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Sinicropi, M.S.; Franchini, C. Diarylureas as antitumor agents. Appl. Sci. 2021, 11, 374. [Google Scholar]
- Mercurio, A.; Adriani, G.; Catalano, A.; Carocci, A.; Rao, L.; Lentini, G.; Cavalluzzi, M.M.; Franchini, C.; Vacca, A.; Corbo, F. A mini-review on thalidomide: Chemistry, mechanisms of action, therapeutic potential and anti-angiogenic properties in multiple myeloma. Curr. Med. Chem. 2017, 24, 2736–2744. [Google Scholar] [CrossRef]
- Inaki, M.; Sasamura, T.; Matsuno, K. Cell chirality drives left-right asymmetric morphogenesis. Front. Cell Develop. Biol. 2018, 6, 34. [Google Scholar]
- Carrisi, C.; Madeo, M.; Morciano, P.; Dolce, V.; Cenci, G.; Cappello, A.R.; Mazzeo, G.; Iacopetta, D.; Capobianco, L. Identification of the Drosophila melanogaster mitochondrial citrate carrier: Bacterial expression, reconstitution, functional characterization and developmental distribution. J. Biochem. 2008, 144, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Aydin, V.; Bahar, A.; Vizdiklar, C.; Akici, A. The association of chiral characteristic with drug withdrawal due to safety: A comparative analysis. Br. J. Clin. Pharmacol. 2022, in press. [CrossRef] [PubMed]
- Barreiro, J.C.; Tiritan, M.E.; Cass, Q.B. Challenges and innovations in chiral drugs in an environmental and bioanalysis perspective. Trends Anal. Chem. 2021, 142, 116326. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug resistance (MDR): A widespread phenomenon in pharmacological therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef]
- Barton, A.K.; McGowan, M.; Smyth, A.; Wright, G.A.; Gardner, R.S. Classification and choice of antiarrhythmic therapies. Prescriber 2020, 31, 11–17. [Google Scholar] [CrossRef]
- Catalano, A.; Carocci, A. Antiarrhythmic mexiletine: A review on synthetic routes to racemic and homochiral mexiletine and its enantioseparation. Curr. Med. Chem. 2016, 23, 3227–3244. [Google Scholar] [CrossRef]
- De Luca, A.; Talon, S.; de Bellis, M.; Desaphy, J.-F.; Franchini, C.; Lentini, G.; Catalano, A.; Corbo, F.; Tortorella, V.; Conte-Camerino, D. Inhibition of skeletal muscle sodium currents by mexiletine analogues: Specific hydrophobic interactions rather than lipophilia per se account for drug therapeutic profile. Naunyn Schmiedeberg’s Arch. Pharmacol. 2003, 367, 318–327. [Google Scholar] [CrossRef]
- Catalano, A.; Desaphy, J.F.; Lentini, G.; Carocci, A.; Di Mola, A.; Bruno, C.; Carbonara, R.; de Palma, A.; Budriesi, R.; Ghelardini, C.; et al. Synthesis and toxicopharmacological evaluation of m-hydroxymexiletine, the first metabolite of mexiletine more potent than the parent compound on voltage-gated sodium channels. J. Med. Chem. 2012, 55, 1418–1422. [Google Scholar] [CrossRef]
- Catalano, A.; Carocci, A.; Sinicropi, M.S. Mexiletine metabolites: A review. Curr. Med. Chem. 2015, 22, 1400–1413. [Google Scholar] [CrossRef]
- De Bellis, M.; De Luca, A.; Rana, F.; Cavalluzzi, M.M.; Catalano, A.; Lentini, G.; Franchini, C.; Tortorella, V.; Conte Camerino, D. Evaluation of the pharmacological activity of the major mexiletine metabolites on skeletal muscle sodium currents. Br. J. Pharmacol. 2006, 149, 300–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraglia, M.; de Bellis, M.; Catalano, A.; Carocci, A.; Franchini, C.; Carrieri, A.; Fortugno, C.; Bertucci, C.; Desaphy, J.F.; De Luca, A.; et al. N-aryl-2,6-dimethylbenzamides, a new generation of tocainide analogues as blockers of skeletal muscle voltage-gated sodium channels. J. Med. Chem. 2014, 57, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Carocci, A.; Corbo, F.; Franchini, C.; Muraglia, M.; Scilimati, A.; de Bellis, M.; De Luca, A.; Conte Camerino, D.; Sinicropi, M.S.; et al. Constrained analogues of tocainide as potent skeletal muscle sodium channel blockers towards the development of antimyotonic agents. Eur. J. Med. Chem. 2008, 43, 2535–2540. [Google Scholar] [CrossRef] [PubMed]
- Osei, K.; Taskesen, T.; Goerbig-Campbell, J.; Hounshell, T. Ivabradine toxicity: A case report and review. HeartRhythm Case Rep. 2020, 6, 183–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency (EMEA). Annual Report of the European Medicines Agency 2005; EMEA/MB/63019/2006; European Medicines Agency: London, UK, 2005. [Google Scholar]
- Liu, X.; Liu, Y.; He, H.; Cai, Z.; Yang, Y. New synthetic route to (1S)-4, 5-dimethoxy-1-[(methylamino) methyl] benzocyclobutane, a key intermediate of ivabradine. Synth. Commun. 2014, 44, 451–456. [Google Scholar] [CrossRef]
- Amariei, G.; Jiménez-Jiménez, S.; García, M.Á.; Marina, M.L.; Boltes, K. First eco-toxicological evidence of ivabradine effect on the marine bacterium Vibrio fischeri: A chiral view. Sci. Tot. Environ. 2022, 838, 156617. [Google Scholar] [CrossRef]
- Ondetti, M.A.; Rubin, B.; Cushman, D.W. Design of specific inhibitors of angiotensin-converting enzyme: New class of orally active antihypertensive agents. Science 1977, 196, 441–444. [Google Scholar] [CrossRef]
- Zhan, L.; Wang, X.; Zhang, Y.; Zhu, G.; Ding, Y.; Chen, X.; Jiang, W.; Wu, S. Benazepril hydrochloride protects against doxorubicin cardiotoxicity by regulating the PI3K/Akt pathway. Exp. Ther. Med. 2021, 22, 1082. [Google Scholar] [CrossRef]
- Domínguez, F.; Lalaguna, L.; López-Olañeta, M.; Villalba-Orero, M.; Padrón-Barthe, L.; Román, M.; Bello-Arroyo, E.; Briceño, A.; Gonzalez-Lopez, E.; Segovia-Cubero, J.; et al. Early preventive treatment with enalapril improves cardiac function and delays mortality in mice with arrhythmogenic right ventricular cardiomyopathy type 5. Circ. Heart Fail. 2021, 14, e007616. [Google Scholar] [CrossRef]
- Cirilli, R.; La Torre, F. Stereoselective analysis of benazepril and its stereoisomers by reversed-phase high-performance liquid chromatography on a chiral AGP column. J. Chromatog. A 1998, 818, 53–60. [Google Scholar] [CrossRef]
- Stefan, R.I.; Aboul-Enein, H.Y.; Radu, G.L. Biosensors for the enantioselective analysis of S-enalapril and S-ramipril. Prep. Biochem. Biotechnol. 1998, 28, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, H.A.; Attique, S.A.; Yan, W.; Arooj, A.; Albulym, O.; Zhu, D.; Bilal, M.; Nawaz, M.Z. Repurposing the inhibitors of COVID-19 key proteins through molecular docking approach. Proc. Biochem. 2021, 110, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Ladhari, A.; La Mura, G.; Di Marino, C.; Di Fabio, G.; Zarrelli, A. Sartans: What they are for, how they degrade, where they are found and how they transform. Sustain. Chem. Pharm. 2021, 20, 100409. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Sohrabi, M.R.; Motiee, F.; Davallo, M. L and D enantiomer binary mixture determination simultaneously by spectrophotometric method without separation step based on artificial neural network and least squares support vector machine in valsartan pharmaceutical production. Optik 2021, 247, 168011. [Google Scholar] [CrossRef]
- Bayes-Genis, A. Neprilysin inhibitors. In Encyclopedia of Molecular Pharmacology; Offermanns, S., Rosenthal, W., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Monteil, T.; Danvy, D.; Sihel, M.; Leroux, R.; Plaquevent, J.C. Strategies for access to enantiomerically pure ecadotril, dexecadotril and fasidotril: A review. Mini Rev. Med. Chem. 2002, 2, 209–217. [Google Scholar] [CrossRef]
- Mehvar, R.; Brocks, D.R. Stereospecific pharmacokinetics and pharmacodynamics of beta-adrenergic blockers in humans. J. Pharm. Pharm. Sci. 2001, 4, 185–200. [Google Scholar] [PubMed]
- Diaconu, C.C.; Marcu, D.R.; Bratu, O.G.; Stanescu, A.M.A.; Gheorghe, G.; Hlescu, A.A.; Mischianu, D.L.; Manea, M. Beta-blockers in cardiovascular therapy: A review. J. Mind Med. Sci. 2019, 6, 216–223. [Google Scholar] [CrossRef]
- Liang, Y.; Mak, J.C. Inhaled therapies for asthma and chronic obstructive pulmonary disease. Curr. Pharm. Des. 2021, 27, 1469–1481. [Google Scholar] [CrossRef]
- Yang, A.; Yu, G.; Wu, Y.; Wang, H. Role of β2-adrenergic receptors in chronic obstructive pulmonary disease. Life Sci. 2020, 265, 118864. [Google Scholar] [CrossRef]
- Jacobson, G.A.; Hostrup, M.; Narkowicz, C.K.; Nichols, D.S.; Walters, E.H. Enantioselective disposition of (R,R)-formoterol, (S,S)-formoterol and their respective glucuronides in urine following single inhaled dosing and application to doping control. Drug Test. Anal. 2019, 11, 950–956. [Google Scholar] [CrossRef]
- Garzon-Siatoya, W.T.; Carrillo-Martin, I.; Chiarella, S.E.; Gonzalez-Estrada, A. State-of-the-art beta-adrenoreceptor agonists for the treatment of asthma. Exp. Opin. Pharmacother. 2022, 23, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Aparici, M.; Gavaldà, A.; Ramos, I.; Carcasona, C.; Otal, R.; Fernández-Blanco, J.A.; Montero, J.L.; García, V.M.; López, R.; de Alba, J.; et al. In vitro and in vivo preclinical profile of abediterol (LAS100977), an inhaled long-acting β2-adrenoceptor agonist, compared with indacaterol, olodaterol and vilanterol. Eur. J. Pharmacol. 2016, 770, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Saganuwan, S.A. Chirality of central nervous system (CNS) acting drugs: A formidable therapeutic hurdle against CNS diseases. Centr. Nerv. System Ag. Med. Chem. 2019, 19, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, J.; Lam, S.; Carlton, M.; Boehm, M.A.; Gomez, J.L.; Solís, O.; Sánchez-Soto, M.; Morris, P.J.; Fredriksson, I.; Thomas, C.J.; et al. Pharmacological and behavioral divergence of ketamine enantiomers: Implications for abuse liability. Mol. Psychiatry 2021, 26, 6704–6722. [Google Scholar] [CrossRef] [PubMed]
- Cristea, I.A.; Naudet, F. US Food and Drug Administration approval of esketamine and brexanolone. Lancet Psych. 2019, 6, 975–977. [Google Scholar] [CrossRef]
- Popik, P.; Khoo, S.Y.S.; Kuziak, A.; Golebiowska, J.; Potasiewicz, A.; Hogendorf, A.; Popik, O.; Matloka, M.; Moszczynski, R.; Nikiforuk, A.; et al. Distinct cognitive and discriminative stimulus effects of ketamine enantiomers in rats. Pharmacol. Biochem. Behav. 2020, 197, 173011. [Google Scholar] [CrossRef]
- Rodrigues-Amorim, D.; Olivares, J.M.; Spuch, C.; Rivera-Baltanás, T. A systematic review of efficacy, safety, and tolerability of duloxetine. Front. Psych. 2020, 11, 554899. [Google Scholar] [CrossRef]
- Vyas, R.; Bhushan, R.; Nagar, H.; Sharma, A. RP-HPLC Enantioseparation and control of enantiomeric purity of duloxetine using a new chiral reagent and recovery of enantiomers. Biomed. Chromatog. 2021, 35, e5228. [Google Scholar] [CrossRef]
- Khoodoruth, M.A.S.; Ouanes, S.; Khan, Y.S. A systematic review of the use of atomoxetine for management of comorbid anxiety disorders in children and adolescents with attention-deficit hyperactivity disorder. Res. Develop. Disabil. 2022, 128, 104275. [Google Scholar] [CrossRef]
- Messineo, L.; Taranto-Montemurro, L.; Calianese, N.; Gell, L.K.; Azarbarzin, A.; Labarca, G.; Vena, D.; Yang, H.C.; Wang, T.Y.; Wellman, A.; et al. Atomoxetine and fesoterodine combination improves obstructive sleep apnoea severity in patients with milder upper airway collapsibility. Respirology 2022, 27, 975–982. [Google Scholar] [CrossRef]
- Abram, M.; Jakubiec, M.; Kaminski, K. Chirality as an important factor for the development of new antiepileptic drugs. ChemMedChem 2019, 14, 1744–1761. [Google Scholar] [CrossRef] [PubMed]
- Bialer, M. Chemical properties of antiepileptic drugs (AEDs). Adv. Drug Deliv. Rev. 2012, 64, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Barel, S.; Yagen, B.; Schurig, V.; Soback, S.; Pisani, F.; Perucca, E.; Bialer, M. Stereoselective pharmacokinetic analysis of valnoctamide in healthy subjects and in patients with epilepsy. Clin. Pharmacol. Ther. 1997, 61, 442–449. [Google Scholar] [CrossRef]
- Kaufmann, D.; Yagen, B.; Minert, A.; Wlodarczyk, B.; Finnell, R.H.; Schurig, V.; Devor, M.; Bialer, M. Evaluation of the antiallodynic, teratogenic and pharmacokinetic profile of stereoisomers of valnoctamide, an amide derivative of a chiral isomer of valproic acid. Neuropharmacology 2010, 52, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Čižmáriková, R.; Čižmárik, J.; Valentová, J.; Habala, L.; Markuliak, M. Chiral aspects of local anesthetics. Molecules 2020, 25, 2738. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.H.; Markham, A. Levobupivacaine. Drugs 2000, 59, 551–579. [Google Scholar] [CrossRef]
- Catalano, A. The 2,6-xylyl moiety as a privileged scaffold of pharmaceutical significance. Curr. Med. Chem. 2022, 29, 3984–3990. [Google Scholar] [CrossRef]
- Ekatodramis, G.; Borgeat, A. The enantiomers: Revolution or evolution. Curr. Top. Med. Chem. 2001, 1, 205–206. [Google Scholar] [CrossRef]
- Hao, H.; Wang, G.; Sun, J. Enantioselective pharmacokinetics of ibuprofen and involved mechanisms. Drug Metab. Rev. 2005, 37, 215–234. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Saturnino, C.; Sinicropi, M.S. A comprehensive review on pyranoindole-containing agents. Curr. Med. Chem. 2022, 29, 3667–3683. [Google Scholar] [CrossRef]
- Roberts, J.S.; Ma, C.; Robertson, S.Y.; Kang, S.; Han, C.S.; Deng, S.X.; Zheng, J.J. R-etodolac is a more potent Wnt signaling inhibitor than enantiomer, S-etodolac. Biochem. Biophys. Rep. 2022, 30, 101231. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, S.M.R.; Farhadi, T.; Ganjparvar, M. Linezolid: A review of its properties, function, and use in critical care. Drug Des. Develop. Ther. 2018, 12, 1759. [Google Scholar] [CrossRef]
- Pozzi, C.; Ferrari, S.; Cortesi, D.; Luciani, R.; Stroud, R.M.; Catalano, A.; Costi, M.P.; Mangani, S. The structure of Enterococcus faecalis thymidylate synthase provides clues about folate bacterial metabolism. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Jones, R.N. Oxazolidinone antibiotics. Lancet 2001, 358, 1975–1982. [Google Scholar] [CrossRef]
- Parisi, O.I.; Fiorillo, M.; Caruso, A.; Cappello, A.R.; Saturnino, C.; Puoci, F.; Panno, A.; Dolce, V.; El-Kashef, H.; Sinicropi, M.S. Enhanced cellular uptake by “pharmaceutically oriented devices” of new simplified analogs of Linezolid with antimicrobial activity. Int. J. Pharm. 2014, 461, 163–170. [Google Scholar] [CrossRef]
- Iqbal, K.; Milioudi, A.; Wicha, S.G. Pharmacokinetics and pharmacodynamics of tedizolid. Clin. Pharmacokinet. 2022, 61, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat Ashlesha, B.; Khedkar, K.M. Impurity Profiling: A Review. Asian J. Pharm. Res. Develop. 2022, 10, 135–143. [Google Scholar]
- Elshafie, H.S.; Sadeek, S.A.; Camele, I.; Mohamed, A.A. Biochemical characterization of new gemifloxacin Schiff base (GMFX-o-phdn) metal complexes and evaluation of their antimicrobial activity against some phyto-or human pathogens. Int. J. Mol. Sci. 2022, 23, 2110. [Google Scholar] [CrossRef]
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A review on the antimicrobial activity of Schiff bases: Data collection and recent studies. Antibiotics 2022, 11, 191. [Google Scholar] [CrossRef]
- Wei, Z.X.; Tang, T.T.; Jiang, S.P. The antiviral mechanisms, effects, safety and adverse effects of chloroquine. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7164–7172. [Google Scholar]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Pellegrino, M.; Mariconda, A.; Longo, P.; Sinicropi, M.S.; Aquaro, S. COVID-19 at a glance: An up-to-date overview on variants, drug design and therapies. Viruses 2022, 14, 573. [Google Scholar] [CrossRef] [PubMed]
- D’Acquarica, I.; Agranat, I. Chiral switches of chloroquine and hydroxychloroquine: Potential drugs to treat COVID-19. Drug Discov. Today 2020, 25, 1121. [Google Scholar] [CrossRef] [PubMed]
- Delany-Moretlwe, S.; Hughes, J.P.; Bock, P.; Ouma, S.G.; Hunidzarira, P.; Kalonji, D.; Kayange, N.; Makhema, J.; Mandima, P.; Mathew, C.; et al. Cabotegravir for the prevention of HIV-1 in women: Results from HPTN 084, a phase 3, randomised clinical trial. Lancet 2022, 399, 1779–1789. [Google Scholar] [CrossRef]
- Götte, M. Remdesivir for the treatment of COVID-19: The value of biochemical studies. Curr. Opin. Virol. 2021, 49, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Sinicropi, M.S.; Baldino, N.; Ceramella, J.; Iacopetta, D.; Scali, E.; Basile, G.; Saturnino, C.; Catalano, A. Opuntia ficus indica (L.) Mill. An Ancient Plant Source of Nutraceuticals. Curr. Top. Med. Chem. 2022, 22, 1736–1749. [Google Scholar]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. In silico investigation on the interaction of chiral phytochemicals from Opuntia ficus-indica with SARS-CoV-2 Mpro. Symmetry 2021, 13, 1041. [Google Scholar] [CrossRef]
- Nehra, A.K.; Alexander, J.A.; Loftus, C.G.; Nehra, V. Proton pump inhibitors: Review of emerging concerns. Mayo Clinic Proc. 2018, 93, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Olbe, L.; Carlsson, E.; Lindberg, P. A proton-pump inhibitor expedition: The case histories of omeprazole and esomeprazole. Nat. Rev. Drug Discov. 2003, 2, 132–139. [Google Scholar] [CrossRef]
- McKeage, K.; Blick, S.K.; Croxtall, J.D.; Lyseng-Williamson, K.A.; Keating, G.M. Esomeprazole. Drugs 2008, 68, 1571–1607. [Google Scholar] [CrossRef]
- Hershcovici, T.; Jha, L.K.; Fass, R. Dexlansoprazole MR—A review. Ann. Med. 2011, 43, 366–374. [Google Scholar] [CrossRef]
- Valentová, J.; Lintnerová, L. Chirality in Anticancer Agents. In Current Topics in Chirality-From Chemistry to Biology; IntechOpen: London, UK, 2021; Chapter 8; ISBN 978-1-83968-954-3. [Google Scholar]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Rui, M.; Siciliano, A.M.; Collina, S. Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorganic Med. Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015, 124, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and naringenin: Their mechanisms of action and the potential anticancer activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef]
- Pinto, C.; Cidade, H.; Pinto, M.; Tiritan, M.E. Chiral flavonoids as antitumor agents. Pharmaceuticals 2021, 14, 1267. [Google Scholar] [CrossRef] [PubMed]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, M.G.; Catalano, A.; Finelli, F.; Giuzio, F.; Iacopetta, D.; Ceramella, J.; Sinicropi, M.S.; Capasso, A.; Saturnino, C. Nutraceutical functions of green tea. Pharmacologyonline 2021, 3, 1156–1166. [Google Scholar]
- Scott, L.J. Larotrectinib: First global approval. Drugs 2019, 79, 201–206. [Google Scholar] [CrossRef]
- Chu, X.; Bu, Y.; Yang, X. Recent Research Progress of Chiral Small Molecular Antitumor-Targeted Drugs Approved by the FDA From 2011 to 2019. Front. Oncol. 2021, 11, 785855. [Google Scholar]
- Lee, A. Niraparib: A review in first-line maintenance therapy in advanced ovarian cancer. Targ. Oncol. 2021, 16, 839–845. [Google Scholar] [CrossRef]
- Sanchez-Borges, M.; Ansotegui, I.J. Second generation antihistamines: An update. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 358–364. [Google Scholar] [CrossRef]
- Wegler, C.; Saleh, A.; Lindqvist, A.; Nordeng, H.; Smeraglia, J.; Baranczewski, P. Simple and rapid quantification of cetirizine, venlafaxine, and O-desmethylvenlafaxine in human breast milk, and metformin in human milk and plasma with UHPLC-MS/MS. J. Chromatog. B 2022, 1205, 123340. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.R.; Hermans, A.N.; Gallacher, D.J. Does terfenadine-induced ventricular tachycardia/fibrillation directly relate to its QT prolongation and Torsades de Pointes? Br. J. Pharmacol. 2012, 166, 1490–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craun, K.L.; Schury, M.P. Fexofenadine. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Meltzer, E.O.; Rosario, N.A.; van Bever, H.; Lucio, L. Fexofenadine: Review of safety, efficacy and unmet needs in children with allergic rhinitis. Allergy Asthma Clin. Immun. 2021, 17, 113. [Google Scholar] [CrossRef]
- Van Eeckhaut, A.; Detaevernier, M.R.; Crommen, J.; Michotte, Y. Differential effects of organic modifiers on the enantioseparation of dimetindene maleate with carboxymethyl-β-cyclodextrin in capillary electrophoresis. J. Sep. Sci. 2004, 27, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Salman, E.K.; Ghoniem, E.K.; Badr, E.S.; Emeran, A.A. The potential of dimetindene maleate inducing resistance to blast fungus Magnaporthe oryzae through activating the salicylic acid signaling pathway in rice plants. Pest Manag. Sci. 2021, 78, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Vashistha, V.K.; Sethi, S.; Tyagi, I.; Das, D.K. Chirality of antidepressive drugs: An overview of stereoselectivity. Asian Biomed. 2022, 16, 55–69. [Google Scholar] [CrossRef]
- Chaubey, N.R.; Ghosh, S.K. An enantiodivergent and formal synthesis of paroxetine enantiomers by asymmetric desymmetrization of 3-(4-fluorophenyl) glutaric anhydride with a chiral SuperQuat oxazolidin-2-one. Tetrahedron Asymm. 2012, 23, 1206–1212. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; de Maio, A.C.; Basile, G.; Giuzio, F.; Bonomo, M.G.; Aquaro, S.; Walsh, T.J.; Sinicropi, M.S.; et al. Are nutraceuticals effective in COVID-19 and post-COVID prevention and treatment? Foods 2022, 11, 2884. [Google Scholar] [CrossRef]
- Catalano, A. COVID-19: Could irisin become the handyman myokine of the 21st century. Coronaviruses 2020, 1, 32–41. [Google Scholar] [CrossRef]
- Otvos, S.B.; Kappe, C.O. Continuous flow asymmetric synthesis of chiral active pharmaceutical ingredients and their advanced intermediates. Green Chem. 2021, 23, 6117–6138. [Google Scholar] [CrossRef]
- Schubart, A.; Anderson, K.; Mainolfi, N.; Sellner, H.; Ehara, T.; Adams, C.M.; Mac Sweeney, A.; Liao, S.-M.; Crowley, M.; Littlewood-Evans, A.; et al. Small-molecule factor B inhibitor for the treatment of complement-mediated diseases. Proc. Natl. Acad. Sci. USA 2019, 116, 7926–7931. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Deng, Y.; Shang, S.; Tang, L.; Li, Q.; Bai, X.; Chen, X. Complement factor B inhibitor LNP023 improves lupus nephritis in MRL/lpr mice. Biomed. Pharmacother. 2022, 153, 113433. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, J.P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug repositioning: A brief overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef] [Green Version]
- De Maio, A.C.; Basile, G.; Iacopetta, D.; Catalano, A.; Ceramella, J.; Cafaro, D.; Saturnino, C.; Sinicropi, M.S. The significant role of nutraceutical compounds in ulcerative colitis treatment. Curr. Med. Chem. 2022, 29, 4216–4234. [Google Scholar]
- Srivastava, R.; Singh, R. Synthesis of Four Heterocyclic Drug Molecules Repurposed for COVID-19. Mini Rev. Org. Chem. 2022, 19, 180–187. [Google Scholar]
- Verma, A.; Vimalesvaran, S.; Lampejo, T.; Deep, A.; Dhawan, A. Use of cidofovir in recent outbreak of adenovirus-associated acute liver failure in children. Lancet Gastroenterol. Hepatol. 2022, 7, 700–702. [Google Scholar] [CrossRef]
- Eberhardt, K.A.; Mischlinger, J.; Jordan, S.; Groger, M.; Günther, S.; Ramharter, M. Ribavirin for the treatment of Lassa fever: A systematic review and meta-analysis. Int. J. Infect. Dis. 2019, 87, 15–20. [Google Scholar] [CrossRef]
- Ajayi, S.; Becker, H.; Reinhardt, H.; Engelhardt, M.; Zeiser, R.; Von Bubnoff, N.; Wasch, R. Ruxolitinib. Recent Results Cancer Res. 2018, 212, 119–132. [Google Scholar]
| Structure | Name | Number of Compounds |
|---|---|---|
| Antiarrhythmic drugs | ||
 | Quinidine (Class IA) | 1 |
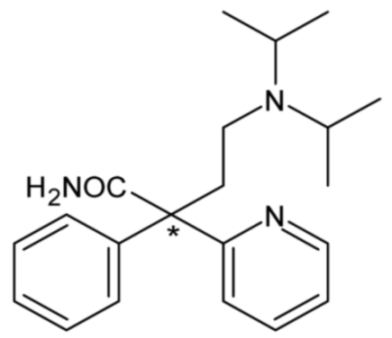 | Disopyramide (Class IA) | 2 |
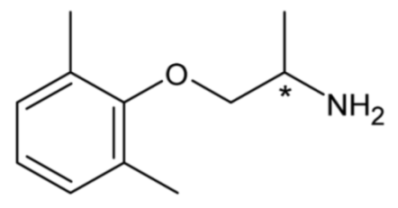 | Mexiletine (Class IB) | 3 |
 | Tocainide (Class IB) | 4 |
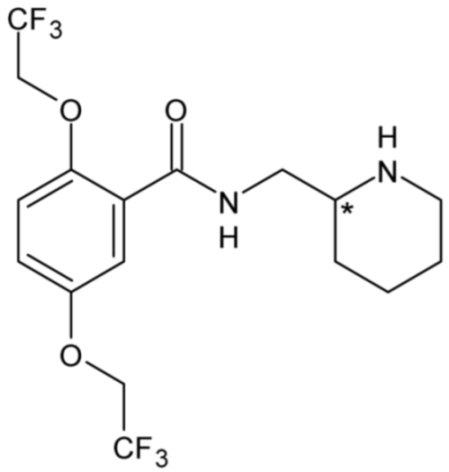 | Flecainide (Class IC) | 5 |
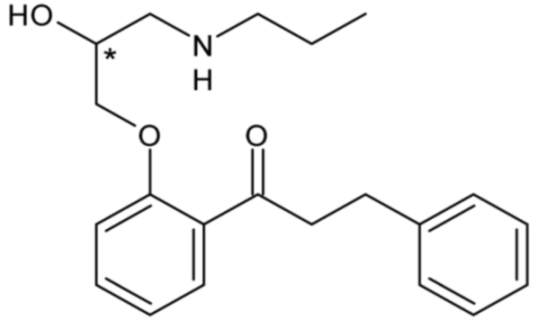 | Propafenone (Class IC) | 6 |
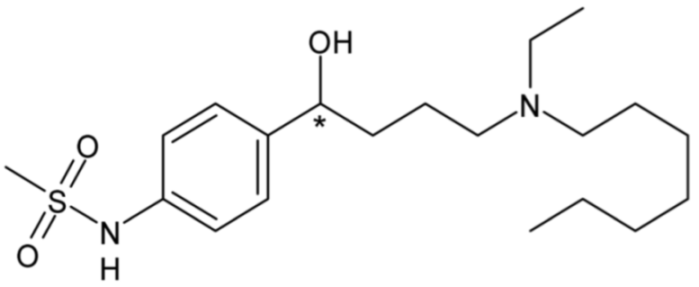 | Ibutilide (Class III) | 7 |
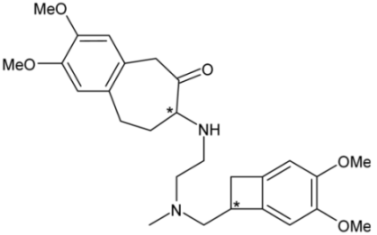 | Ivabradine | 8 |
| Antihypertensive drugs | ||
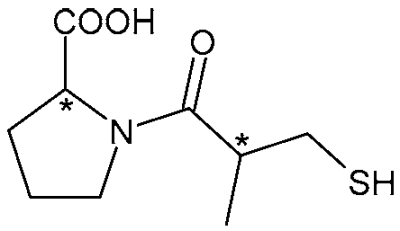 | Captopril (ACE-inhibitor) | 9 |
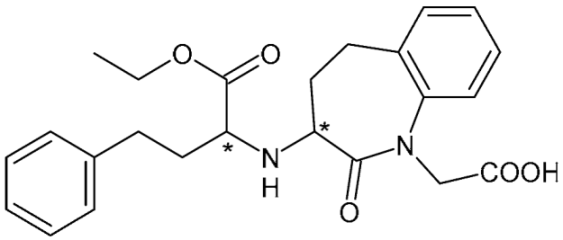 | Benazepril (ACE-inhibitor) | 10 |
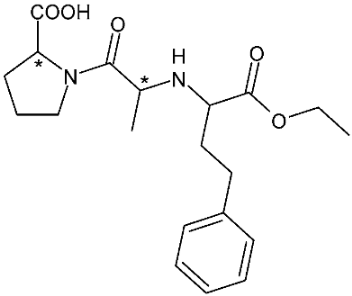 | Enalapril (ACE-inhibitor) | 11 |
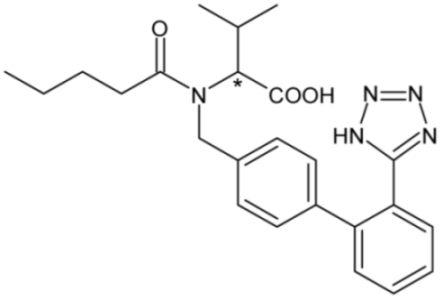 | Valsartan (angiotensin II receptor antagonist) | 12 |
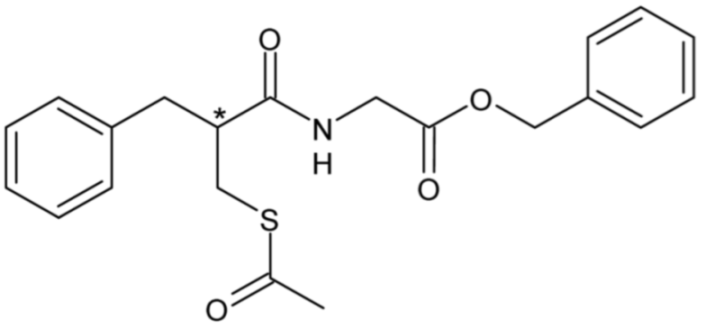 | Ecadotril | 13 |
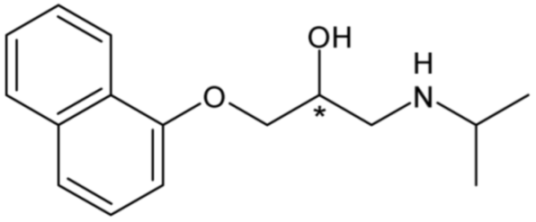 | Propranolol (non-selective β-blocker) | 14 |
 | Bisoprolol (β1-blocker) | 15 |
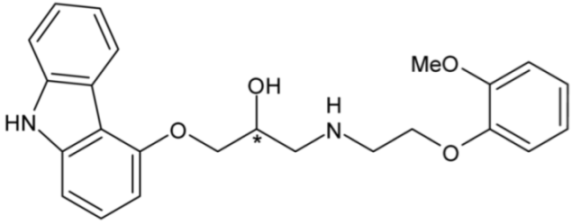 | Carvedilol (non-selective α- and β-blocker) | 16 |
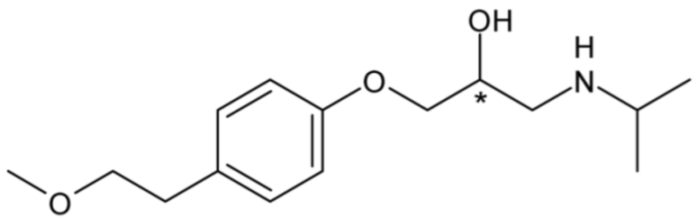 | Metoprolol (β1-blocker) | 17 |
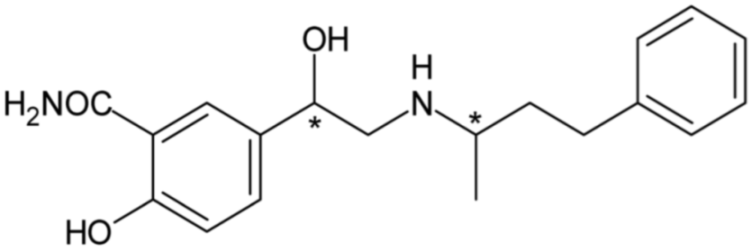 | Labetalol (non-selective α- and β-blocker) | 18 |
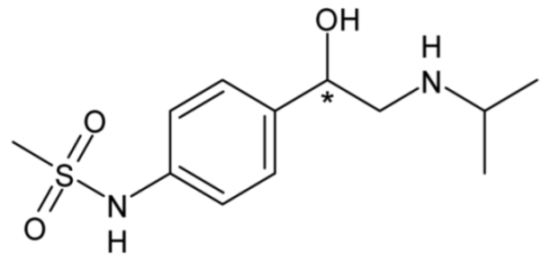 | Sotalol (non-selective β-blocker) | 19 |
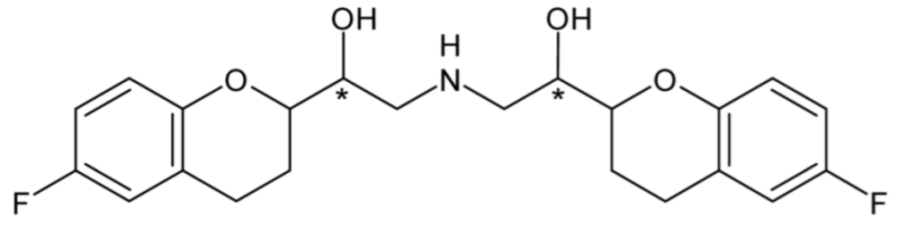 | Nebivolol (β1-blocker) | 20 |
| β2-Agonists (bronchodilators) | ||
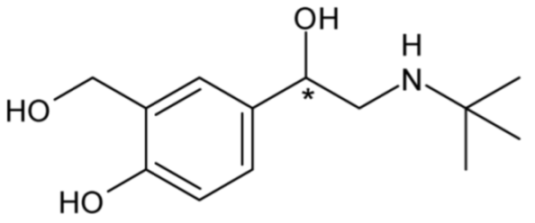 | Salbutamol or albuterol | 21 |
 | Formoterol | 22 |
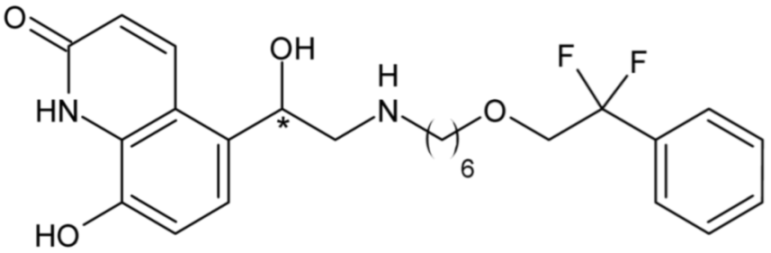 | Abediterol | 23 |
| Drugs acting on CNS | ||
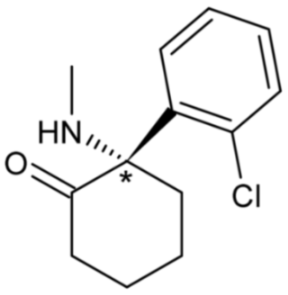 | Esketamine [(S)-isomer of ketamine] | 24 |
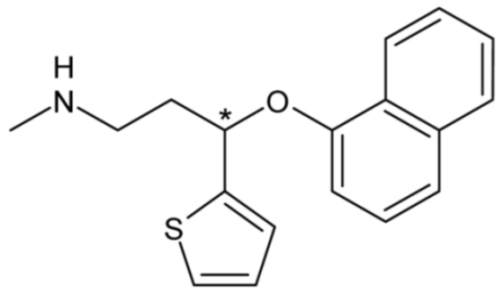 | Duloxetine | 25 |
 | Atomoxetine | 26 |
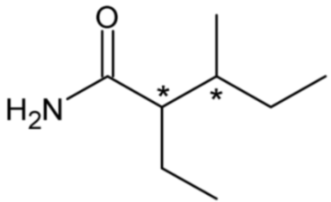 | Valnoctamide | 27 |
| Local anesthetics | ||
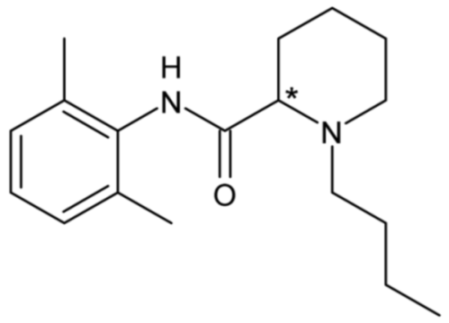 | Bupivacaine | 28 |
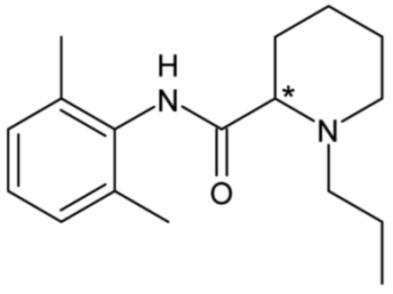 | Ropivacaine | 29 |
| Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) | ||
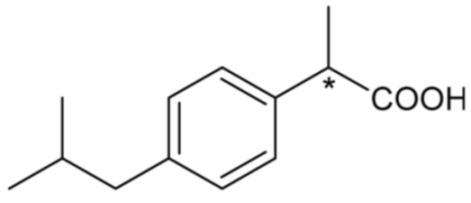 | Ibuprofen | 30 |
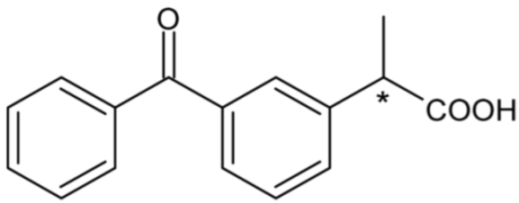 | Ketoprofen | 31 |
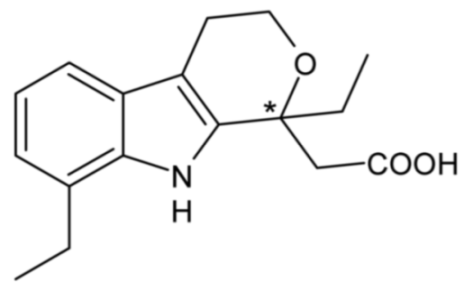 | Etodolac | 32 |
| Antibacterials | ||
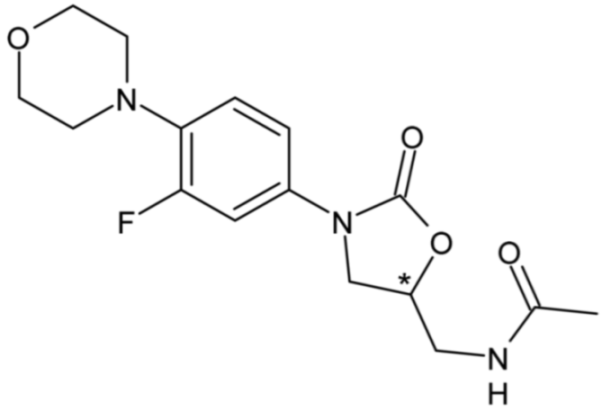 | Linezolid | 33 |
 | Tedizolid | 34 |
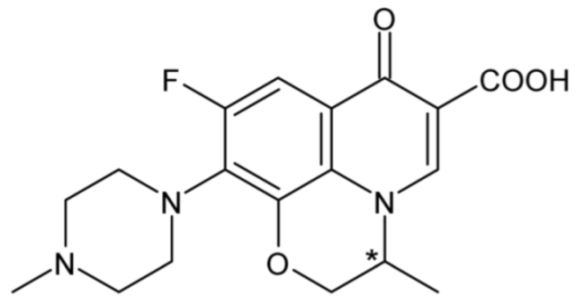 | Ofloxacin | 35 |
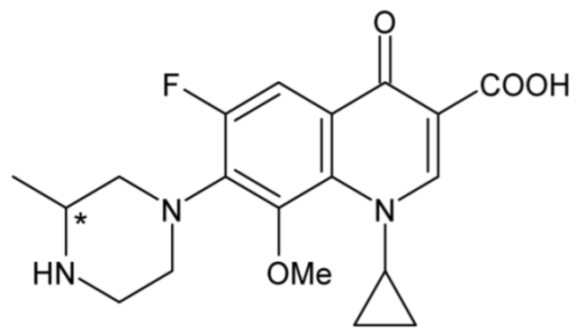 | Gatifloxacin | 36 |
| Antimalarial and antivirals | ||
 | Chloroquine | 37 |
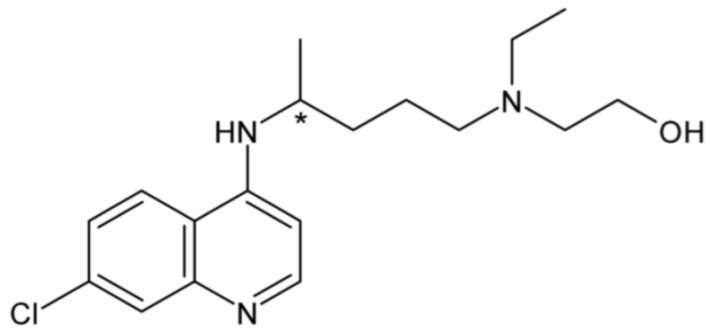 | Hydroxychloroquine | 38 |
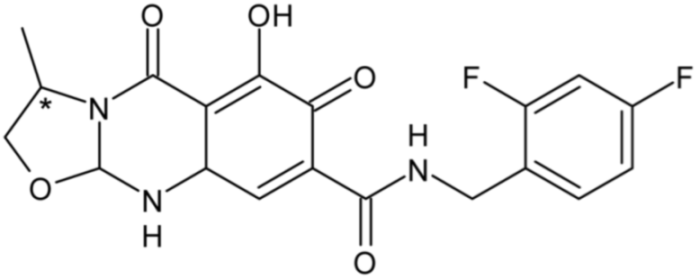 | Cabotegravir | 39 |
| Proton Pump Inhibitors | ||
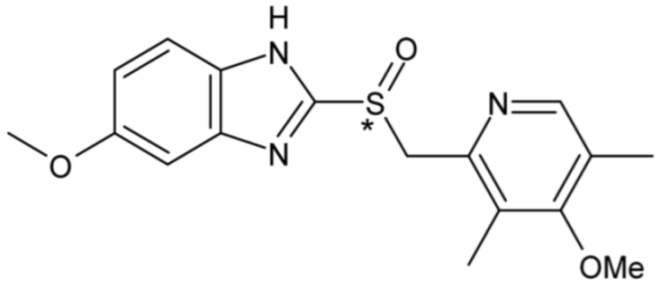 | Omeprazole | 40 |
 | Lansoprazole | 41 |
| Anticancer drugs | ||
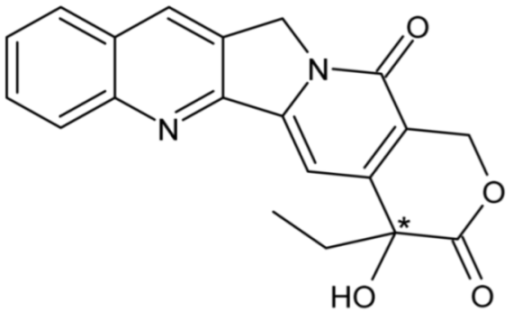 | Camptothecin | 42 |
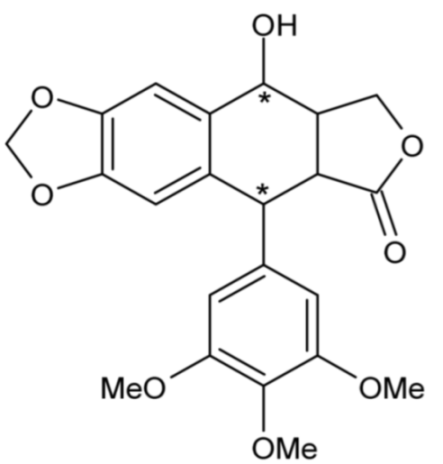 | Epipodophyllotoxin | 43 |
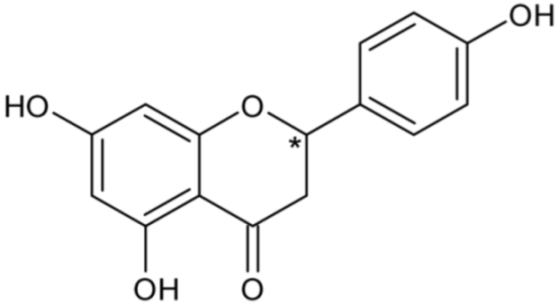 | Naringenin | 44 |
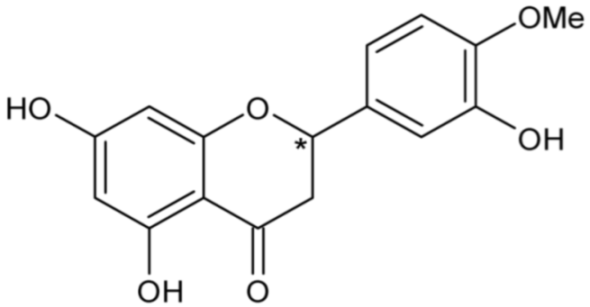 | Hesperetin | 45 |
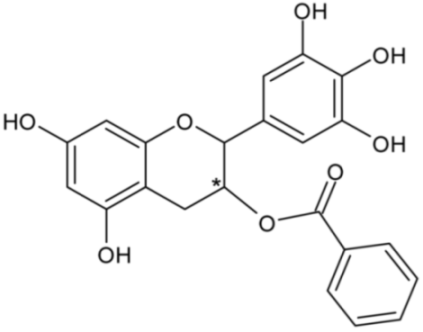 | Epigallocatechin-3-gallate | 46 |
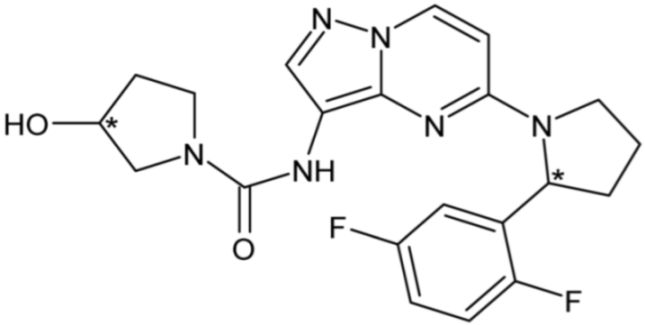 | Larotrectinib (Tropomyosin receptor kinase inhibitor) | 47 |
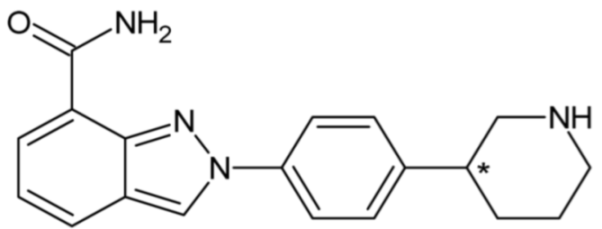 | Niraparib (PARP inhibitor) | 48 |
| Anti-Histamine drugs | ||
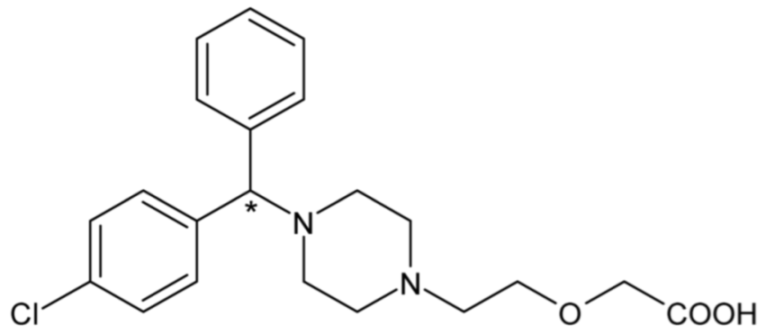 | Cetirizine | 49 |
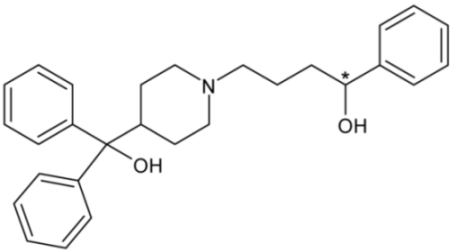 | Terfenadine | 50 |
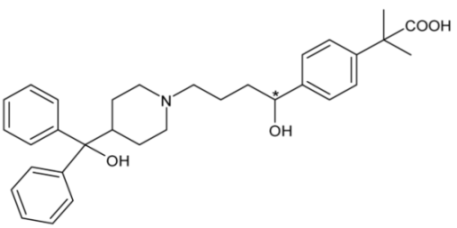 | Fexofenadine | 51 |
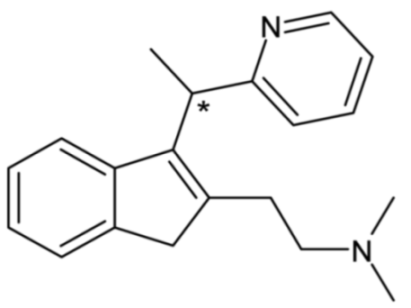 | Dimetindene | 52 |
| Selective Serotonin Reuptake Inhibitors | ||
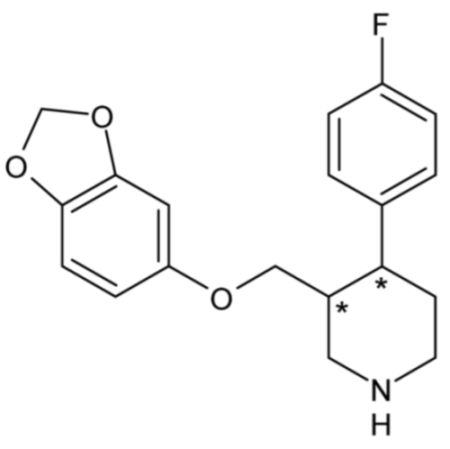 | Paroxetine | 53 |
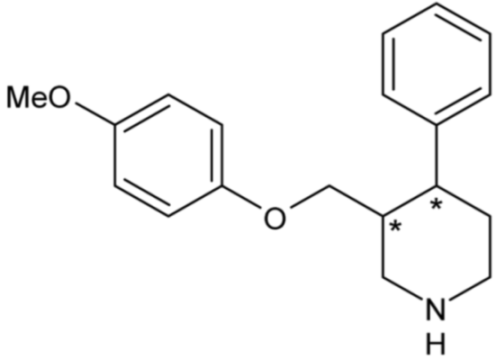 | Femoxetine | 54 |
| Serine Protease Factor B Inhibitors | ||
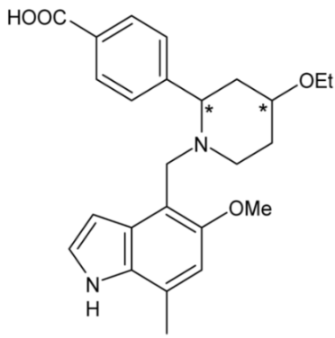 | LNP023 | 55 |
| Repositioned drugs | ||
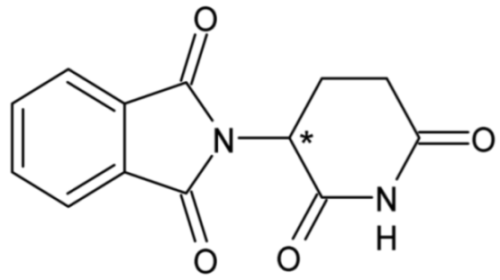 | Thalidomide | 56 |
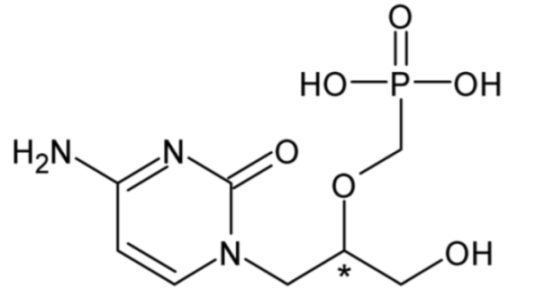 | Cidofovir | 57 |
 | Ribavirin | 58 |
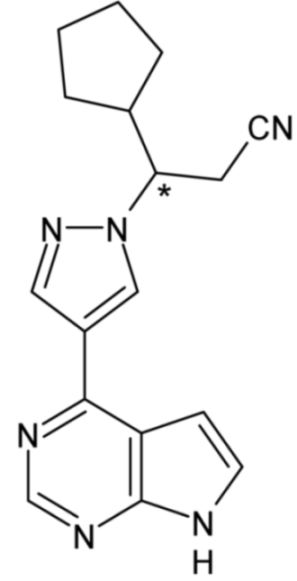 | Ruxolitinib | 59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceramella, J.; Iacopetta, D.; Franchini, A.; De Luca, M.; Saturnino, C.; Andreu, I.; Sinicropi, M.S.; Catalano, A. A Look at the Importance of Chirality in Drug Activity: Some Significative Examples. Appl. Sci. 2022, 12, 10909. https://doi.org/10.3390/app122110909
Ceramella J, Iacopetta D, Franchini A, De Luca M, Saturnino C, Andreu I, Sinicropi MS, Catalano A. A Look at the Importance of Chirality in Drug Activity: Some Significative Examples. Applied Sciences. 2022; 12(21):10909. https://doi.org/10.3390/app122110909
Chicago/Turabian StyleCeramella, Jessica, Domenico Iacopetta, Angelica Franchini, Michele De Luca, Carmela Saturnino, Inmaculada Andreu, Maria Stefania Sinicropi, and Alessia Catalano. 2022. "A Look at the Importance of Chirality in Drug Activity: Some Significative Examples" Applied Sciences 12, no. 21: 10909. https://doi.org/10.3390/app122110909
APA StyleCeramella, J., Iacopetta, D., Franchini, A., De Luca, M., Saturnino, C., Andreu, I., Sinicropi, M. S., & Catalano, A. (2022). A Look at the Importance of Chirality in Drug Activity: Some Significative Examples. Applied Sciences, 12(21), 10909. https://doi.org/10.3390/app122110909










