Multi-Organ Transcriptome Response of Lumpfish (Cyclopterus lumpus) to Aeromonas salmonicida Subspecies salmonicida Systemic Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain, Culture Media, and Reagents
2.2. Bacteria Inoculum Preparation
2.3. Ethics Statement
2.4. Fish Holding
2.5. Lumpfish Infection and Lethal Dose 50 (LD50) Determination
2.6. A. salmonicida Tissue Colonization
2.7. Histopathology
2.8. RNA Purification
2.9. Library Preparation and RNA-Seq
2.10. Reference Transcriptome Assembly and Downstream Analysis
2.11. De Novo Transcriptome Assembly, Contig Abundance, and Functional Annotation
2.12. Gene Filtration and Gene Ontology (GO) Enrichment Analysis
2.13. Real-Time Quantitative Polymerase Chain Reaction (qPCR) Analyses
3. Results
3.1. LD50 Determination and A. salmonicida Infection Kinetics in Lumpfish
3.2. Raw Sequencing Data and Quality Statistics
3.3. Global Profile of Differentially Expressed Genes and Transcripts Identified Using the Lumpfish Reference Genome
3.4. Global Profile of Differentially Expressed Transcripts Identified by De Novo Transcriptome
3.5. Gene Ontology Enrichment Analysis
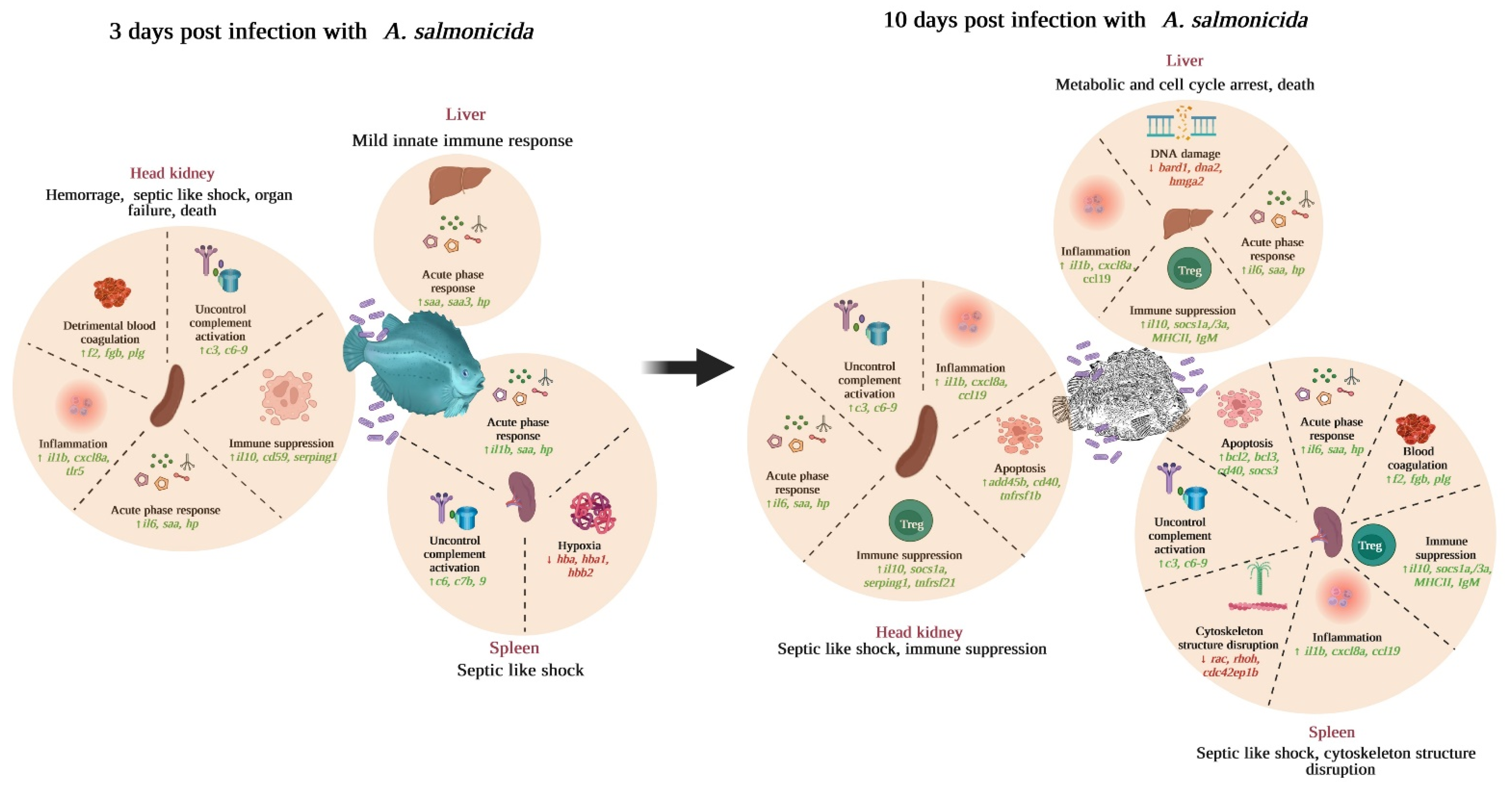
3.6. Analysis of the Most Significant DEGs
3.7. qPCR Verification Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eliasen, K.; Danielsen, E.; Johannesen, Á.; Joensen, L.L.; Patursson, E.J. The cleaning efficacy of lumpfish (Cyclopterus lumpus L.) in Faroese salmon (Salmo salar L.) farming pens in relation to lumpfish size and seasonality. Aquaculture 2018, 488, 61–65. [Google Scholar] [CrossRef]
- Imsland, A.K.D.; Frogg, N.; Stefansson, S.O.; Reynolds, P. Improving sea lice grazing of lumpfish (Cyclopterus lumpus L.) by feeding live feeds prior to transfer to Atlantic salmon (Salmo salar L.) net-pens. Aquaculture 2019, 511, 734224. [Google Scholar] [CrossRef]
- Powell, A.; Treasurer, J.W.; Pooley, C.L.; Keay, A.J.; Lloyd, R.; Imsland, A.K.; Garcia de Leaniz, C. Use of lumpfish for sea-lice control in salmon farming: Challenges and opportunities. Rev. Aquac. 2018, 10, 683–702. [Google Scholar] [CrossRef] [Green Version]
- Brooker, A.J.; Papadopoulou, A.; Gutierrez, C.; Rey, S.; Davie, A.; Migaud, H. Sustainable production and use of cleaner fish for the biological control of sea lice: Recent advances and current challenges. Vet. Reccord 2018, 183, 383. [Google Scholar] [CrossRef] [Green Version]
- Imsland, A.K.; Reynolds, P.; Eliassen, G.; Hangstad, T.A.; Foss, A.; Vikingstad, E.; Elvegård, T.A. The use of lumpfish (Cyclopterus lumpus L.) to control sea lice (Lepeophtheirus salmonis Krøyer) infestations in intensively farmed Atlantic salmon (Salmo salar L.). Aquaculture 2014, 424–425, 18–23. [Google Scholar] [CrossRef]
- Imsland, A.K.D.; Hanssen, A.; Nytro, A.V.; Reynolds, P.; Jonassen, T.M.; Hangstad, T.A.; Elvegard, T.A.; Urskog, T.C.; Mikalsen, B. It works! Lumpfish can significantly lower sea lice infestation in large-scale salmon farming. Biol. Open 2018, 7, bio036301. [Google Scholar] [CrossRef] [Green Version]
- Fish, F.F. Furunculosis in Wild Trout. Copeia 1937, 1937, 37–40. [Google Scholar] [CrossRef]
- Duijn, J.C.V. Taxonomy of the Fish Furunculosis Organism. Nature 1962, 4846, 1127. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clininal Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [Green Version]
- Beaz-Hidalgo, R.; Figueras, M.J. Aeromonas spp. whole genomes and virulence factors implicated in fish disease. J. Fish Dis. 2013, 36, 371–388. [Google Scholar] [CrossRef]
- Gulla, S.; Duodu, S.; Nilsen, A.; Fossen, I.; Colquhoun, D.J. Aeromonas salmonicida infection levels in pre- and post-stocked cleaner fish assessed by culture and an amended qPCR assay. J. Fish Dis. 2016, 39, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Rouleau, F.D.; Vincent, A.T.; Charette, S.J. Genomic and phenotypic characterization of an atypical Aeromonas salmonicida strain isolated from a lumpfish and producing unusual granular structures. J. Fish Dis. 2018, 41, 673–681. [Google Scholar] [CrossRef]
- Lin, B.; Chen, S.; Cao, Z.; Lin, Y.; Mo, D.; Zhang, H.; Gu, J.; Dong, M.; Liu, Z.; Xu, A. Acute phase response in zebrafish upon Aeromonas salmonicida and Staphylococcus aureus infection: Striking similarities and obvious differences with mammals. Mol. Immunol. 2007, 44, 295–301. [Google Scholar] [CrossRef]
- Long, M.; Nielsen, T.K.; Leisner, J.J.; Hansen, L.H.; Shen, Z.X.; Zhang, Q.Q.; Li, A. Aeromonas salmonicida subsp. salmonicida strains isolated from Chinese freshwater fish contain a novel genomic island and possible regional-specific mobile genetic elements profiles. FEMS Microbiol. Lett. 2016, 363, fnw190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewart, K.V.; Belanger, J.C.; Williams, J.; Karakach, T.; Penny, S.; Tsoi, S.C.; Richards, R.C.; Douglas, S.E. Identification of genes differentially expressed in Atlantic salmon (Salmo salar) in response to infection by Aeromonas salmonicida using cDNA microarray technology. Dev. Comp. Immunol. 2005, 29, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Haugland, G.T.; Jakobsen, R.A.; Vestvik, N.; Ulven, K.; Stokka, L.; Wergeland, H.I. Phagocytosis and respiratory burst activity in lumpsucker (Cyclopterus lumpus L.) leucocytes analysed by flow cytometry. PLoS ONE 2012, 7, e47909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronneseth, A.; Ghebretnsae, D.B.; Wergeland, H.I.; Haugland, G.T. Functional characterization of IgM+ B cells and adaptive immunity in lumpfish (Cyclopterus lumpus L.). Dev. Comp. Immunol. 2015, 52, 132–143. [Google Scholar] [CrossRef]
- Patel, D.M.; Brinchmann, M.F. Skin mucus proteins of lumpsucker (Cyclopterus lumpus). Biochem. Biophys. Rep. 2017, 9, 217–225. [Google Scholar] [CrossRef]
- Eggestøl, H.Ø.; Lunde, H.S.; Rønneseth, A.; Fredman, D.; Petersen, K.; Mishra, C.K.; Furmanek, T.; Colquhoun, D.J.; Wergeland, H.I.; Haugland, G.T. Transcriptome-wide mapping of signaling pathways and early immune responses in lumpfish leukocytes upon in vitro bacterial exposure. Sci. Rep. 2018, 8, 5261. [Google Scholar] [CrossRef] [Green Version]
- Gnanagobal, H.; Cao, T.; Hossain, A.; Dang, M.; Hall, J.R.; Kumar, S.; Van Cuong, D.; Boyce, D.; Santander, J. Lumpfish (Cyclopterus lumpus) is susceptible to Renibacterium salmoninarum infection and induces cell-mediated immunity in the chronic stage. Front. Immunol. 2021, 4647. [Google Scholar] [CrossRef]
- Ronneseth, A.; Haugland, G.T.; Colquhoun, D.J.; Brudal, E.; Wergeland, H.I. Protection and antibody reactivity following vaccination of lumpfish (Cyclopterus lumpus L.) against atypical Aeromonas salmonicida. Fish. Shellfish Immunol. 2017, 64, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Erkinharju, T.; Lundberg, M.R.; Isdal, E.; Hordvik, I.; Dalmo, R.A.; Seternes, T. Studies on the antibody response and side effects after intramuscular and intraperitoneal injection of Atlantic lumpfish (Cyclopterus lumpus L.) with different oil-based vaccines. J. Fish Dis. 2017, 40, 1805–1813. [Google Scholar] [CrossRef] [Green Version]
- Dang, M.; Cao, T.; Vasquez, I.; Hossain, A.; Gnanagobal, H.; Kumar, S.; Hall, J.R.; Monk, J.; Boyce, D.; Westcott, J.; et al. Oral immunization of larvae and juvenile of lumpfish (Cyclopterus lumpus) against Vibrio anguillarum does not influence systemic immunity. Vaccines 2021, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Zapata, A.; Diez, B.; Cejalvo, T.; Gutiérrez-de Frías, C.; Cortés, A. Ontogeny of the immune system of fish. Fish. Shellfish Immunol. 2006, 20, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Crispe, I.N. The liver as a lymphoid organ. Annu. Rev. Immunol. 2009, 27, 147–163. [Google Scholar] [CrossRef]
- Eckert, C.; Klein, N.; Kornek, M.; Lukacs-Kornek, V. The complex myeloid network of the liver with diverse functional capacity at steady state and in inflammation. Front. Immunol. 2015, 6, 179. [Google Scholar] [CrossRef] [Green Version]
- Thomson, A.W.; Knolle, P.A. Antigen-presenting cell function in the tolerogenic liver environment. Nat. Rev. Immunol. 2010, 10, 753–766. [Google Scholar] [CrossRef]
- González-Silvera, D.; Cuesta, A.; Esteban, M.Á. Immune defence mechanisms presented in liver homogenates and bile of gilthead seabream (Sparus aurata). J. Fish. Biol. 2021, 99, 1958–1967. [Google Scholar] [CrossRef]
- Frey, J.; Origgi, F.C. Type III secretion system of Aeromonas salmonicida undermining the host’s immune response. Front. Mar. Sci. 2016, 3, 130. [Google Scholar] [CrossRef]
- Valderrama, K.; Saravia, M.; Santander, J. Phenotype of Aeromonas salmonicida sp. salmonicida cyclic adenosine 3’,5’-monophosphate receptor protein (Crp) mutants and its virulence in rainbow trout (Oncorhynchus mykiss). J. Fish Dis. 2017, 40, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Soto-Dávila, M.; Hossain, A.; Chakraborty, S.; Rise, M.L.; Santander, J. Aeromonas salmonicida subsp. salmonicida early infection and immune response of Atlantic cod (Gadus morhua L.) primary macrophages. Front. Immunol. 2019, 10, 1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto-Dávila, M.; Valderrama, K.; Inkpen, S.M.; Hall, J.R.; Rise, M.L.; Santander, J. Effects of vitamin D2 (Ergocalciferol) and D3 (Cholecalciferol) on Atlantic salmon (Salmo salar) primary macrophage immune response to Aeromonas salmonicida subsp. salmonicida infection. Front. Immunol. 2020, 10, 3011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasquez, I.; Hossain, A.; Gnanagobal, H.; Valderrama, K.; Campbell, B.; Ness, M.; Charette, S.J.; Gamperl, A.K.; Cipriano, R.; Segovia, C.; et al. Comparative Genomics of Typical and Atypical Aeromonas salmonicida Complete Genomes Revealed New Insights into Pathogenesis Evolution. Microorganisms 2022, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Leboffe, M.J.; Pierce, B.E. Microbiology: Laboratory Theory & Application, 4th ed.; Morton Publishing: Englewood, CO, USA, 2015; p. 896. [Google Scholar]
- Sambrook, J.; Russell, W. Molecular Cloning, A Laboratory Manual; Cold Spring Harbor Press.: Long Island, NY, USA, 2001. [Google Scholar]
- Chakraborty, S.; Woldemariam, N.T.; Visnovska, T.; Rise, M.L.; Boyce, D.; Santander, J.; Andreassen, R. Characterization of miRNAs in Embryonic, Larval, and Adult Lumpfish Provides a Reference miRNAome for Cyclopterus lumpus. Biology 2022, 11, 130. [Google Scholar] [CrossRef]
- Vasquez, I.; Cao, T.; Hossain, A.; Valderrama, K.; Gnanagobal, H.; Dang, M.; Leeuwis, R.H.J.; Ness, M.; Campbell, B.; Gendron, R.; et al. Aeromonas salmonicida infection kinetics and protective immune response to vaccination in sablefish (Anoplopoma fimbria). Fish. Shellfish Immunol. 2020, 104, 557–566. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Ahmed, M. Acute Toxicity (Lethal Dose 50 Calculation) of Herbal Drug Somina in Rats and Mice. Pharmacol. Pharm. 2015, 06, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Chandler, D.; Roberson, R.W. Bioimaging: Current Concepts in Light and Electron. Microscopy; Jones and Bartlett Publishers: Sudbury, MA, USA, 2009; p. 440. [Google Scholar]
- Bibert, S.; Guex, N.; Lourenco, J.; Brahier, T.; Papadimitriou-Olivgeris, M.; Damonti, L.; Manuel, O.; Liechti, R.; Götz, L.; Tschopp, J.; et al. Transcriptomic signature differences between SARS-CoV-2 and Influenza Virus infected patients. Front. Immunol. 2021, 12, 666163. [Google Scholar] [CrossRef]
- Jia, Z.; Wu, N.; Jiang, X.; Li, H.; Sun, J.; Shi, M.; Li, C.; Ge, Y.; Hu, X.; Ye, W.; et al. Integrative transcriptomic analysis reveals the immune mechanism for a CyHV-3-Resistant common carp strain. Front. Immunol. 2021, 12, 687151. [Google Scholar] [CrossRef]
- Prokop, J.W.; Hartog, N.L.; Chesla, D.; Faber, W.; Love, C.P.; Karam, R.; Abualkheir, N.; Feldmann, B.; Teng, L.; McBride, T.; et al. High-density blood transcriptomics reveals precision immune signatures of SARS-CoV-2 infection in hospitalized individuals. Front. Immunol. 2021, 12, 2844. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.; Kim, N.; Lee, Y.; Park, J.; Kim, B.S.; Lee, M.K.; Park, C.-I.; Kim, D.-H. Dual-organ transcriptomic analysis of rainbow trout infected with Ichthyophthirius multifiliis through co-expression and machine learning. Front. Immunol. 2021, 12, 677730. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Kumar, S.; Navaneethaiyer, U.; Caballero-Solares, A.; Carvalho, L.A.; Whyte, S.K.; Purcell, S.L.; Gagne, N.; Hori, T.S.; Allen, M.; et al. Transcriptome analysis of Atlantic salmon (Salmo salar) skin in response to sea lice and infectious Salmon Anemia Virus co-infection under different experimental functional diets. Front. Immunol. 2022, 12, 787033. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Gihon, I.; Israeli, O.; Tidhar, A.; Sapoznikov, A.; Evgy, Y.; Stein, D.; Aftalion, M.; Gur, D.; Orr, I.; Zvi, A.; et al. Transcriptome analysis of lungs in a mouse model of severe COVID-19. Front. Virol. 2022, 2, 848465. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Bioinformatics, Babraham Institute: Cambridge, UK, 2010. [Google Scholar]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Ruotti, V.; Stewart, R.M.; Thomson, J.A.; Dewey, C.N. RNA-Seq gene expression estimation with read mapping uncertainty. Bioinformatics 2009, 26, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Teng, M.; Love, M.I.; Davis, C.A.; Djebali, S.; Dobin, A.; Graveley, B.R.; Li, S.; Mason, C.E.; Olson, S.; Pervouchine, D.; et al. A benchmark for RNA-seq quantification pipelines. Genome Biol. 2016, 17, 74. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.B.; Wallroth, M.; Jonsson, V.; Kristiansson, E. Comparison of normalization methods for the analysis of metagenomic gene abundance data. BMC Genom. 2018, 19, 274. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. Genome Project Data Processing, S., The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, J.T.; Thorvaldsdottir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef] [Green Version]
- Simao, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Bryant, D.M.; Johnson, K.; DiTommaso, T.; Tickle, T.; Couger, M.B.; Payzin-Dogru, D.; Lee, T.J.; Leigh, N.D.; Kuo, T.-H.; Davis, F.G.; et al. A tissue-mapped axolotl de novo transcriptome enables identification of limb regeneration factors. Cell Rep. 2017, 18, 762–776. [Google Scholar] [CrossRef] [Green Version]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef] [Green Version]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2020, 49, D412–D419. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [Green Version]
- Sonnhammer, E.L.; von Heijne, G.; Krogh, A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1998, 6, 175–182. [Google Scholar] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- St-Pierre, J.; Grégoire, J.-C.; Vaillancourt, C. A simple method to assess group difference in RT-qPCR reference gene selection using GeNorm: The case of the placental sex. Sci. Rep. 2017, 7, 16923. [Google Scholar] [CrossRef] [Green Version]
- Soto-Dávila, M.; Chakraborty, S.; Santander, J. Relative expression and validation of Aeromonas salmonicida subsp. salmonicida reference genes during ex vivo and in vivo fish infection. Infect. Genet. Evol. 2022, 103, 105320. [Google Scholar]
- Roberts, A.; Schaeffer, L.; Pachter, L. Updating RNA-Seq analyses after re-annotation. Bioinformatics 2013, 29, 1631–1637. [Google Scholar] [CrossRef] [Green Version]
- Steijger, T.; Abril, J.F.; Engström, P.G.; Kokocinski, F.; Abril, J.F.; Akerman, M.; Alioto, T.; Ambrosini, G.; Antonarakis, S.E.; Behr, J.; et al. Assessment of transcript reconstruction methods for RNA-seq. Nat. Methods 2013, 10, 1177–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjørgen, H.; Koppang, E.O. Anatomy of teleost fish immune structures and organs. Immunogenetics 2021, 73, 53–63. [Google Scholar] [CrossRef]
- Zwollo, P.; Cole, S.; Bromage, E.; Kaattari, S. B cell heterogeneity in the teleost kidney: Evidence for a maturation gradient from anterior to posterior kidney. J. Immunol. 2005, 174, 6608–6616. [Google Scholar] [CrossRef] [Green Version]
- Flajnik, M.F. A cold-blooded view of adaptive immunity. Nat. Rev. Immunol. 2018, 18, 438–453. [Google Scholar] [CrossRef]
- Koppang, E.O.; Fischer, U.; Moore, L.; Tranulis, M.A.; Dijkstra, J.M.; Köllner, B.; Aune, L.; Jirillo, E.; Hordvik, I. Salmonid T cells assemble in the thymus, spleen and in novel interbranchial lymphoid tissue. J. Anat. 2010, 217, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Hong, S. Characterization of Aeromonas salmonicida and A. sobria isolated from cultured salmonid fish in Korea and development of a vaccine against furunculosis. J. Fish Dis. 2020, 43, 609–620. [Google Scholar]
- Bricknell, I.R.; Bowden, T.J.; Bruno, D.W.; MacLachlan, P.; Johnstone, R.; Ellis, A.E. Susceptibility of Atlantic halibut, Hippoglossus hippoglossus (L.) to infection with typical and atypical Aeromonas salmonicida. Aquaculture 1999, 175, 1–13. [Google Scholar] [CrossRef]
- Lin, Q.; Li, J.; Fu, X.; Liu, L.; Liang, H.; Niu, Y.; Huang, C.; Huang, Z.; Mo, Z.; Li, N. Hemorrhagic gill disease of Chinese perch caused by Aeromonas salmonicida subsp. salmonicida in China. Aquaculture 2020, 519, 734775. [Google Scholar] [CrossRef]
- Farto, R.; Milton, D.L.; Bermudez, M.B.; Nieto, T.P. Colonization of turbot tissues by virulent and avirulent Aeromonas salmonicida subsp. salmonicida strains during infection. Dis. Aquat. Org. 2011, 95, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Braden, L.M.; Whyte, S.K.; Brown, A.B.J.; Iderstine, C.V.; Letendre, C.; Groman, D.; Lewis, J.; Purcell, S.L.; Hori, T.; Fast, M.D. Vaccine-induced protection against furunculosis involves pre-emptive priming of humoral immunity in Arctic Charr. Front. Immunol. 2019, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.H.; Everett, J.; Trivedi, U.; Rumbaugh, K.P.; Whiteley, M. Requirements for Pseudomonas aeruginosa acute burn and chronic surgical wound infection. PLoS Genet. 2014, 10, e1004518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Wang, X.; Zhang, X. Using non-uniform read distribution models to improve isoform expression inference in RNA-Seq. Bioinformatics 2011, 27, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Glaus, P.; Honkela, A.; Rattray, M. Identifying differentially expressed transcripts from RNA-seq data with biological variation. Bioinformatics 2012, 28, 1721–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cloonan, N.; Forrest, A.R.; Kolle, G.; Gardiner, B.; Faulkner, G.J.; Brown, M.K.; Taylor, D.F.; Steptoe, A.L.; Wani, S.; Bethel, G. Stem cell transcriptome profiling via massive-scale mRNA sequencing. Nat. Methods 2008, 5, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Marioni, J.C.; Mason, C.E.; Mane, S.M.; Stephens, M.; Gilad, Y. RNA-seq: An assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 2008, 18, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Z.; Zhang, X. Isoform abundance inference provides a more accurate estimation of gene expression levels in RNA-seq. J. Bioinform. Comput. Biol. 2010, 8, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Yi, L.; Pimentel, H.; Bray, N.L.; Pachter, L. Gene-level differential analysis at transcript-level resolution. Genome Biol. 2018, 19, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.A.; Wang, Z. Next-generation transcriptome assembly. Nat. Rev. Genet. 2011, 12, 671–682. [Google Scholar] [CrossRef]
- Kovi, M.R.; Abdelhalim, M.; Kunapareddy, A.; Ergon, Å.; Tronsmo, A.M.; Brurberg, M.B.; Hofgaard, I.S.; Asp, T.; Rognli, O.A. Global transcriptome changes in perennial ryegrass during early infection by pink snow mould. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Ward, J.A.; Ponnala, L.; Weber, C.A. Strategies for transcriptome analysis in non-model plants. Am. J. Bot. 2012, 99, 267–276. [Google Scholar] [CrossRef]
- Lee, S.G.; Na, D.; Park, C. Comparability of reference-based and reference-free transcriptome analysis approaches at the gene expression level. BMC Bioinform. 2021, 22, 310. [Google Scholar] [CrossRef]
- Kovi, M.R.; Amdahl, H.; Alsheikh, M.; Rognli, O.A. De novo and reference transcriptome assembly of transcripts expressed during flowering provide insight into seed setting in tetraploid red clover. Sci. Rep. 2017, 7, 44383. [Google Scholar] [CrossRef]
- Lu, B.; Zeng, Z.; Shi, T. Comparative study of de novo assembly and genome-guided assembly strategies for transcriptome reconstruction based on RNA-Seq. Sci. China. Life Sci. 2013, 56, 143–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijay, N.; Poelstra, J.W.; Künstner, A.; Wolf, J.B. Challenges and strategies in transcriptome assembly and differential gene expression quantification. A comprehensive in silico assessment of RNA-seq experiments. Mol. Ecol. 2013, 22, 620–634. [Google Scholar] [CrossRef]
- Kumar, R.; Joy, K.P.; Singh, S.M. Morpho-histology of head kidney of female catfish Heteropneustes fossilis: Seasonal variations in melano-macrophage centers, melanin contents and effects of lipopolysaccharide and dexamethasone on melanins. Fish. Physiol. Biochem. 2016, 42, 1287–1306. [Google Scholar] [CrossRef]
- Morales-Lange, B.; Agboola, J.O.; Hansen, J.Ø.; Lagos, L.; Øyås, O.; Mercado, L.; Mydland, L.T.; Øverland, M. The Spleen as a Target to Characterize Immunomodulatory Effects of Down-Stream Processed Cyberlindnera jadinii Yeasts in Atlantic salmon exposed to a dietary soybean meal challenge. Front. Immunol. 2021, 12, 708747. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Wang, Q.; Lai, Y.; Zhao, C.; Sun, C.; Chen, Z.; Tao, J.; Huang, Z. Study on immune response of organs of Epinephelus coioides and Carassius auratus after immersion vaccination with inactivated Vibrio harveyi vaccine. Front. Immunol. 2021, 11, 622387. [Google Scholar] [CrossRef]
- Bronte, V.; Pittet, M.J. The spleen in local and systemic regulation of immunity. Immunity 2013, 39, 806–818. [Google Scholar] [CrossRef] [Green Version]
- Rui, L. Energy metabolism in the liver. Comp. Physiol. 2014, 4, 177–197. [Google Scholar]
- Charlie-Silva, I.; Klein, A.; Gomes, J.M.M.; Prado, E.J.R.; Moraes, A.C.; Eto, S.F.; Fernandes, D.C.; Fagliari, J.J.; Junior, J.D.C.; Lima, C.; et al. Acute-phase proteins during inflammatory reaction by bacterial infection: Fish-model. Sci. Rep. 2019, 9, 4776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlar, C.M.; Whitehead, A.S. Serum amyloid A, the major vertebrate acute-phase reactant. Eur. J. Biochem. 1999, 265, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebl, A.; Korytar, T.; Kobis, J.M.; Verleih, M.; Krasnov, A.; Jaros, J.; Kuhn, C.; Kollner, B.; Goldammer, T. Transcriptome profiling reveals insight into distinct immune responses to Aeromonas salmonicida in gill of two rainbow trout strains. Mar. Biotechnol. 2014, 16, 333–348. [Google Scholar] [CrossRef]
- Long, M.; Zhao, J.; Li, T.; Tafalla, C.; Zhang, Q.; Wang, X.; Gong, X.; Shen, Z.; Li, A. Transcriptomic and proteomic analyses of splenic immune mechanisms of rainbow trout (Oncorhynchus mykiss) infected by Aeromonas salmonicida subsp. salmonicida. J. Proteom. 2015, 122, 41–54. [Google Scholar] [CrossRef]
- Stearns-Kurosawa, D.J.; Osuchowski, M.F.; Valentine, C.; Kurosawa, S.; Remick, D.G. The pathogenesis of sepsis. Annu. Rev. Pathol. 2011, 6, 19–48. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, J.M.; Ali, N.A.; Aberegg, S.K.; Abraham, E. Sepsis. Am. J. Med. 2007, 120, 1012–1022. [Google Scholar] [CrossRef]
- Smith, N.C.; Rise, M.L.; Christian, S.L. A Comparison of the innate and adaptive immune systems in cartilaginous fish, ray-finned fish, and lobe-finned fish. Front. Immunol. 2019, 10, 2292. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef]
- Amara, U.; Flierl, M.A.; Rittirsch, D.; Klos, A.; Chen, H.; Acker, B.; Brückner, U.B.; Nilsson, B.; Gebhard, F.; Lambris, J.D.; et al. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 2010, 185, 5628–5636. [Google Scholar] [CrossRef] [Green Version]
- Antoniak, S. The coagulation system in host defense. Res. Pract. Thromb. Haemost. 2018, 2, 549–557. [Google Scholar] [CrossRef]
- Semeraro, N.; Ammollo, C.T.; Semeraro, F.; Colucci, M. Sepsis-associated disseminated intravascular coagulation and thromboembolic disease. Mediterr. J. Hematol. Infect. Dis. 2010, 2, e2010024. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Coagulation and sepsis. Thromb. Res. 2017, 149, 38–44. [Google Scholar] [CrossRef]
- Goeijenbier, M.; van Wissen, M.; van de Weg, C.; Jong, E.; Gerdes, V.E.A.; Meijers, J.C.M.; Brandjes, D.P.M.; van Gorp, E.C.M. Review: Viral infections and mechanisms of thrombosis and bleeding. J. Med. Virol. 2012, 84, 1680–1696. [Google Scholar] [CrossRef]
- Lupu, F.; Keshari, R.S.; Lambris, J.D.; Mark Coggeshall, K. Crosstalk between the coagulation and complement systems in sepsis. Thromb. Res. 2014, 133, S28–S31. [Google Scholar] [CrossRef] [Green Version]
- Pierrakos, C.; Vincent, J.-L. Sepsis biomarkers: A review. Crit. Care 2010, 14, R15. [Google Scholar] [CrossRef] [Green Version]
- Fast, M.D.; Tse, B.; Boyd, J.M.; Johnson, S.C. Mutations in the Aeromonas salmonicida subsp. salmonicida type III secretion system affect Atlantic salmon leucocyte activation and downstream immune responses. Fish. Shellfish Immunol. 2009, 27, 721–728. [Google Scholar] [CrossRef]
- Vanden Bergh, P.; Frey, J. Aeromonas salmonicida subsp. salmonicida in the light of its type-three secretion system. Microb. Biotechnol. 2014, 7, 381–400. [Google Scholar]
- Vanden Bergh, P.; Heller, M.; Braga-Lagache, S.; Frey, J. The Aeromonas salmonicida subsp. salmonicida exoproteome: Global analysis, moonlighting proteins and putative antigens for vaccination against furunculosis. Proteome Sci. 2013, 11, 44. [Google Scholar] [CrossRef] [Green Version]
- Vanden Bergh, P.; Heller, M.; Braga-Lagache, S.; Frey, J. The Aeromonas salmonicida subsp. salmonicida exoproteome: Determination of the complete repertoire of Type-Three Secretion System effectors and identification of other virulence factors. Proteome Sci. 2013, 11, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radaev, S.; Zou, Z.; Tolar, P.; Nguyen, K.; Nguyen, A.; Krueger, P.D.; Stutzman, N.; Pierce, S.; Sun, P.D. Structural and functional studies of Ig alpha beta and its assembly with the B cell antigen receptor. Structure 2010, 18, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Sun, L. Tongue Sole CD209: A Pattern-Recognition Receptor that Binds a Broad Range of Microbes and Promotes Phagocytosis. Int. J. Mol. Sci. 2017, 18, 1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallaire-Dufresne, S.; Tanaka, K.H.; Trudel, M.V.; Lafaille, A.; Charette, S.J. Virulence, genomic features, and plasticity of Aeromonas salmonicida subsp. salmonicida, the causative agent of fish furunculosis. Vet. Microbiol. 2014, 169, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.M.; Figueiredo, C.; Seruca, R.; Rasmussen, L.J. Helicobacter pylori infection generates genetic instability in gastric cells. Biochim. Biophys. Acta 2010, 1806, 58–65. [Google Scholar] [CrossRef]
- Rich, T.; Allen, R.L.; Wyllie, A.H. Defying death after DNA damage. Nature 2000, 407, 777–783. [Google Scholar] [CrossRef]
- Zgur-Bertok, D. DNA damage repair and bacterial pathogens. PLoS Pathog. 2013, 9, e1003711. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; van Dissel, D.; Mo, I.; Boysen, P.; Haslene-Hox, H.; Lund, H. Identification of novel biomarkers of inflammation in Atlantic salmon (Salmo salar L.) by a plasma proteomic approach. Dev. Comp. Immunol 2022, 127, 104268. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Generoso, J.S.; Singer, M.; Dal-Pizzol, F. Biomarkers for sepsis: More than just fever and leukocytosis—a narrative review. Crit. Care 2022, 26, 14. [Google Scholar] [CrossRef]
- Schrödl, W.; Büchler, R.; Wendler, S.; Reinhold, P.; Muckova, P.; Reindl, J.; Rhode, H. Acute phase proteins as promising biomarkers: Perspectives and limitations for human and veterinary medicine. Proteomics. Clin. Appl. 2016, 10, 1077–1092. [Google Scholar] [CrossRef]
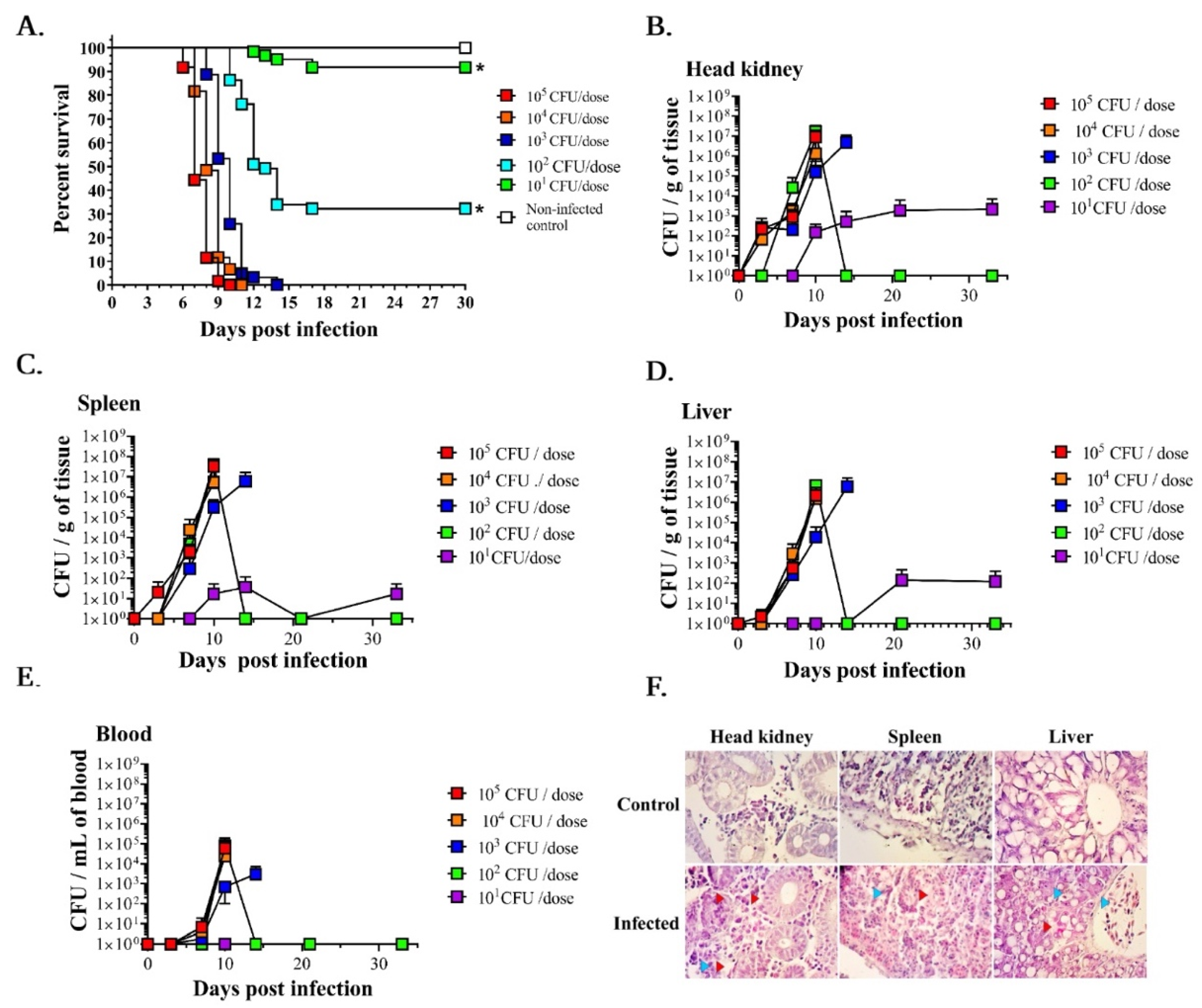
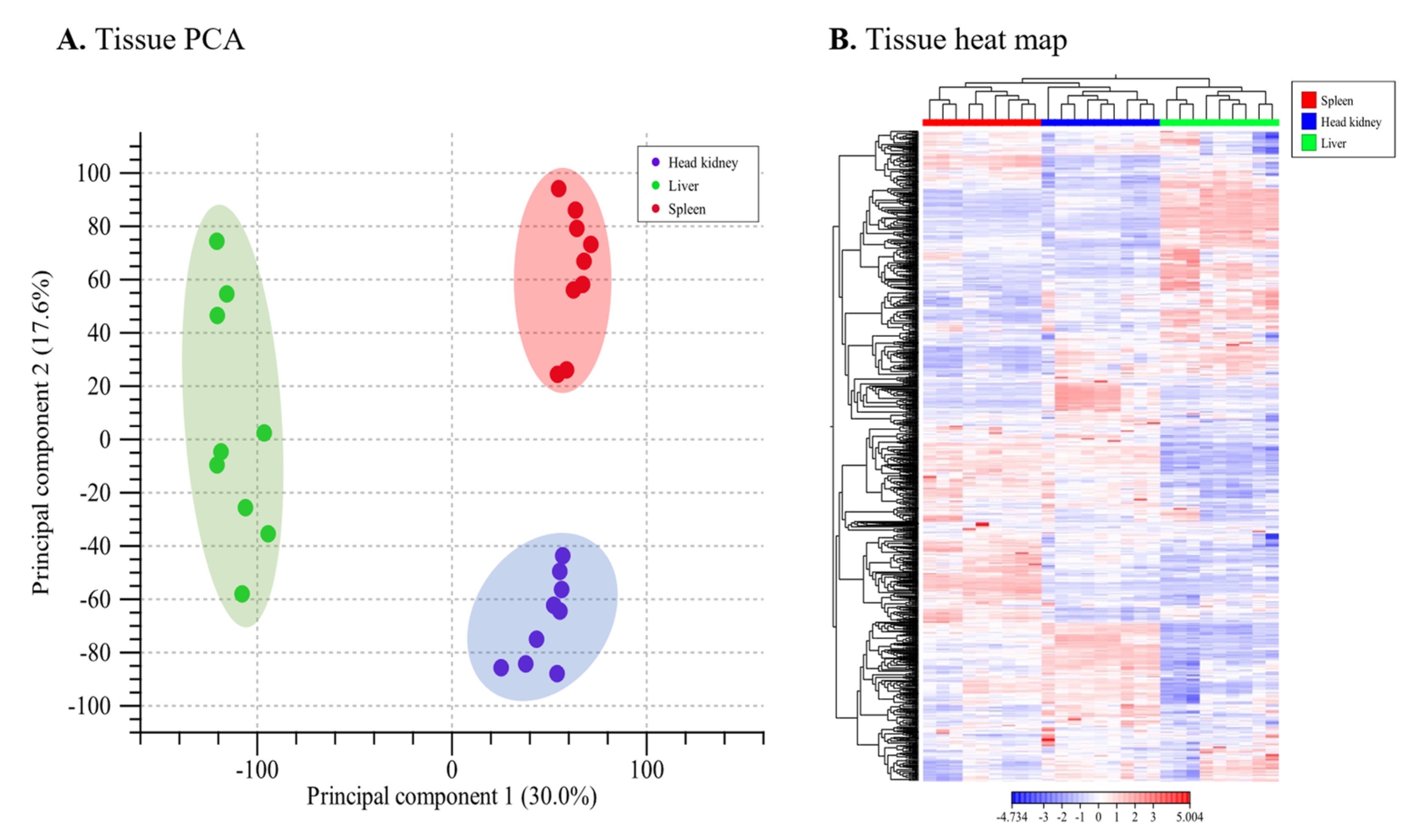
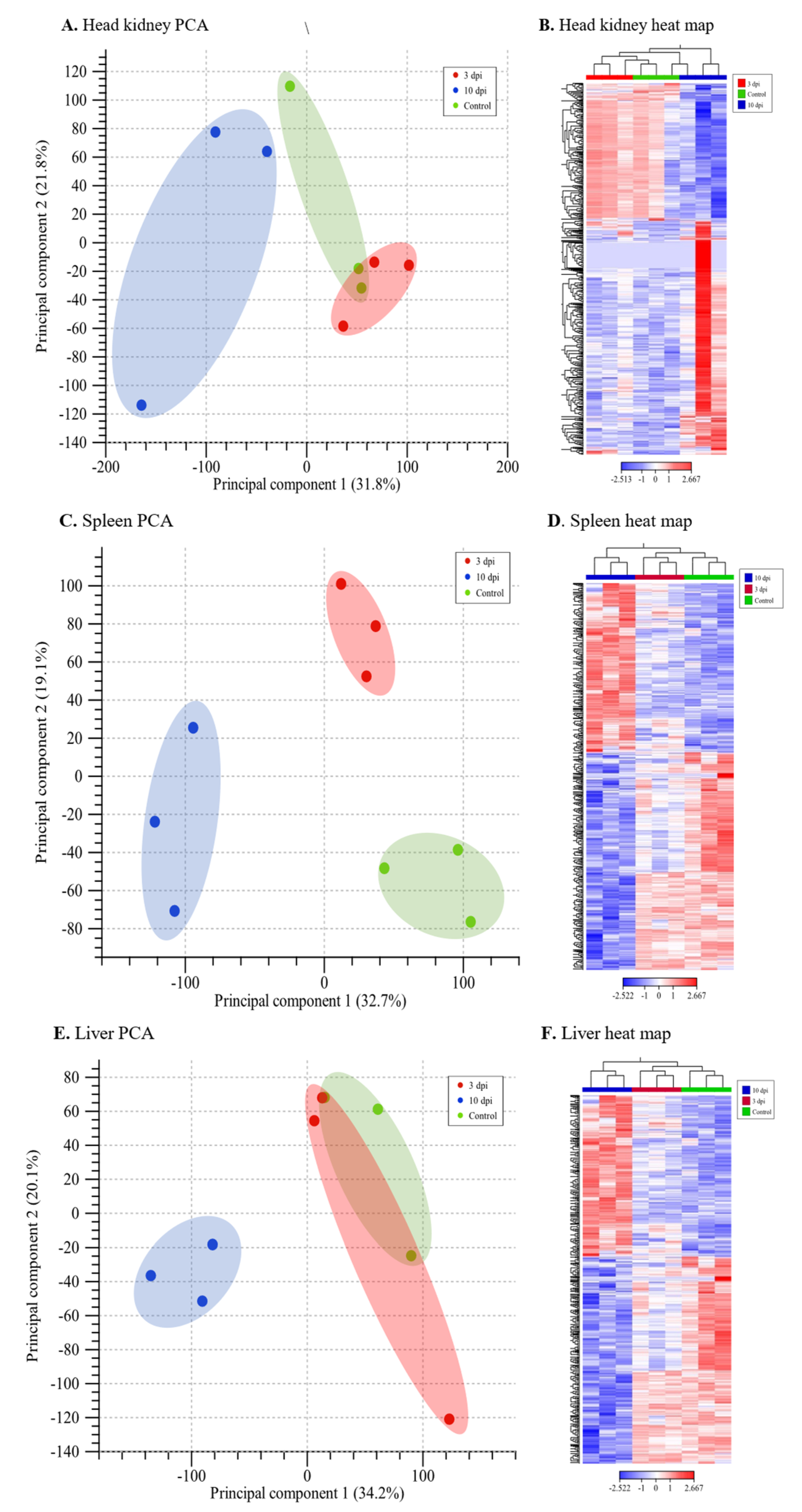



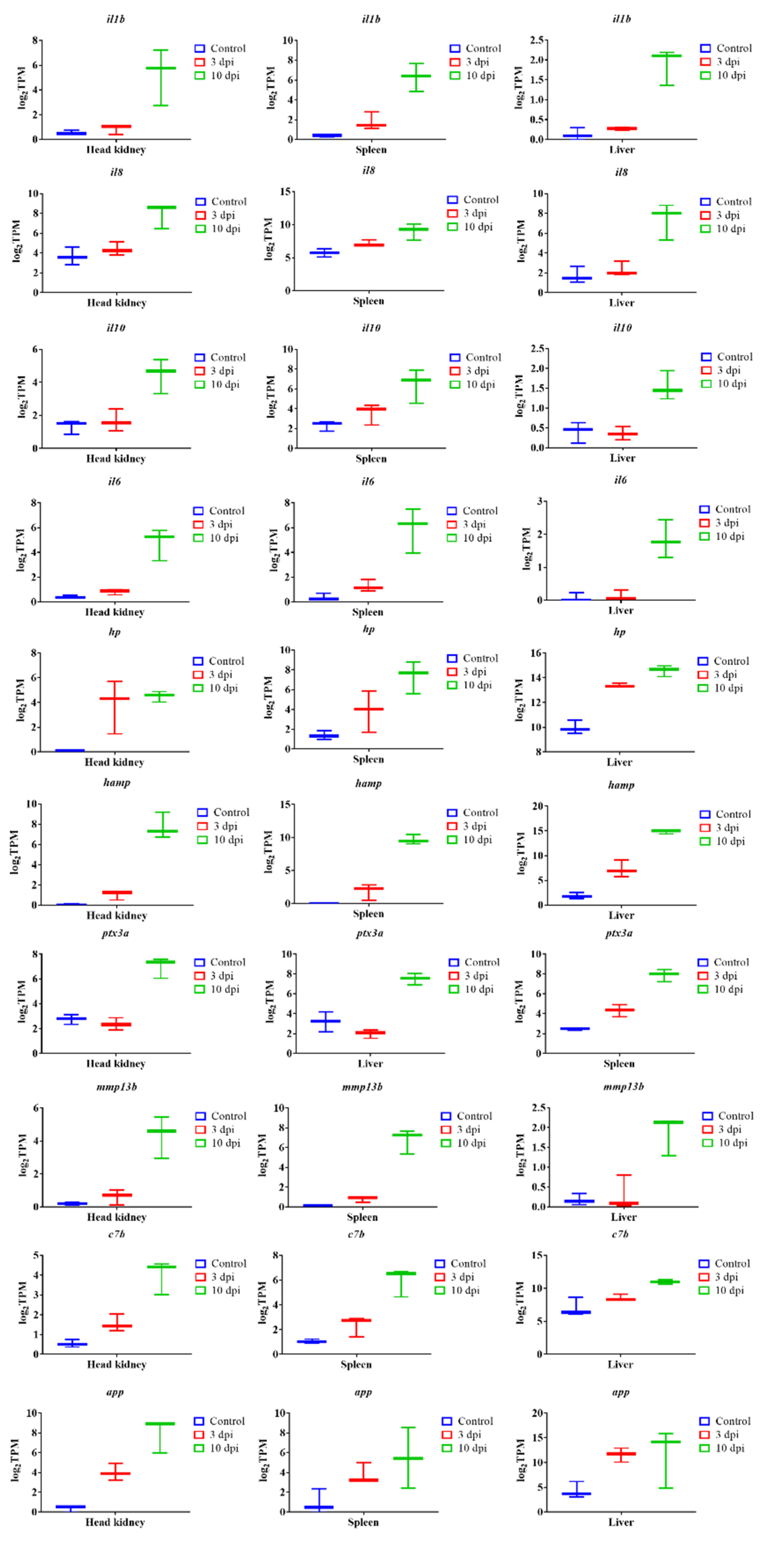
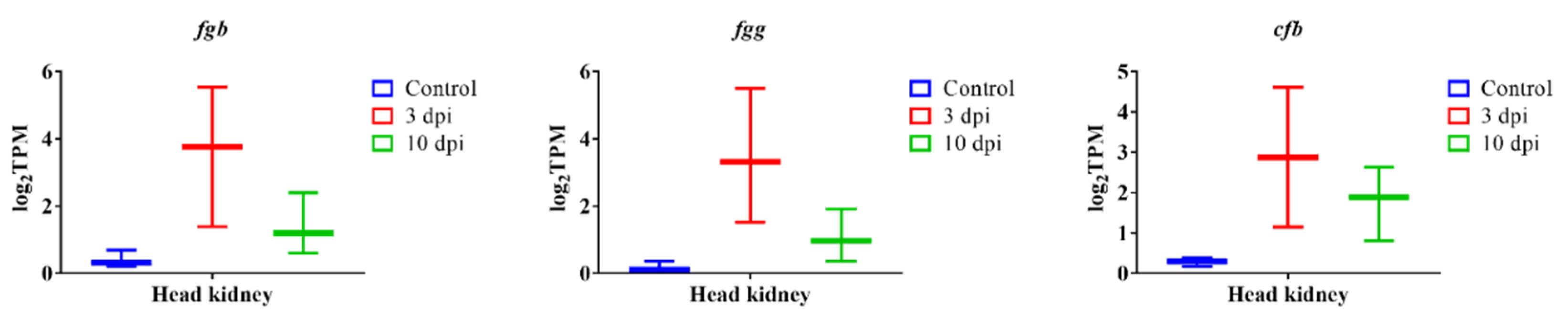
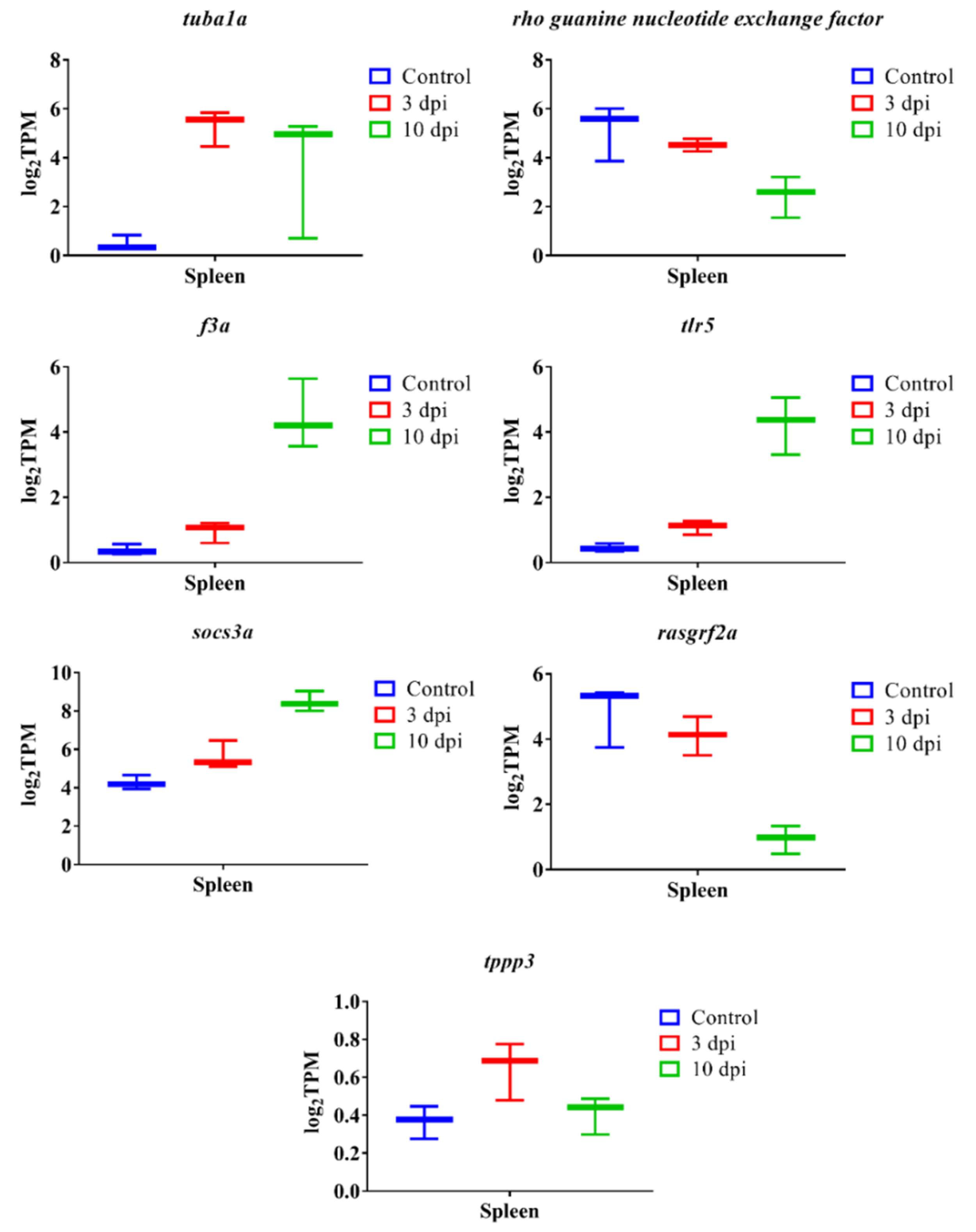
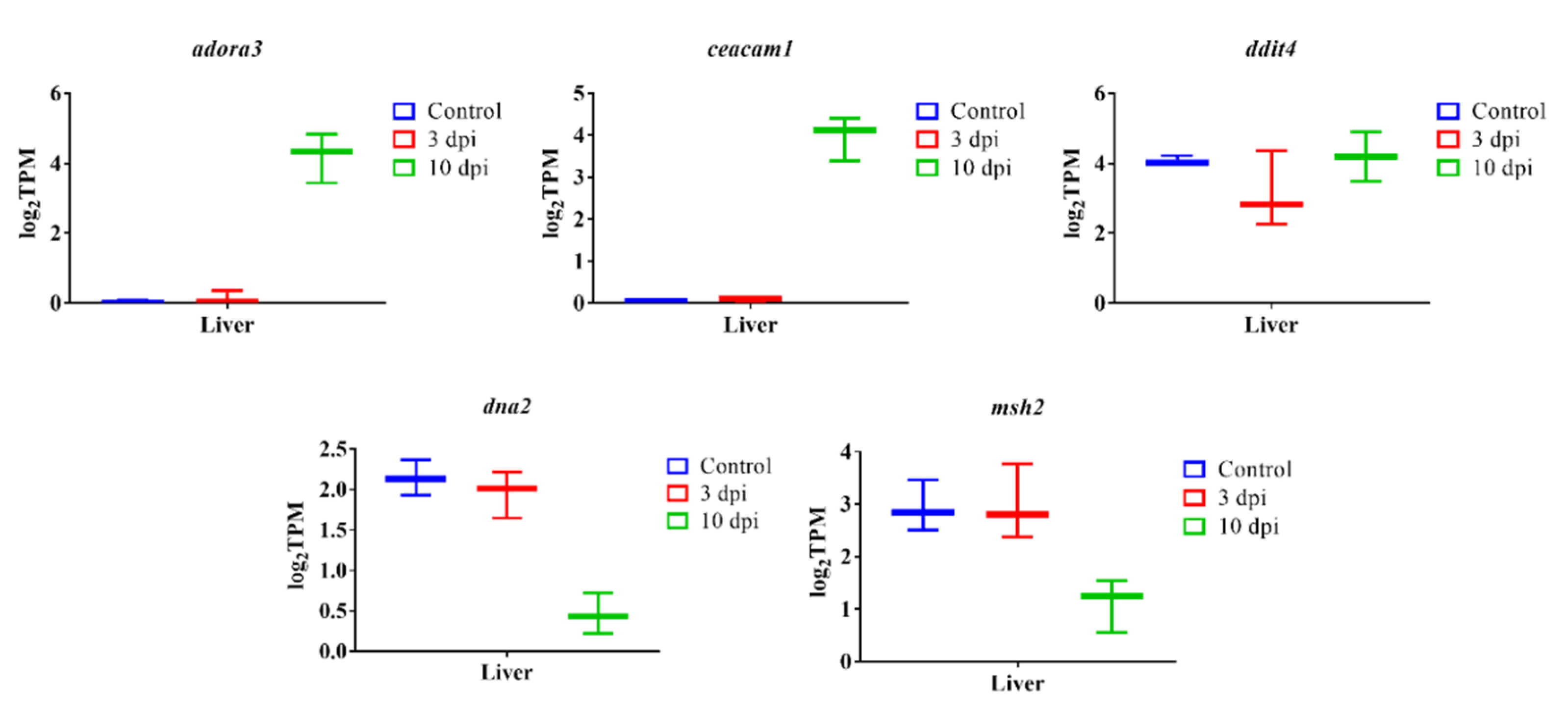

| Tissues | Days Post-Infection (dpi) with A. salmonicida | Upregulated Genes | Downregulated Genes | Total Genes |
|---|---|---|---|---|
| Head-kidney | 3 | 94 | 8 | 102 |
| Head-kidney | 10 | 530 | 1392 | 1922 |
| Spleen | 3 | 253 | 384 | 637 |
| Spleen | 10 | 1368 | 1765 | 3133 |
| Liver | 3 | 44 | 14 | 58 |
| Liver | 10 | 1360 | 1406 | 2766 |
| Tissues | Days Post-Infection (dpi) with A. salmonicida | Upregulated Transcripts | Downregulated Transcripts | Total Transcripts |
|---|---|---|---|---|
| Head-kidney | 3 | 89 | 44 | 133 |
| Head-kidney | 10 | 451 | 248 | 699 |
| Spleen | 3 | 237 | 377 | 614 |
| Spleen | 10 | 1173 | 979 | 2152 |
| Liver | 3 | 27 | 6 | 33 |
| Liver | 10 | 887 | 810 | 1697 |
| Tissues | Days Post-Infection (dpi) | edgeR Upregulated Transcripts | edgeR Downregulated Transcripts | Total Transcripts |
|---|---|---|---|---|
| Head-kidney | 3 | 138 | 148 | 286 |
| Head-kidney | 10 | 204 | 273 | 477 |
| Spleen | 3 | 214 | 287 | 501 |
| Spleen | 10 | 1005 | 1410 | 2415 |
| Liver | 3 | 56 | 77 | 133 |
| Liver | 10 | 1053 | 1040 | 2093 |
| Gene symbol | Description | Log2 Fold Change | |||||
|---|---|---|---|---|---|---|---|
| Head-Kidney | Spleen | Liver | |||||
| 3 dpi | 10 dpi | 3 dpi | 10 dpi | 3 dpi | 10 dpi | ||
| LOC117737678 | H-2 class II histocompatibility antigen gamma chain | - | - | - | −1.91 | −1.37 | −1.68 |
| LOC117747618 | H-2 class II histocompatibility antigen, E-S beta chain | - | - | −1.12 | −2.35 | −1.58 | −1.76 |
| LOC117731450 | H-2 class II histocompatibility antigen, A-U alpha chain | - | - | −1.03 | −2.75 | −1.16 | −2.12 |
| LOC117747619 | H-2 class II histocompatibility antigen, A-U alpha chain | - | - | - | −2.79 | −1.76 | −1.65 |
| LOC117745568 | H-2 class II histocompatibility antigen, A-U alpha chain | - | 1.23 | - | - | - | −3.18 |
| LOC117742904 | V-set and immunoglobulin domain-containing protein 1 | - | - | −2.20 | −5.07 | −1.92 | −3.44 |
| si:ch211-139g16.8 | immunoglobulin superfamily member 6 | - | - | - | −1.33 | −1.04 | −1.58 |
| Sema3fa | Immunoglobulin (Ig) | - | −1.13 | - | −1.81 | - | −2.23 |
| vsig10 | V-set and immunoglobulin domain-containing protein 10 | - | - | - | −1.85 | - | −1.18 |
| LOC117747962 | immunoglobulin kappa light chain | - | - | - | −1.94 | - | −1.34 |
| LOC117737972 | polymeric immunoglobulin receptor | - | - | - | −1.97 | - | −1.68 |
| igsf8 | immunoglobulin superfamily member 8 | - | - | - | −2.04 | - | - |
| igsf5b | immunoglobulin superfamily member 5 | - | −2.05 | −1.64 | −2.24 | - | - |
| tmigd1 | transmembrane and immunoglobulin domain containing 1 | 1.07 | −4.07 | −1.28 | −2.56 | - | - |
| LOC117750007 | immunoglobulin-like domain-containing receptor 1 | - | −2.14 | −1.42 | - | - | - |
| vsig8b | V-set and immunoglobulin domain containing 8b | - | −2.61 | −1.94 | - | - | - |
| cd79a | B-cell receptor complex-associated protein alpha | - | - | −2.27 | −5.76 | −1.33 | −2.49 |
| cd79b | B-cell receptor complex-associated protein beta | - | - | −1.21 | −4.75 | −1.87 | −5.44 |
| LOC117735721 | CD209 | −1.08 | −1.69 | - | −1.96 | - | −2.53 |
| LOC117750415 | CD209 | - | - | - | −2.17 | −1.31 | −3.50 |
| LOC117750406 | CD209 | - | - | - | −2.90 | - | −3.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, S.; Hossain, A.; Cao, T.; Gnanagobal, H.; Segovia, C.; Hill, S.; Monk, J.; Porter, J.; Boyce, D.; Hall, J.R.; et al. Multi-Organ Transcriptome Response of Lumpfish (Cyclopterus lumpus) to Aeromonas salmonicida Subspecies salmonicida Systemic Infection. Microorganisms 2022, 10, 2113. https://doi.org/10.3390/microorganisms10112113
Chakraborty S, Hossain A, Cao T, Gnanagobal H, Segovia C, Hill S, Monk J, Porter J, Boyce D, Hall JR, et al. Multi-Organ Transcriptome Response of Lumpfish (Cyclopterus lumpus) to Aeromonas salmonicida Subspecies salmonicida Systemic Infection. Microorganisms. 2022; 10(11):2113. https://doi.org/10.3390/microorganisms10112113
Chicago/Turabian StyleChakraborty, Setu, Ahmed Hossain, Trung Cao, Hajarooba Gnanagobal, Cristopher Segovia, Stephen Hill, Jennifer Monk, Jillian Porter, Danny Boyce, Jennifer R. Hall, and et al. 2022. "Multi-Organ Transcriptome Response of Lumpfish (Cyclopterus lumpus) to Aeromonas salmonicida Subspecies salmonicida Systemic Infection" Microorganisms 10, no. 11: 2113. https://doi.org/10.3390/microorganisms10112113
APA StyleChakraborty, S., Hossain, A., Cao, T., Gnanagobal, H., Segovia, C., Hill, S., Monk, J., Porter, J., Boyce, D., Hall, J. R., Bindea, G., Kumar, S., & Santander, J. (2022). Multi-Organ Transcriptome Response of Lumpfish (Cyclopterus lumpus) to Aeromonas salmonicida Subspecies salmonicida Systemic Infection. Microorganisms, 10(11), 2113. https://doi.org/10.3390/microorganisms10112113







