25-Hydroxyvitamin D and Peripheral Immune Mediators: Results from Two Nationwide Danish Pediatric Cohorts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Sample Population, Data Sources and Variables
2.1.1. The Birth Cohort Samples
2.1.2. The Newly Diagnosed Cohort Samples
2.2. Outcome Assessment
2.2.1. Assessment of Peripheral Immune Mediators on Dried Blood Spots
2.2.2. Assessment of Peripheral Immune Mediators in Serum
2.3. Exposure Assessment
2.3.1. Assessment of 25(OH)D on Dried Blood Spots
2.3.2. Assessment of 25(OH)D in Serum
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Basic Characteristics for both Cohorts are Presented in Table 1
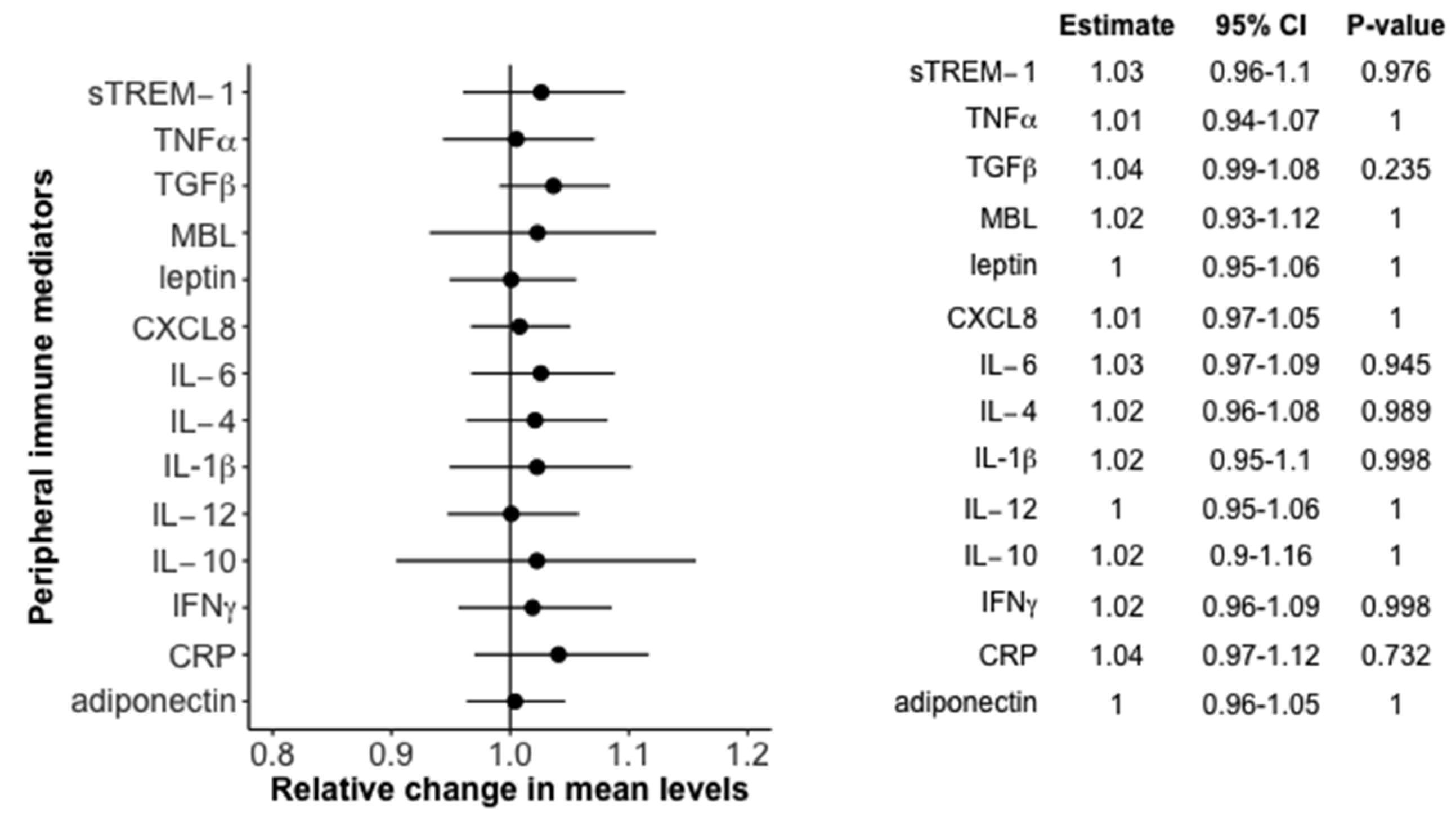
3.2. Association between Peripheral Immune Mediators and 25(OH)D in the Birth Cohort
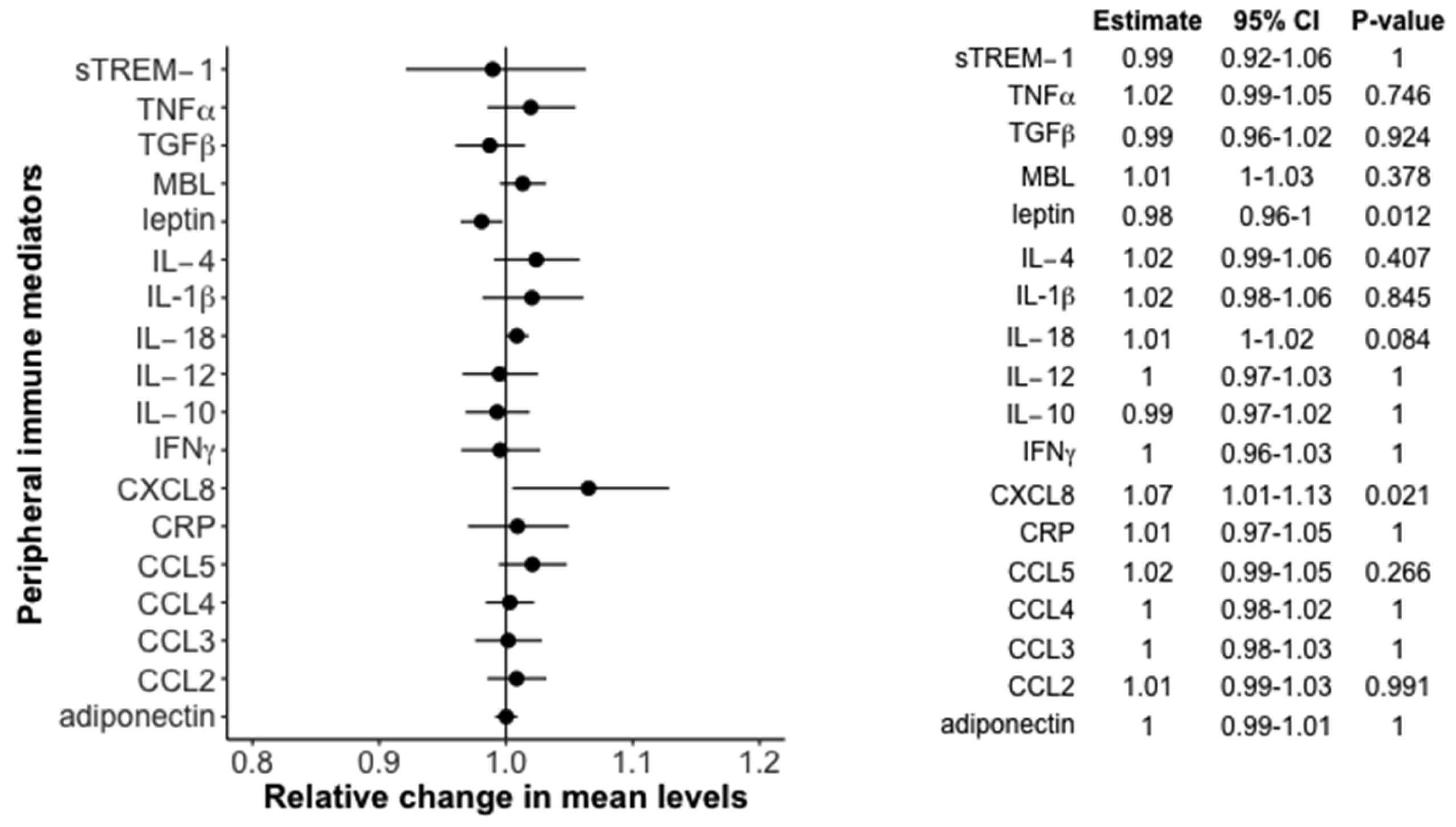
3.3. Association between Peripheral Immune Mediators and 25(OH)D in the Newly Diagnosed Cohort
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rewers, M.; Ludvigsson, J. Environmental risk factors for type 1 diabetes. Lancet Lond. Engl. 2016, 387, 2340–2348. [Google Scholar] [CrossRef]
- Paul, D.S.; Teschendorff, A.E.; Dang, M.A.N.; Lowe, R.; Hawa, M.I.; Ecker, S.; Beyan, H.; Cunningham, S.; Fouts, A.R.; Ramelius, A.; et al. Increased DNA methylation variability in type 1 diabetes across three immune effector cell types. Nat. Commun. 2016, 7, 13555. [Google Scholar] [CrossRef] [PubMed]
- Pociot, F.; Lernmark, Å. Genetic risk factors for type 1 diabetes. Lancet Lond. Engl. 2016, 387, 2331–2339. [Google Scholar] [CrossRef]
- Wolden-Kirk, H.; Overbergh, L.; Christesen, H.T.; Brusgaard, K.; Mathieu, C. Vitamin D and diabetes: Its importance for beta cell and immune function. Mol. Cell. Endocrinol. 2011, 347, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Badenhoop, K.; Kahles, H.; Penna-Martinez, M. Vitamin D, immune tolerance, and prevention of type 1 diabetes. Curr. Diabetes Rep. 2012, 12, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.-Y.; Zhang, W.-G.; Chen, J.J.; Zhang, Z.-L.; Han, S.-F.; Qin, L.-Q. Vitamin D intake and risk of type 1 diabetes: A meta-analysis of observational studies. Nutrients 2013, 5, 3551–3562. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, S.U.; Mortensen, H.B.; Carstensen, B.; Fenger, M.; Thuesen, B.H.; Husemoen, L.L.; Bergholdt, R.; Brorsson, C.; Pociot, F.; Linneberg, A.; et al. No difference in vitamin d levels between children newly diagnosed with type 1 diabetes and their healthy siblings: A 13-year nationwide Danish study. Diabetes Care 2013, 36, e157–e158. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, S.U.; Pipper, C.B.; Eising, S.; Skogstrand, K.; Hougaard, D.M.; Svensson, J.; Pociot, F. Neonatal levels of adiponectin, interleukin-10 and interleukin-12 are associated with the risk of developing type 1 diabetes in childhood and adolescence: A nationwide Danish case-control study. Clin. Immunol. 2017, 174, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, R.; Thorsen, S.U.; Cohen, A.S.; Lundqvist, M.; Frederiksen, P.; Pipper, C.B.; Pociot, F.; Thygesen, L.C.; Ascherio, A.; Svensson, J.; et al. Neonatal vitamin D status is not associated with later risk of type 1 diabetes: Results from two large Danish population-based studies. Diabetologia 2016, 59, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, S.U.; Pipper, C.B.; Mortensen, H.B.; Skogstrand, K.; Pociot, F.; Johannesen, J.; Svensson, J.; Danish Childhood Diabetes Register. Levels of soluble TREM-1 in children with newly diagnosed type 1 diabetes and their siblings without type 1 diabetes: A Danish case-control study. Pediatr. Diabetes 2016. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Cerqueira, C.; Kjærsgaard, P.; Lyngsøe, L.; Hertel, N.T.; Madsen, M.; Mortensen, H.B.; Johannesen, J. Danish Registry of Childhood and Adolescent Diabetes. Clin. Epidemiol. 2016, 8, 679. [Google Scholar] [CrossRef] [PubMed]
- Eising, S.; Svensson, J.; Skogstrand, K.; Nilsson, A.; Lynch, K.; Andersen, P.S.; Lernmark, Å.; Hougaard, D.M.; Pociot, F.; Nørgaard-Pedersen, B.; et al. Type 1 diabetes risk analysis on dried blood spot samples from population-based newborns: Design and feasibility of an unselected case–control study. Paediatr. Perinat. Epidemiol. 2007, 21, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Hollegaard, M.V.; Grauholm, J.; Nielsen, R.; Grove, J.; Mandrup, S.; Hougaard, D.M. Archived neonatal dried blood spot samples can be used for accurate whole genome and exome-targeted next-generation sequencing. Mol. Genet. Metab. 2013, 110, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Skogstrand, K.; Thorsen, P.; Nørgaard-Pedersen, B.; Schendel, D.E.; Sørensen, L.C.; Hougaard, D.M. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin. Chem. 2005, 51, 1854–1866. [Google Scholar] [CrossRef] [PubMed]
- Leek, J.T.; Scharpf, R.B.; Bravo, H.C.; Simcha, D.; Langmead, B.; Johnson, W.E.; Geman, D.; Baggerly, K.; Irizarry, R.A. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat. Rev. Genet. 2010, 11, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Skogstrand, K. Multiplex assays of inflammatory markers, a description of methods and discussion of precautions—Our experience through the last ten years. Methods San Diego Calif. 2012, 56, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.; Anderson, C.; Ko, P.; Jones, A.; Thomas, A.; Burne, T.; Mortensen, P.B.; Nø rgaard-Pedersen, B.; Hougaard, D.M.; McGrath, J. A sensitive LC/MS/MS assay of 25OH vitamin D3 and 25OH vitamin D2 in dried blood spots. Clin. Chim. Acta 2009, 403, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Morley, R.; Anderson, C.; Ko, P.; Burne, T.; Permezel, M.; Mortensen, P.B.; Nørgaard-Pedersen, B.; Hougaard, D.M.; McGrath, J.J. The utility of neonatal dried blood spots for the assessment of neonatal vitamin D status. Paediatr. Perinat. Epidemiol. 2010, 24, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Thuesen, B.; Husemoen, L.; Fenger, M.; Jakobsen, J.; Schwarz, P.; Toft, U.; Ovesen, L.; Jø rgensen, T.; Linneberg, A. Determinants of vitamin D status in a general population of Danish adults. Bone 2012, 50, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Pipper, C.B.; Ritz, C.; Bisgaard, H. A versatile method for confirmatory evaluation of the effects of a covariate in multiple models. J. R. Stat. Soc. Ser. C Appl. Stat. 2012, 61, 315–326. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biometr. J. Biometr. Z. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Vandercappellen, J.; Van Damme, J.; Struyf, S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008, 267, 226–244. [Google Scholar] [CrossRef] [PubMed]
- Ryynänen, J.; Carlberg, C. Primary 1,25-Dihydroxyvitamin D3 Response of the Interleukin 8 Gene Cluster in Human Monocyte- and Macrophage-Like Cells. PLoS ONE 2013, 8, e78170. [Google Scholar] [CrossRef] [PubMed]
- Greiller, C.L.; Martineau, A.R. Modulation of the Immune Response to Respiratory Viruses by Vitamin D. Nutrients 2015, 7, 4240–4270. [Google Scholar] [CrossRef] [PubMed]
- Dauletbaev, N.; Herscovitch, K.; Das, M.; Chen, H.; Bernier, J.; Matouk, E.; Bérubé, J.; Rousseau, S.; Lands, L.C. Down-regulation of IL-8 by high-dose vitamin D is specific to hyperinflammatory macrophages and involves mechanisms beyond up-regulation of DUSP1. Br. J. Pharmacol. 2015, 172, 4757–4771. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, S.U.; Eising, S.; Mortensen, H.B.; Skogstrand, K.; Pociot, F.; Johannesen, J.; Svensson, J.; Danish Childhood Diabetes Register. Systemic Levels of CCL2, CCL3, CCL4 and CXCL8 Differ According to Age, Time Period and Season among Children Newly Diagnosed with type 1 Diabetes and their Healthy Siblings. Scand. J. Immunol. 2014, 80, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Riejos, P.; Najib, S.; Santos-Alvarez, J.; Martín-Romero, C.; Pérez-Pérez, A.; González-Yanes, C.; Sánchez-Margalet, V. Role of leptin in the activation of immune cells. Mediat. Inflamm. 2010, 2010, 568343. [Google Scholar] [CrossRef] [PubMed]
- Safai, N.; Eising, S.; Hougaard, D.M.; Mortensen, H.B.; Skogstrand, K.; Pociot, F.; Johannesen, J.; Svensson, J. Levels of adiponectin and leptin at onset of type 1 diabetes have changed over time in children and adolescents. Acta Diabetol. 2015, 52, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Hajimohammadi, M.; Shab-Bidar, S.; Neyestani, T.R. Vitamin D and serum leptin: A systematic review and meta-analysis of observational studies and randomized controlled trials. Eur. J. Clin. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Peakman, M. Immunological pathways to β-cell damage in Type 1 diabetes. Diabet. Med. 2013, 30, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Jörns, A.; Arndt, T.; Meyer zu Vilsendorf, A.; Klempnauer, J.; Wedekind, D.; Hedrich, H.-J.; Marselli, L.; Marchetti, P.; Harada, N.; Nakaya, Y.; et al. Islet infiltration, cytokine expression and beta cell death in the NOD mouse, BB rat, Komeda rat, LEW. 1AR1-iddm rat and humans with type 1 diabetes. Diabetologia 2014, 57, 512–521. [Google Scholar] [CrossRef] [PubMed]
| Variables | Birth Cohort | Newly Diagnosed Cohort | ||||
|---|---|---|---|---|---|---|
| Patient | Control | p-Value 1 | Patient | Sibling | p-Value | |
| (n = 470) | (n = 500) | (n = 460) | (n = 453) | |||
| Basic characteristics | ||||||
| Sex | ||||||
| Female, n/% of total | 231/49.1 | 231/46.2 | 0.39 | 218/47.4 | 198/43.7 | 0.29 |
| Male, n/% of total | 239/50.9 | 269/53.8 | 242/52.6 | 255/56.3 | ||
| Age at onset 2 | ||||||
| Median/ | 8.2/ | 10.4/ | 10.3/ | 0.41 | ||
| Q1–Q3 3, years | 4.8–11.0 | 7.4–12.5 | 7.8–12.8 | |||
| Pregnancy and birth | ||||||
| Gestational age | ||||||
| Median/Q1–Q3, weeks | 40/39–41 | 40/39–40 | 0.58 | |||
| Birth weight, n/% of total | ||||||
| <2500 g | 19/4.0 | 30/6.0 | ||||
| 2500–4499 g | 441/93.8 | 460/92.0 | 0.38 | |||
| >4500 g | 10/2.1 | 10/2.0 | ||||
| Birth length | ||||||
| Median/Q1–Q3, cm | 52/51–53 | 52/50–53 | 0.37 | |||
| Mother’s age at child’s birth | ||||||
| Median/Q1–Q3, years | 28/25–31 | 28/25–31 | 0.84 | |||
| Season and time period of blood sampling | ||||||
| Season, n/% of total 4 | ||||||
| Winter | 104/22.1 | 106/21.2 | 117/25.4 | 118/26.0 | ||
| Spring | 112/23.8 | 125/25.0 | 0.49 | 115/25.0 | 116/25.6 | 0.11 |
| Summer | 113/24.0 | 137/27.4 | 105/22.8 | 126/27.8 | ||
| Autumn | 141/30.0 | 132/26.4 | 123/26.7 | 93/20.5 | ||
| Time period, n/% of total 5 | ||||||
| 1. 1981–1987 or 1997–1999 | 177/37.7 | 171/34.2 | 143/31.1 | 210/46.4 | ||
| 2. 1987–1991 or 2000–2002 | 152/32.3 | 177/35.4 | 0.48 | 94/20.4 | 45/10.0 | <0.0001 |
| 3. 1991–1999 or 2003–2005 | 141/30.0 | 152/30.4 | 223/48.5 | 198/43.7 | ||
| Peripheral Immune Mediators | Birth Cohort | Newly Diagnosed Cohort | ||
|---|---|---|---|---|
| Patient | Control | Patient | Sibling | |
| (n = 470) | (n = 500) | (n = 460) | (n = 453) | |
| IL-1β | ||||
| Median/Q1–Q3, ng/L | 45.1/22.8–80.0 | 40.3/21.3–77.1 | 23.0/10.3–71.3 | 18.2/8.1–58.9 |
| IL-4 | ||||
| Median/Q1–Q3, ng/L | 20.1/12.4–31.0 | 20.7/12.8–31.7 | 9.5/2.0–21.7 | 8.4/2.0–17.2 |
| IL-6 | ||||
| Median/Q1–Q3, ng/L | 33.7/21.3–60.0 | 37.5/22.1–65.5 | ||
| CXCL8 | ||||
| Median/Q1–Q3, ng/L | 85.2/58.7–138.4 | 89.8/63.7–138.3 | 2517.3/433.5–4914.1 | 1923.5/305.6–4593.0 |
| IL-10 | ||||
| Median/Q1–Q3, ng/L | 242.9/64.5–793.8 | 245.3/72.5–721.2 | 47.2/25.8–92.8 | 44.2/20.9–79.7 |
| IL-12 | ||||
| Median/Q1–Q3, ng/L | 94.8/56.5–147.8 | 92.9/52.5–146.5 | 27.5/12.3–56.1 | 21.7/10.2–45.6 |
| IL-18 | ||||
| Median/Q1–Q3, ng/L | 183.8/119.8–285.9 | 142.4/104.0–212.0 | ||
| IFNγ | ||||
| Median/Q1–Q3, ng/L | 34.7/17.1–61.9 | 39.8/19.8–65.2 | 106.1/57.9–211.3 | 93.9/50.1–177.3 |
| TNFα | ||||
| Median/Q1–Q3, ng/L | 43.7/25.8–67.7 | 42.7/26.1–68.0 | 69.4/26.3–134.8 | 65.0/16.1–131.9 |
| TGFβ | ||||
| Median/Q1–Q3, ng/L | 927.2/603.5–1385.3 | 1006.6/687.7–1414.9 | 233.6/142.6–341.3 | 166.9/104.2–256.4 |
| Leptin | ||||
| Median/Q1–Q3, ng/L | 3079/1953–4678 | 3143/2022–4588 | 368.1/174.5–772.1 | 430.6/192.1–930.7 |
| Adiponectin | ||||
| Median/Q1–Q3, mg/L | 13.4/9.3–20.1 | 13.3/9.1–19.8 | 14.9/11.0–19.5 | 14.4/11.1–19.7 |
| CRP | ||||
| Median/Q1–Q3, mg/L | 0.9/0.3–2.0 | 1.0/0.4–2.0 | 0.04/0.01–0.16 | 0.02/0.01–0.09 |
| MBL | ||||
| Median/Q1–Q3, mg/L | 0.6/0.2–1.0 | 0.5/0.18–1.1 | 1.9/0.7–3.3 | 1.2/0.5–2.3 |
| sTREM-1 | ||||
| Median/Q1–Q3, ng/L | 2868.5/1533.4–4930.9 | 2693.5/1524.9–5180.5 | 3368/244–16411 | 1085/244–9442 |
| CCL2 | ||||
| Median/Q1–Q3, ng/L | 303.3/174.3–725.6 | 261.1/146.1–683.5 | ||
| CCL3 | ||||
| Median/Q1–Q3, ng/L | 234.5/120.4–520.3 | 202.2/107.7–517.7 | ||
| CCL4 | ||||
| Median/Q1–Q3, ng/L | 330.7/187.1–704.5 | 320.1/186.8–739.6 | ||
| CCL5 | ||||
| Median/Q1–Q3, ng/mL | 10.8/6.7–29.2 | 13.7/7.2–36.3 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorsen, S.U.; Pipper, C.B.; Skogstrand, K.; Pociot, F.; Svensson, J. 25-Hydroxyvitamin D and Peripheral Immune Mediators: Results from Two Nationwide Danish Pediatric Cohorts. Nutrients 2017, 9, 365. https://doi.org/10.3390/nu9040365
Thorsen SU, Pipper CB, Skogstrand K, Pociot F, Svensson J. 25-Hydroxyvitamin D and Peripheral Immune Mediators: Results from Two Nationwide Danish Pediatric Cohorts. Nutrients. 2017; 9(4):365. https://doi.org/10.3390/nu9040365
Chicago/Turabian StyleThorsen, Steffen U., Christian B. Pipper, Kristin Skogstrand, Flemming Pociot, and Jannet Svensson. 2017. "25-Hydroxyvitamin D and Peripheral Immune Mediators: Results from Two Nationwide Danish Pediatric Cohorts" Nutrients 9, no. 4: 365. https://doi.org/10.3390/nu9040365
APA StyleThorsen, S. U., Pipper, C. B., Skogstrand, K., Pociot, F., & Svensson, J. (2017). 25-Hydroxyvitamin D and Peripheral Immune Mediators: Results from Two Nationwide Danish Pediatric Cohorts. Nutrients, 9(4), 365. https://doi.org/10.3390/nu9040365





