Understanding the Antilymphoma Activity of Annona macroprophyllata Donn and Its Acyclic Terpenoids: In Vivo, In Vitro, and In Silico Studies
Abstract
:1. Introduction
2. Results
2.1. Brine Shrimp Lethality Assay
2.2. Analysis of GC-MS, and 1H-13C-NMR Spectra of Geranylgeraniol (P6), Phytol (P7) and Farnesyl Acetate (P10)
2.3. Citotoxic Activity
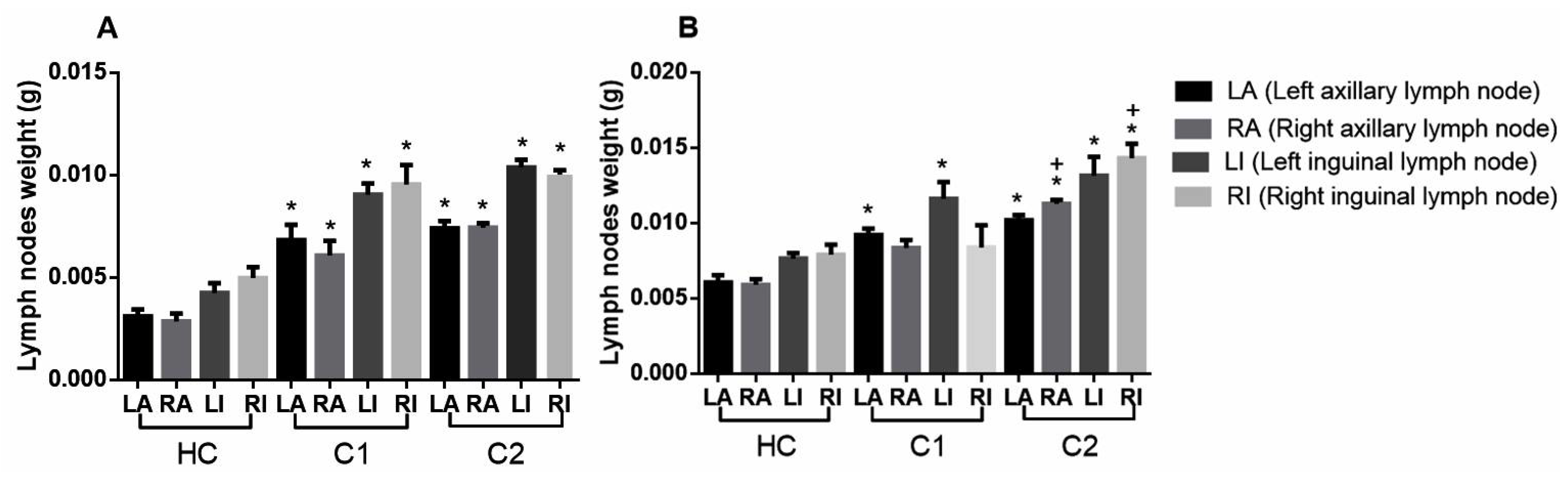
2.4. Antilymphoma Activity
2.5. Acute Oral Toxicity
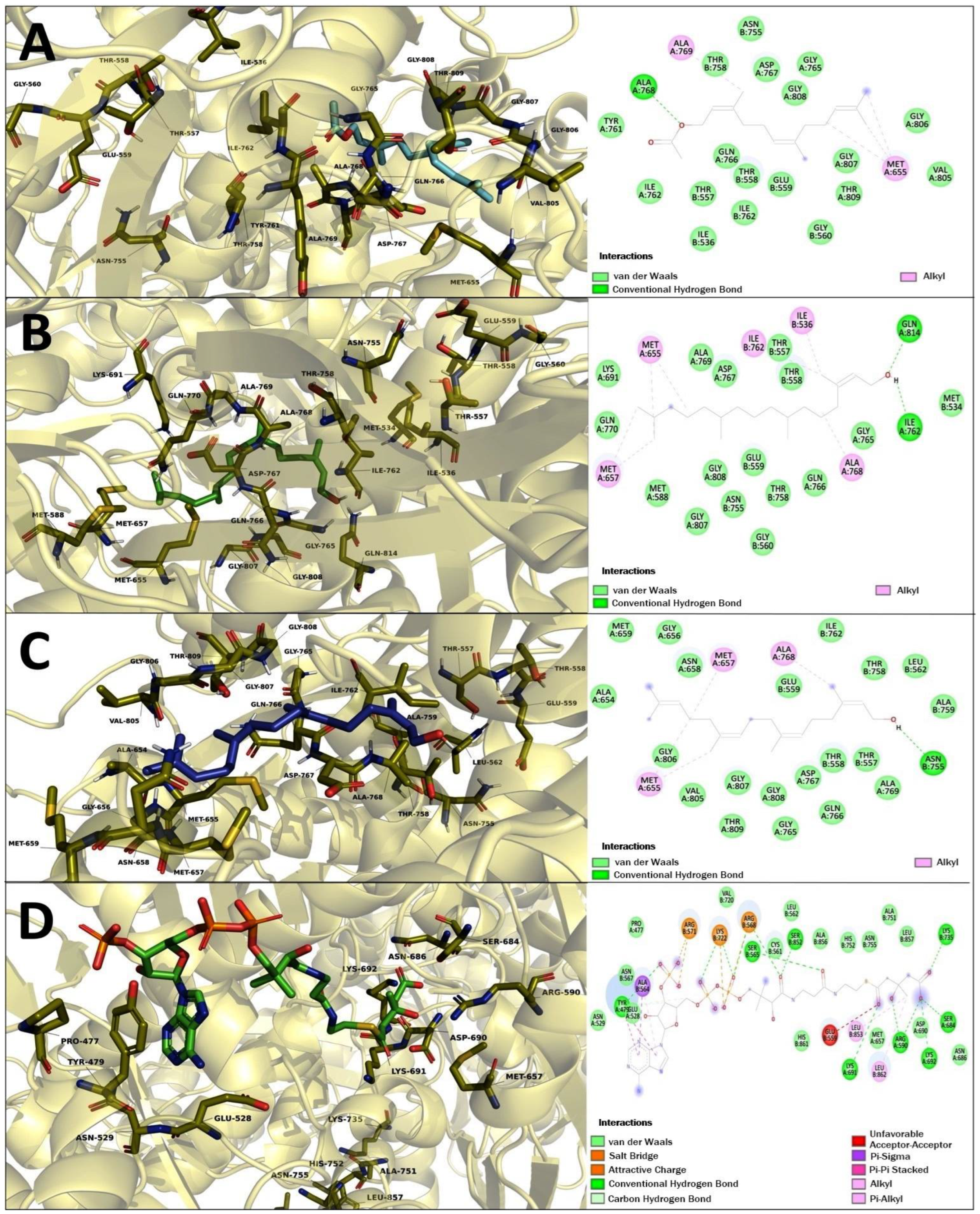
2.6. Molecular Docking Studies of Geranylgeraniol (Gg), Farnesyl Acetate (FA), and Phytol (PT)
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Extraction and Isolation of Products
4.3. Identification of Geranylgeraniol, Phytol and Farnesyl Acetate
4.4. Brine Shrimp Lethality Test
4.5. Cell-Based Assay
4.5.1. Culture
4.5.2. Citotoxic Activity
4.6. Animals
Antilymphoma Activity
4.7. Acute Oral Toxicity
4.8. Molecular Docking Studies of Geranylgeraniol, Farnesyl Acetate, and Phytol
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Trujano, M.; Navarrete, A.; Reyes, B.; Cedillo-Portugal, E.; Hong, E. Anticonvulsant Properties and Bio-Guided Isolation of Palmitone from Leaves of Annona diversifolia. Planta Med. 2001, 67, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Calzada, F.; Ramirez-Santos, J.; Valdes, M.; Garcia-Hernandez, N.; Pina-Jiménez, E.; Ordoñez-Razo, R.M. Evaluation of acute oral toxicity, brine shrimp lethality, and antilymphoma activity of geranylgeraniol and Annona macroprophyllata Leaf extracts. Rev. Bras. Farmacogn. 2020, 30, 301–304. [Google Scholar] [CrossRef]
- Ballesteros, G.; Rodríguez, L.; De la Paz, R.; Zvala, F.; Urieta, M.; Ballesteros, N.; Martinez, J. Diversidad en las ilamas (Annona diversifolia Saff.) de la tierra caliente del Balsas; Instituto Tecnologico de Cd. Altamirano: Ciudad Altamirano, México, 2010; ISBN 978-607-7814-04-7. [Google Scholar]
- Comision Nacional para el Conocimiento y Uso de la Biodiversidad. 2019. Available online: www.conabio.gob.mx (accessed on 26 February 2022).
- González-Trujano, M.; Tapia, E.; López-Meraz, L.; Navarrete, A.; Reyes-Ramírez, A.; Martínez, A. Anticonvulsant effect of Annona diversifolia Saff. and palmitone on penicillin-induced convulsive activity. A behavioral and EEG study in rats. Epilepsia 2006, 47, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Carballo, I.; Martínez, L.; González-Trujano, M.; Pellicer, F.; Ventura-Martínez, R.; Díaz-Reval, I.; López-Muñoz, J. Antinociceptive activity of Annona diversifolia Saff. leaf extracts and palmitone as a bioactive compound. Pharmacol. Biochem. Behav. 2010, 95, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Luna-Cazares, M.; González-Esquinca, R. Susceptibility of complete bacteria and spheroplasts of Escherichia coli, Pseudomonas aeruginosa and Salmonella typhi to rolliniastatin-2. Nat. Prod Res. 2010, 24, 1139–1145. [Google Scholar] [CrossRef]
- De la Cruz, I.; González-Esquinca, R. Liriodenine alkaloid in Annona diversifolia during early development. Nat. Prod. Res. 2012, 26, 42–49. [Google Scholar] [CrossRef]
- Angeles-López, E.; González-Trujano, M.; Déciga, M.; Ventura, R. Neuroprotective evaluation of tilia americana and Annona diversifolia in the neuronal damage induced by intestinal ischemia. Neurochem. Res. 2013, 38, 1632–1640. [Google Scholar] [CrossRef]
- Shile-Guzmán, M.; García-Carrancá, A.; González-Esquinca, A. In vitro and in vivo antiproliferative activity of laherradurin and cherimolin-2 of Annona diversifolia Saff. Phytother. Res. 2009, 23, 1128–1133. [Google Scholar] [CrossRef]
- Brindis, F.; González-Trujano, M.; González-Andrade, M.; Aguirre-Hernández, E.; Villalobos-Molina, A. Aqueous Extract of Annona macroprophyllata A Potential ∝-Glucosidase Inhibitor. BioMed Res. Int. 2013, 2013, 591313. [Google Scholar] [CrossRef] [Green Version]
- Calzada, F.; Valdes, M.; Garcia-Hernandez, N.; Velázquez, C.; Barbosa, E.; Bustos-Brito, C.; Mendieta-Wejebe, J.E. Antihyperglycemic activity of the leaves from Annona diversifolia Safford. and farnesol on normal and alloxan-induced diabetic mice. Pharmacogn. Mag. 2019, 15, 5. [Google Scholar]
- Ansari, I.; Akhtar, M. Current insights on the role of terpenoids as anticancer agents: A perspective on cancer prevention and treatment. In Natural Bio-Active Compounds; Springer: Singapore, 2019; Volume 2, pp. 53–80. [Google Scholar]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Ther. Exp. 2007, 55, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Thulasingam, S.; Nagarajan, S. Terpenoids as anti-colon cancer agents–A comprehensive review on its mechanistic perspectives. Eur. J. Pharmacol. 2017, 795, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Al-Naeeb, A.; Ajithkumar, T.; Behan, S.; Hodson, D. Non-Hodgkin lymphoma. BMJ 2018, 362, k3204. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. GLOBOCAN 2020. Global Cancer Observatory, 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/34-Non-hodgkin-lymphoma-fact-sheet.pdf (accessed on 20 March 2022).
- Instituto Nacional de Estadística. Geografía e Informática. Estadísticas a Propósito del día Mundial Contra el Cáncer. 2020. Available online: https://www.inegi.org.mx/contenidos/saladeprensa/aproposito/2022/EAP_CANCER22.pdf (accessed on 3 March 2022).
- International Agency for Research on Cancer. GLOBOCAN 2020. Global Cancer Observatory, 2020. Available online: https://gco.iarc.fr/today/data/factsheets/populations/484-mexico-fact-sheets.pdf (accessed on 3 March 2022).
- Ansell, S. Non-Hodgkin lymphoma: Diagnosis and treatment. Mayo Clin. Proc. 2015, 90, 1152–1163. [Google Scholar] [CrossRef] [Green Version]
- de Moura Espíndola, R.; Mazzantini, R.; Ong, T.; de Conti, A.; Heidor, R.; Moreno, F. Geranylgeraniol and β-ionone inhibit hepatic preneoplastic lesions, cell proliferation, total plasma cholesterol and DNA damage during the initial phases of hepatocarcinogenesis, but only the former inhibits NF-κB activation. Carcinogenesis 2005, 26, 1091–1099. [Google Scholar] [CrossRef] [Green Version]
- Soltanian, S.; Sheikhbahaei, M.; Ziasistani, M. Phytol down-regulates expression of some cancer stem cell markers and decreases side population proportion in human embryonic carcinoma NCCIT cells. Nutr Cancer. 2021, 73, 1520–1533. [Google Scholar] [CrossRef]
- Mo, H.; Jeter, R.; Bachmann, A.; Yount, S.T.; Shen, C.L.; Yeganehjoo, H. The potential of isoprenoids in adjuvant cancer therapy to reduce adverse effects of statins. Front. Pharmacol. 2019, 9, 1515. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, R.; Cuny, E. Terpenoids with special pharmacological significance: A review. Nat. Prod. Commun. 2016, 11, 1373–1390. [Google Scholar] [CrossRef] [Green Version]
- Calzada, F.; Merlin-Lucas, V.; Valdes, M.; Solares-Pascasio, J.; Garcia-Hernandez, N.; Pina-Jimenez, E.; Ordoñez-Razo, R. Secondary metabolites and biological properties of Annona muricata. Rev. Bras. Farmacogn. 2020, 30, 305–311. [Google Scholar] [CrossRef]
- Al-Shaya, H.; Li, H.; Beg, O.; Hamama, A.; Witiak, S.; Kaseloo, P.; Siddiqui, R. Phytochemical profile and antioxidation activity of annona fruit and its effect on lymphoma cell proliferation. Food Sci. Nutr. 2020, 8, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Calzada, F.; Bautista, E.; Hidalgo-Figueroa, S.; García-Hernández, N.; Barbosa, E.; Velázquez, C.; Arietta-García, A. Antilymphoma Effect of Incomptine A: In Vivo, In Silico, and Toxicological Studies. Molecules 2021, 26, 6646. [Google Scholar] [PubMed]
- Zhuang, Q.; Ruan, L.; Jin, T.; Zheng, X.; Jin, Z. Anti-leukaemia effects of leonurine in vitro and in vivo. Gen. Physiol. Biophys. 2021, 40, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Calzada, F.; Solares-Pascasio, J.I.; Valdes, M.; Garcia-Hernandez, N.; Velazquez, C.; Ordoñez-Razo, R.M.; Barbosa, E. Antilymphoma potential of the ethanol extract and rutin obtained of the leaves from Schinus molle linn. Pharmacogn. Res. 2018, 10, 119–123. [Google Scholar] [CrossRef]
- Ayaz, M.; Junaid, M.; Ullah, F.; Sadiq, A.; Subhan, F.; Khan, M.; Ahmad, S. Molecularly characterized solvent extracts and saponins from Polygonum hydropiper L. show high anti-angiogenic, anti-tumor, brine shrimp, and fibroblast NIH/3T3 cell line cytotoxicity. Front. Pharmacol. 2016, 7, 74. [Google Scholar] [CrossRef] [Green Version]
- Meyer, B.; Ferrigni, N.; Putnam, J.; Jacobsen, L.; Nichols, D.; McLaughlin, J. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Merlín-Lucas, V.; Ordoñez-Razo, R.; Calzada, F.; Solís, A.; García-Hernández, N.; Barbosa, E.; Valdés, M. Antitumor Potential of Annona muricata Linn. An Edible and Medicinal Plant in Mexico: In Vitro, In Vivo, and Toxicological Studies. Molecules 2021, 26, 7675. [Google Scholar] [CrossRef]
- Valdés, M.; Calzada, F.; Mendieta-Wejebe, J.E.; Merlín-Lucas, V.; Velázquez, C.; Barbosa, E. Antihyperglycemic Effects of Annona Diversifolia Safford and Its Acyclic Terpenoids: α-Glucosidase and Selective SGLT1 Inhibitiors. Molecules 2020, 25, 3361. [Google Scholar] [CrossRef]
- Hamidi, M.R.; Jovanova, B.; Panovska, T.K. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced. Pharm. Bull. 2014, 60, 9–18. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.; Huang, M.; Bao, J.; Chen, X.; Wang, Y. Terpenoids: Natural products for cancer therapy. Expert Opin. Investig. Drugs 2012, 21, 1801–1818. [Google Scholar]
- Pina-Jiménez, E.; Calzada, F.; Bautista, E.; Ordoñez-Razo, R.M.; Velázquez, C.; Barbosa, E.; García-Hernández, N. Incomptine a induces apoptosis, ROS production and a differential protein expression on non-Hodgkin’s lymphoma cells. Int. J. Mol. Sci. 2021, 22, 10516. [Google Scholar] [CrossRef]
- Cascaes, M.; Carneiro, O.; Nascimento, L.; de Moraes, Â.; de Oliveira, M.; Cruz, J.; Andrade, E. Essential Oils from Annonaceae Species from Brazil: A Systematic Review of Their Phytochemistry, and Biological Activities. Int. J. Mol. Sci. 2021, 22, 12140. [Google Scholar] [CrossRef] [PubMed]
- Bourgou, S.; Pichette, A.; Marzouk, B.; Legault, J. Bioactivities of black cumin essential oil and its main terpenes from Tunisia. South Afr. J. Bot. 2010, 76, 210–216. [Google Scholar] [CrossRef]
- Thoppil, R.J.; Bishayee, A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 2011, 3, 228. [Google Scholar] [CrossRef]
- OECD. Guideline for Testing of Chemicals 423. Acute Oral Toxicity-Acute Toxic Class Method. Organización para la Cooperación y el Desarrollo Economicos, OECD/OCDE. 2001. Available online: https://www.oecd.org/chemicalsafety/risk-assessment/1948378.pdf (accessed on 8 April 2022).
- Koźmiński, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Overview of dual-acting drug methotrexate in different neurological diseases, autoimmune pathologies and cancers. Int. J. Mol. Sci. 2020, 21, 3483. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef] [Green Version]
- Siraj, M.A.; Islam, M.; Al Fahad, M.; Kheya, H.; Xiao, J.; Simal-Gandara, J. Cancer Chemopreventive Role of Dietary Terpenoids by Modulating Keap1-Nrf2-ARE Signaling System—A Comprehensive Update. Appl. Sci. 2021, 11, 10806. [Google Scholar] [CrossRef]
- Bonikowski, R.; Świtakowska, P.; Sienkiewicz, M.; Zakłos-Szyda, M. Selected compounds structurally related to acyclic sesquiterpenoids and their antibacterial and cytotoxic activity. Molecules 2015, 20, 11272–11296. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, N.V.; Yeganehjoo, H.; Katuru, R.; DeBose-Boyd, R.; Morris, L.; Michon, R.; Mo, H. Geranylgeraniol suppresses the viability of human DU145 prostate carcinoma cells and the level of HMG CoA reductase. Exp. Biol. Med. 2013, 238, 1265–1274. [Google Scholar] [CrossRef]
- Yeganehjoo, H.; DeBose-Boyd, R.; McFarlin, B.; Mo, H. Synergistic impact of d-δ-tocotrienol and geranylgeraniol on the growth and HMG CoA reductase of human DU145 prostate carcinoma cells. Nutr. Cancer 2017, 69, 682–691. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Yamada, J.; Tsuno, N.; Okaji, Y.; Kawai, K.; Tsuchiya, T.; Takahashi, K. Plaunotol and geranylgeraniol induce caspase-mediated apoptosis in colon cancer. J. Surg. Res. 2009, 153, 246–253. [Google Scholar] [CrossRef]
- Ohizumi, H.; Masuda, Y.; Nakajo, S.; Sakai, I.; Ohsawa, S.; Nakaya, K. Geranylgeraniol is a potent inducer of apoptosis in tumor cells. J. Biochem. 1995, 117, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Mullen, P.J.; Yu, R.; Longo, J.; Archer, M.C.; Penn, L.Z. The interplay between cell signalling and the mevalonate pathway in cancer. Nat. Rev. Cancer 2016, 16, 718–731. [Google Scholar] [CrossRef]
- Chen, M.; Knifley, T.; Subramanian, T.; Spielmann, H.P.; O’Connor, K.L. Use of synthetic isoprenoids to target protein prenylation and Rho GTPases in breast cancer invasion. PLoS ONE. 2014, 9, e89892. [Google Scholar] [CrossRef] [Green Version]
- Mo, H.; Elson, C.E. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp. Biol. Med. 2004, 229, 567–585. [Google Scholar] [CrossRef]
- Juarez, D.; Fruman, D.A. Targeting the mevalonate pathway in cancer. Trends Cancer 2021, 7, 525–540. [Google Scholar] [CrossRef]
- Istvan, E.S.; Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, E.A.; Wang, M.; Radwan, M.M.; Wanas, A.S.; Majumdar, C.G.; Avula, B.; ElSohly, M. Analysis of terpenes in Cannabis sativa L. using GC/MS: Method development, validation, and application. Planta Med. 2019, 85, 431–438. [Google Scholar] [CrossRef]
- Joo, K.M.; Kim, S.; Koo, Y.J.; Lee, M.; Lee, S.H.; Choi, D.; Lim, K.M. Development and validation of UPLC method for WST-1 cell viability assay and its application to MCTT HCE™ eye irritation test for colorful substances. Toxicol. Vitro. 2019, 60, 412–419. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana. NOM-062-ZOO-1999: Especificaciones Técnicas Para la Producción, Cuidado y Uso de Los Animales de Laboratorio. 1999. Available online: https://www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF (accessed on 9 April 2022).
- Hanwell, M.; Curtis, D.; Lonie, D.; Vandermeersch, T.; Zurek, E.; Hutchison, G. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.; Lindstrom, W.; Sanner, M.; Belew, R.; Goodshell, D.; Olson, A. Autodock4 and AutodockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]





| Sample | LD50 (µg mL−1) a |
|---|---|
| PEAm | 18.84 ± 0.58 * |
| P1 | 20.75 ± 0.30 |
| P2 | 8.07 ± 0.59 * |
| P3 | 9.06 ± 0.45 * |
| P4 | 45.32 ± 0.33 * |
| P5 | 8.58 ± 0.28 * |
| Gg (P6) | 1.41 ± 0.42 * |
| PT (P7) | 3.03 ± 0.33 * |
| P8 | 25.88 ± 0.31 |
| P9 | 9.90 ± 0.41 * |
| FA (P10) | 5.82 ± 0.58 * |
| P11 | 13.34 ± 0.34 * |
| P12 | 13.79 ± 0.56 * |
| P13 | 9.98 ± 0.88 * |
| MTX | 24.66 ± 0.27 |
| Compound Name | IUPAC Name | R.T * (Min) | Area % | Molecular Weight | Molecular Formula |
|---|---|---|---|---|---|
| Farnesyl acetate | [(E,E)-3,7,11-trimethyldodeca-2,6,10-trienyl] acetate | 17.95 | 94.67 | 264 | C17H28O2 |
| Phytol | (2E,7R,11R)-3,7,11,15-tetramethylhexadec-2-en-1-ol | 23.09 | 1.96 | 296 | C20H40O |
| Geranylgeraniol | (2E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-ol | 24.12 | 3.39 | 290 | C20H34O |
| Position | Geranylgeraniol (P6) | Phytol (P7) | Farnesyl Acetate (P10) | |||
|---|---|---|---|---|---|---|
| δH, Mult. (J in Hz) | δC, Type | δH, Mult. (J in Hz) | δC, Type | δH, Mult. (J in Hz) | δC, Type | |
| 1 | 4.14 (2H, m, 6.9) | 59.4 (CH2) | 4.14 (2H, m, 0.2) | 59.4 (CH2) | 171.21 (C) | |
| 2 | 5.41 (1H, t. 7.29) | 123.3 (CH) | 5.4 (1H, t. 0.1) | 123.4 (CH) | 2.06 (3H, s) | 21.15 (CH3) |
| 3 | 139.8 (C) | 25.1 (C) | 4.57 (2H, m, 8.1) | 61.5 (CH2) | ||
| 4 | 2.148 (2H, m, 6.6) | 39.7 (CH2) | 1.59 (2H, m, 0.01) | 39.7 (CH2) | 5.34 (1H, m, 6.1) | 118.4 (CH2) |
| 5 | 2.148 (2H, m, 6.6) | 39.6 (CH2) | 1.52 (2H, s) | 25.2 (CH2) | 142.3 (C) | |
| 6 | 5.10 (1H, m, 0.6) | 123.7 (CH) | 5.10 (2H, m, 0.6) | 36.8 (CH2) | 2.10 (2H, m, 3.1) | 39.93 (CH2) |
| 7 | 135.4 (C) | 1.57 (1H, d, 0.6) | 32.71 (CH) | 2.10 (2H, m, 3.1) | 26.6 (CH2) | |
| 8 | 2.148 (2H, m, 6.6) | 26.7 (CH2) | 1.24 (2H, m, 0.06) | 37.2 (CH2) | 4.91 (1H, d, 2.8) | 123.7 (CH2) |
| 9 | 2.148 (2H, m, 6.6) | 26.6 (CH2) | 3.6 (2H, m, 0.04) | 24.5 (CH2) | 135.5 (C) | |
| 10 | 5.10 (1H, m, 0.6) | 124.1 (CH) | 1.24 (2H, m, 0.06) | 37.0 (CH2) | 1.98 (2H, t, 0.04) | 39.8 (CH2) |
| 11 | 134.9 (C) | 1.52 (1H, d, 0.7) | 32.72 (CH) | 2.06 (2H, m, 4.2) | 26.21 (CH2) | |
| 12 | 2.148 (2H, m, 6.6) | 26.3 (CH2) | 1.32 (2H, s) | 36.9 (CH2) | 5.10 (1H, t, 2.2) | 124.3 (CH2) |
| 13 | 2.148 (2H, m, 6.6) | 25.7 (CH2) | 1.29 (2H, m, 0.6) | 24.7 (CH2) | 131.5 (C) | |
| 14 | 5.10 (1H, m, 0.6) | 124.4 (CH) | 1.28 (2H, s) | 38.9 (CH2) | 1.64 (3H, m, 7) | 25.79 (CH3) |
| 15 | 131.3 (C) | 1.52 (1H, d, 0.02) | 28.0 (CH) | 1.62 (3H, m, 2.1) | 21.15 (CH3) | |
| 16 | 1.673 (3H, s) | 17.67 (CH3) | 0.79 (3H, d, 0.1) | 22.72 (CH3) | 1.62 (3H, m, 2.1) | 16.1 (CH3) |
| 17 | 1.673 (3H, s) | 16.28 (CH3) | 0.84 (3H, 0.1) | 22.7 (CH3) | 1.68 (3H, d, 3.1) | 17.78 (CH3) |
| 18 | 1.593 (3H, s) | 16 (CH3) | 0.85 (3H, s) | 19.7 (CH3) | ||
| 19 | 1.593 (3H, s) | 15.87 (CH3) | 0.86 (3H, s) | 19.6 (CH3) | ||
| 20 | 1.593 (3H, s) | 15.97 (CH3) | 1.67 (3H, d, 0.6) | 17 (CH3) | ||
| Sample | CC50 (µg mL−1) a |
|---|---|
| PEAm | 298.30 ± 2.87 |
| CC50 (µM) a | |
| FA | 0.275 ± 0.08 |
| PT | 0.296 ± 0.07 |
| Gg | 0.395 ± 0.005 * |
| MTX | 0.243 ± 0.007 |
| Treatment | ED50 (mg kg−1) a | |
|---|---|---|
| Female | Male | |
| PEAm | 180.42 ± 1.65 * | 166.41 ± 3.8 * |
| FA | 5.89 ± 0.39 * | 5.09 ± 0.66 * |
| PT | 6.71 ± 0.31 * | 5.83 ± 0.50 * |
| Gg | 7.22 ± 0.51 * | 6.98 ± 0.57 * |
| MTX | 1.31 ± 0.34 | 0.99 ± 0.024 |
| Sample | LD50 (mg/kg) a | TI b Female | TI b Male |
|---|---|---|---|
| PEAd | >3000 | 16.62 | 18.02 |
| FA | >1000 | 169.77 | 196.46 |
| PT | >1000 | 149.03 | 171.52 |
| Gg | 742.17 ± 0.34 | 138.5 | 143.26 |
| MTX | 335.04 ± 0.39 | 255.75 | 338.42 |
| Compound | HMG-CoA Reductase | |||
|---|---|---|---|---|
| ΔG (kcal-mol−1) | H-BR | NPI | RMSD | |
| Farnesyl acetate | −7.38 | Ile 536, Thr 557, Thr 558, Glu 559, Gly 560, Asn 755, Thr 758, Tyr 761, Ile 762, Gly 765, Gln 766, Asp 767, Ala 768, Val 805, Gly 806, Gly 807, Gly 808, Thr 809 | Met 655, Ala 769 | - |
| Phytol | −7.55 | Met 534, Thr 557, Thr 558, Glu 559, Gly 560, Met 588, Lys 691, Asn 755, Thr 758, Ile 762, Gly 765 Gln 766, Asp 767, Ala 769, Gln 770, Gly 807, Gly 808, Gln 814 | Ile 536, Met 655, Met 657, Ile 762, Ala 768 | - |
| Geranylgeraniol | −7.95 | Glu 559, Leu 562, Thr 557, Thr 558, Ala 654, Gly 656, Asn 658, Met 659, Asn 755, Thr 758, Ala 759, Ile 762, Gly 765, Gln 766, Asp 767, Ala 769, Val 805, Gly806, Gly 807, Gly 808, Thr 809 | Met 655, Met 657, Ala 768 | - |
| HMG-CoA- substrate | −9.21 | Pro 477, Tyr 479, Glu 528, Asn 529, Cys 561, Leu 562, Ser 565, Asn 567, Arg 590, Met 657, Ser 684, Asn 686, Asp 690, Lys 691, Lys 692, Val 720, Lys 735, Ala 751, His 752, Asn 755, Ser 852, Ala 856, Leu 857, His 861 | Tyr 479, Ala 564, Arg 568, Arg 571, Lys 722, Leu 853, Leu 862 | 1.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Santos, J.; Calzada, F.; Mendieta-Wejebe, J.E.; Ordoñez-Razo, R.M.; Martinez-Casares, R.M.; Valdes, M. Understanding the Antilymphoma Activity of Annona macroprophyllata Donn and Its Acyclic Terpenoids: In Vivo, In Vitro, and In Silico Studies. Molecules 2022, 27, 7123. https://doi.org/10.3390/molecules27207123
Ramírez-Santos J, Calzada F, Mendieta-Wejebe JE, Ordoñez-Razo RM, Martinez-Casares RM, Valdes M. Understanding the Antilymphoma Activity of Annona macroprophyllata Donn and Its Acyclic Terpenoids: In Vivo, In Vitro, and In Silico Studies. Molecules. 2022; 27(20):7123. https://doi.org/10.3390/molecules27207123
Chicago/Turabian StyleRamírez-Santos, Jesica, Fernando Calzada, Jessica Elena Mendieta-Wejebe, Rosa María Ordoñez-Razo, Rubria Marlen Martinez-Casares, and Miguel Valdes. 2022. "Understanding the Antilymphoma Activity of Annona macroprophyllata Donn and Its Acyclic Terpenoids: In Vivo, In Vitro, and In Silico Studies" Molecules 27, no. 20: 7123. https://doi.org/10.3390/molecules27207123
APA StyleRamírez-Santos, J., Calzada, F., Mendieta-Wejebe, J. E., Ordoñez-Razo, R. M., Martinez-Casares, R. M., & Valdes, M. (2022). Understanding the Antilymphoma Activity of Annona macroprophyllata Donn and Its Acyclic Terpenoids: In Vivo, In Vitro, and In Silico Studies. Molecules, 27(20), 7123. https://doi.org/10.3390/molecules27207123










