Regrowth Response and Nutritional Composition of Moringa oleifera to Cutting Back in Three Agro-Ecological Zones in South Africa
Abstract
:1. Introduction
2. Materials and Methods
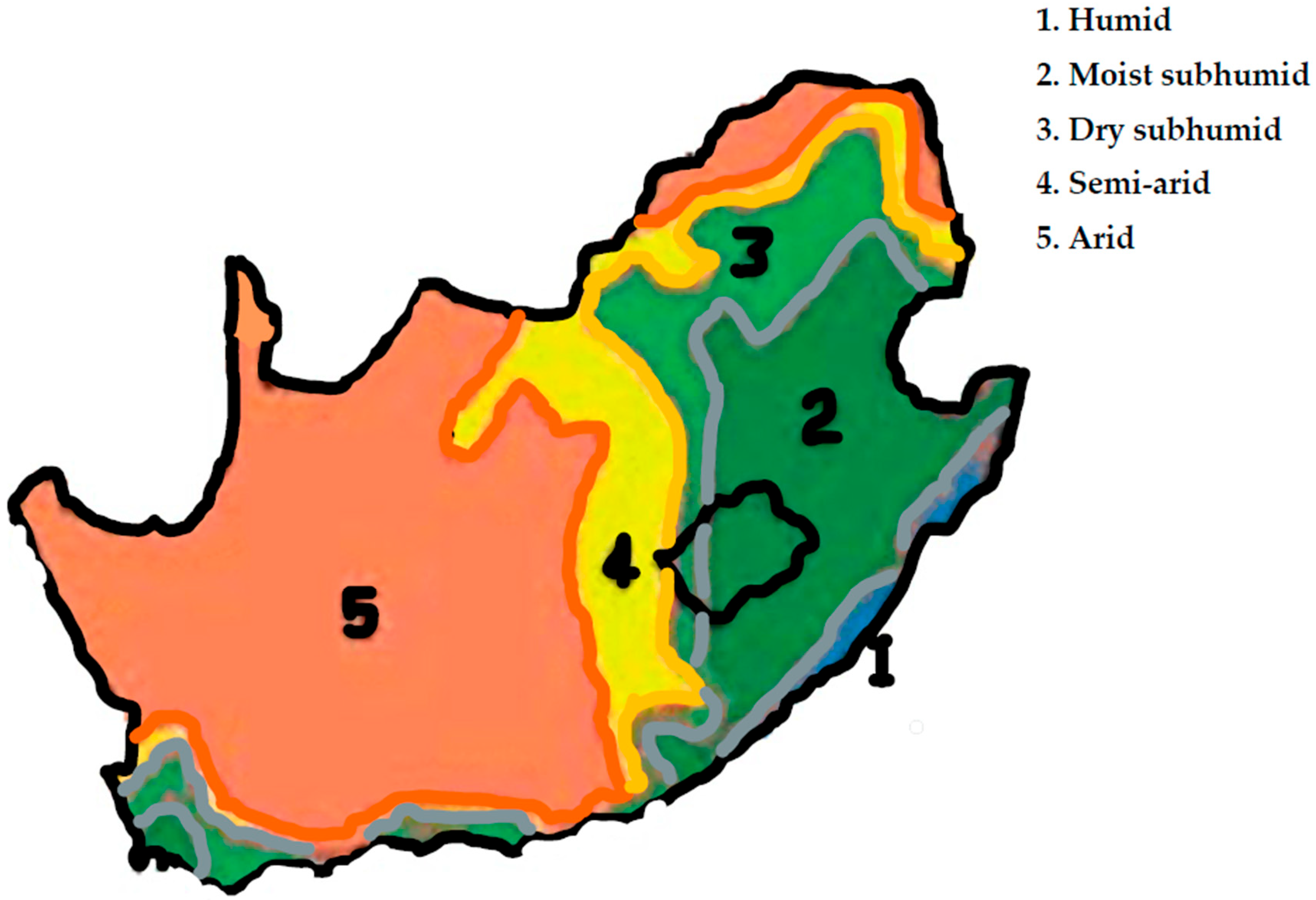
2.1. Study Sites
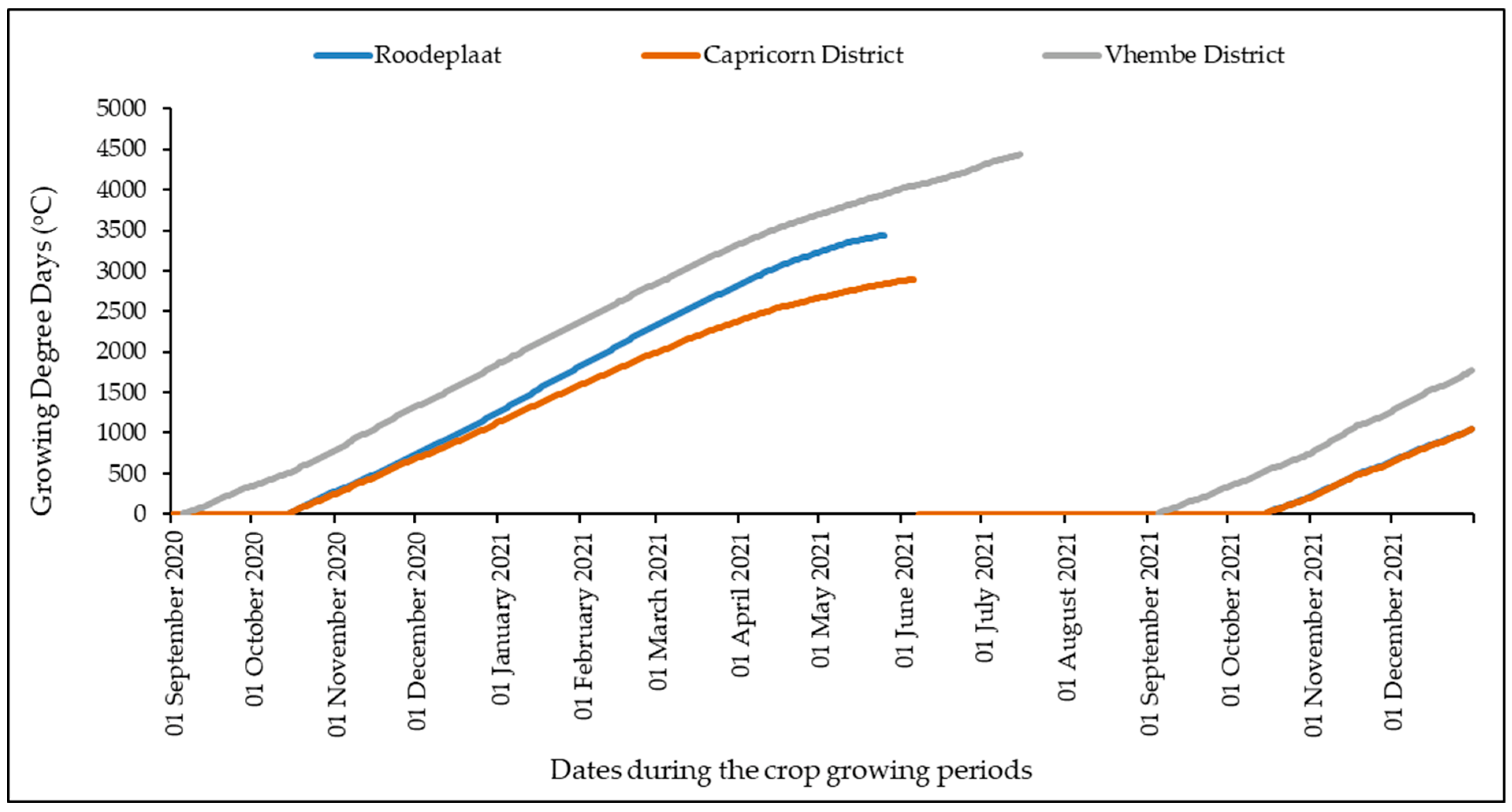
2.2. Growing Degree Days (GDD)
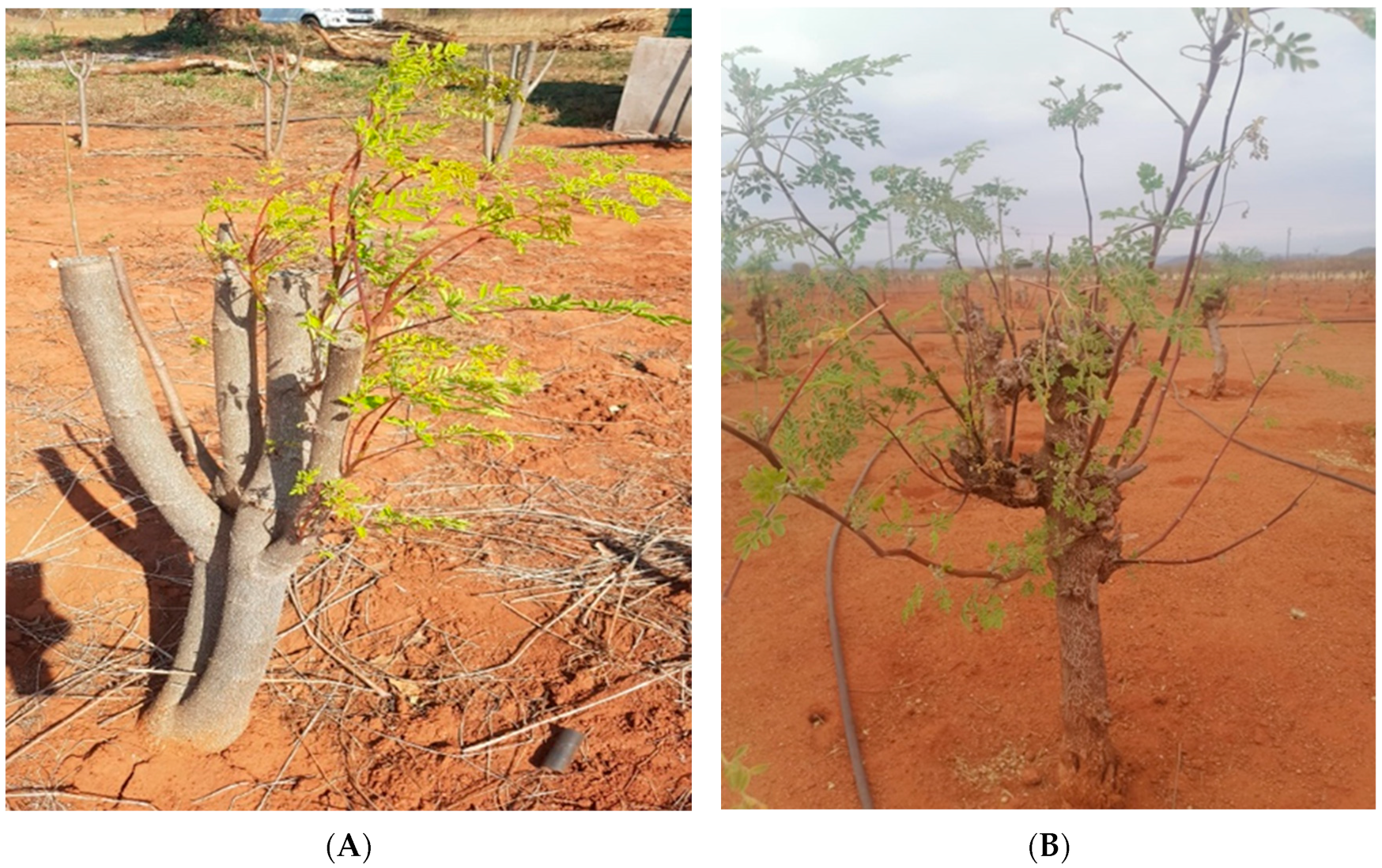
2.3. Measurement of the Number of Sprouts and Sprout Length of M. oleifera Plantation in Three Growing Areas
2.4. Soil Collection and Analysis
2.5. Leaf Nutritional Composition
2.6. Data Analysis
3. Results
3.1. Soil Analysis from Three Agro-Ecological Zones
3.2. Number and Length of Sprouts of Trees from the Three Agro-Ecological Zones
3.3. Thermal Time Accumulation of Moringa oleifera across the Three Agro-Ecological Zones of South Africa
3.4. Nutritional Composition of Moringa oleifera Leaves across the Study Three Agro-Ecological Zones of South Africa
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, S.V.; Mohite, B.V.; Marathe, K.R.; Salunkhe, N.S.; Marathe, V.; Patil, V.S. Moringa Tree, Gift of Nature: A Review on Nutritional and Industrial Potential. Curr. Pharmacol. Rep. 2022, 8, 262–280. [Google Scholar] [CrossRef] [PubMed]
- Korsor, M.; Ntahonshikira, C.; Bello, H.M.; Kwaambwa, H.M. Growth Performance of Moringa oleifera and Moringa ovalifolia in Central Namibia Semi-Arid Rangeland Environment. Agric. Sci. 2019, 10, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Godino, M.; Arias, C.; Izquierdo, M. Moringa oleifera: Potential areas of cultivation on the Iberian Peninsula. Acta Hortic. 2017, 1158, 405–412. [Google Scholar] [CrossRef]
- Milla, P.; Peñalver, R.; Nieto, G. Health Benefits of Uses and Applications of Moringa oleifera in Bakery Products. Plants 2021, 10, 318. [Google Scholar] [CrossRef]
- Saini, R.K.; Sivanesan, I.; Keum, Y.-S. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech 2016, 6, 203. [Google Scholar] [CrossRef] [Green Version]
- Daba, M. Miracle Tree: A Review on Multi-purposes of Moringa oleifera and Its Implication for Climate Change Mitigation. J. Earth Sci. Clim. Chang. 2016, 7, 8. [Google Scholar] [CrossRef]
- Seifu, E.; Teketay, D. Introduction and expansion of Moringa oleifera Lam. in Botswana: Current status and potential for commercialization. S. Afr. J. Bot. 2020, 129, 471–479. [Google Scholar] [CrossRef]
- Tshabalala, T.; Ndhlala, A.; Ncube, B.; Abdelgadir, H.; Van Staden, J. Potential substitution of the root with the leaf in the use of Moringa oleifera for antimicrobial, antidiabetic and antioxidant properties. S. Afr. J. Bot. 2019, 129, 106–112. [Google Scholar] [CrossRef]
- Sarkar, S.; Panda, S. Moringa oleifera Lam.—A multipurpose and great therapeutic tree: A review. In Medicinal Plants: Herbal Wealth of India; Sharma, I.R., Ed.; Agrobios Research: Jodhpur, India, 2021; pp. 103–119. [Google Scholar]
- Glover-Amengor, M.; Aryeetey, R.; Afari, E.; Nyarko, A. Micronutrient composition and acceptability of Moringa oleifera leaf-fortified dishes by children in Ada-East district, Ghana. Food Sci. Nutr. 2016, 5, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Popoola, J.O.; Obembe, O.O. Local knowledge, use pattern and geographical distribution of Moringa oleifera Lam. (Moringaceae) in Nigeria. J. Ethnopharmacol. 2013, 150, 682–691. [Google Scholar] [CrossRef]
- Islam, Z.; Islam, S.M.R.; Hossen, F.; Mahtab-Ul-Islam, K.; Hasan, R.; Karim, R. Moringa oleifera is a Prominent Source of Nutrients with Potential Health Benefits. Int. J. Food Sci. 2021, 2021, 6627265. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Ogunsina, B.S.; Indira, T.N.; Bhatnagar, A.S.; Radha, C.; Debnath, S.; Krishna, A.G.G. Quality characteristics and stability of Moringa oleifera seed oil of Indian origin. J. Food Sci. Technol. 2011, 51, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boukandoul, S.; Casal, S.; Zaidi, F. The Potential of Some Moringa Species for Seed Oil Production. Agriculture 2018, 8, 150. [Google Scholar] [CrossRef] [Green Version]
- Abdullahi, I.N.; Ozobia, A.P. The performance of Soybean using Moringa as an alley to improve soil productivity in North-Central Nigeria. Afr. J. Agric. Res. 2017, 12, 1182–1189. [Google Scholar]
- Reppin, S.; Kuyah, S.; de Neergaard, A.; Oelofse, M.; Rosenstock, T.S. Contribution of agroforestry to climate change mitigation and livelihoods in Western Kenya. Agrofor. Syst. 2019, 94, 203–220. [Google Scholar] [CrossRef] [Green Version]
- Fahey, J. Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Trees Life J. 2005, 1, 5. [Google Scholar]
- Delelegn, A.; Sahile, S.; Husen, A. Water purification and antibacterial efficacy of Moringa oleifera Lam. Agric. Food Secur. 2018, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Mabapa, M.P.; Ayisi, K.K.; Mariga, I.K. Effect of Planting Density and Harvest Interval on the Leaf Yield and Quality of Moringa (Moringa oleifera) under Diverse Agroecological Conditions of Northern South Africa. Int. J. Agron. 2017, 2017, 2941432. [Google Scholar] [CrossRef] [Green Version]
- Bopape-Mabapa, M.P. Yield Characteristics, Carbon Capture and Chemical Composition of Moringa Oleifera under Diverse Planting Populations and Agro-Ecological Conditions of the Limpopo Province. Ph.D. Thesis, University of Limpopo, Mankweng, Africa, 2019. [Google Scholar]
- Pinkard, E.A.; Beadle, C.L. A physiological approach to pruning. Int. For. Rev. 2000, 2, 295–305. [Google Scholar]
- Du Toit, E.S.; Sithole, J.; Vorster, J. Pruning intensity influences growth, flower and fruit development of Moringa oleifera Lam. under sub-optimal growing conditions in Gauteng, South Africa. S. Afr. J. Bot. 2019, 129, 448–456. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organisation). A Perspective on Water Control in Southern Africa. 2003. Available online: http://www.fao.org/docrep/006/y5096e/y5096e00.htm#Contents (accessed on 17 April 2013).
- Serote, B.; Mokgehle, S.; Du Plooy, C.; Mpandeli, S.; Nhamo, L.; Senyolo, G. Factors Influencing the Adoption of Climate-Smart Irrigation Technologies for Sustainable Crop Productivity by Smallholder Farmers in Arid Areas of South Africa. Agriculture 2021, 11, 1222. [Google Scholar] [CrossRef]
- Trigo, C.; Castelló, M.L.; Ortolá, M.D.; García-Mares, F.J.; Soriano, M.D. Moringa oleifera: An unknown crop in developed countries with great potential for industry and adapted to climate change. Foods 2021, 10, 31. [Google Scholar] [CrossRef]
- Ibraimo, N.A.; Taylor, N.J.; Steyn, J.M.; Gush, M.B.; Annandale, J.G. Estimating water use of mature pecan orchards: A six stage crop growth curve approach. Agric. Water Manag. 2016, 177, 359–368. [Google Scholar] [CrossRef]
- Moyo, M.; Amoo, S.O.; Aremu, A.O.; Gruz, J.; Šubrtová, M.; Jarošová, M.; Tarkowski, P.; Doležal, K. Determination of Mineral Constituents, Phytochemicals and Antioxidant Qualities of Cleome gynandra, Compared to Brassica oleracea and Beta vulgaris. Front. Chem. 2018, 5, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Sass-Klaassen, U.; Sterck, F.; Goudzwaard, L.; Akhmetzyanov, L.; Poorter, L. Growth of 19 conifer species is highly sensitive to winter warming, spring frost and summer drought. Ann. Bot. 2021, 128, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Cochard, H.; Badel, E.; Herbette, S.; Delzon, S.; Choat, B.; Jansen, S. Methods for measuring plant vulnerability to cavitation: A critical review. J. Exp. Bot. 2013, 64, 4779–4791. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.J.; Jackson, R.B. Xylem cavitation caused by drought and freezing stress in four co-occurring Juniperus species. Physiol. Plant. 2006, 127, 374–382. [Google Scholar] [CrossRef]
- Del Tredici, P. Sprouting in temperate trees: A morphological and ecological review. Bot. Rev. 2001, 67, 121–140. [Google Scholar] [CrossRef]
- Bond, W.J.; Midgley, J.J. The Evolutionary Ecology of Sprouting in Woody Plants. Bot. Gaz. 2003, 164, S103–S114. [Google Scholar] [CrossRef] [Green Version]
- Vesk, P.A.; Westoby, M. Sprouting ability across diverse disturbances and vegetation types worldwide. J. Ecol. 2004, 92, 310–320. [Google Scholar] [CrossRef]
- Kennard, D.; Gould, K.; Putz, F.; Fredericksen, T.; Morales, F. Effect of disturbance intensity on regeneration mechanisms in a tropical dry forest. For. Ecol. Manag. 2002, 162, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Pauw, A.; Van Bael, S.A.; Peters, H.A.; Allison, S.D.; Camargo, J.L.C.; Cifuentes-Jara, M.; Conserva, A.; Restom, T.G.; Heartsiii-Scalley, T.; Mangan, S.A.; et al. Physical damage in relation to carbon allocation strategies of tropical forest tree saplings. Biotropica 2004, 36, 410–413. [Google Scholar] [CrossRef]
- Rani, N.Z.A.; Husain, K.; Kumolosasi, E. Moringa Genus: A Review of Phytochemistry and Pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbikay, M. Therapeutic Potential of Moringa oleifera Leaves in Chronic Hyperglycemia and Dyslipidemia: A Review. Front. Pharmacol. 2012, 3, 24. [Google Scholar] [CrossRef] [PubMed]



| Location | Clay Content (%) | N | P (Bray 1) | K | Mg | Ca | pH |
|---|---|---|---|---|---|---|---|
| (%) | mg/kg | H2O | |||||
| ARC-Roodeplaat | 22.0 | 0.04 | 54.43 | 287.7 | 275.3 | 919.3 | 7.06 |
| Vhembe district | 24.4 | 8.14 | 168.2 | 166.4 | 475.0 | 893.2 | 5.64 |
| Capricorn district | 14.0 | 5.60 | 132.2 | 123.6 | 74.1 | 2227.0 | 6.50 |
| Source of Variation | Degree of Freedom | Sum of Square | Mean Square | Variance Ratio | p Value |
|---|---|---|---|---|---|
| Number of sprouts | |||||
| Treatments | 2 | 41.0 | 20.5 | 18.7 | ** |
| Residual | 12 | 13.1 | 1.0 | ||
| Total | 14 | 54.2 | |||
| Sprout length | |||||
| Treatments | 2 | 177.7 | 88.8 | 50.4 | ** |
| Residual | 12 | 21.1 | 1.7 | ||
| Total | 14 | 198.8 |
| Location | Number of Sprouts | Length of Sprouts (cm) |
|---|---|---|
| ARC-Roodeplaat | 9.7 b | 13.68 b |
| Vhembe district | 13.4 a | 21.50 a |
| Capricorn district | 10.1 b | 20.32 a |
| CV (%) | 9.4 | 7.2 |
| Parameter | Unit | Nutritional Content for Different Agro-Ecological Regions | ||
|---|---|---|---|---|
| ARC-Roodeplaat | Capricorn District | Vhembe District | ||
| Protein | g/100 g | 27.8 a | 26.5 a | 24.2 a |
| Total fat | g/100 g | 2.3 b | 5.56 a | 4.40 a |
| Calcium | mg/1000 g | 32,652.1 a | 22,255 b | 23,757 b |
| Magnesium | mg/1000 g | 3460.5 b | 4467 a | 4753 a |
| Iron | mg/1000 g | 291.6 a | 236.6 a | 261.1 a |
| Sodium | mg/100 g | 29 c | 118.8 b | 130.8 a |
| Phosphorus | mg/1000 g | 2620.1 b | 3116 a | 3416 a |
| Potassium | mg/1000 g | 11,916.3 b | 16,224 a | 17,191 a |
| Zinc | mg/kg | 17.5 b | 40.8 a | 47.2 a |
| β-carotene | mg/100 g | 0.978 a | 0.855 a | 0.820 a |
| Vitamin C | mg/100 g | 276.3 a | 221 a | 201 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokgehle, S.; Araya, N.; Mofokeng, M.; Makgato, M.; Amoo, S.; Maboka, K.; du Plooy, C.; Araya, H. Regrowth Response and Nutritional Composition of Moringa oleifera to Cutting Back in Three Agro-Ecological Zones in South Africa. Horticulturae 2022, 8, 963. https://doi.org/10.3390/horticulturae8100963
Mokgehle S, Araya N, Mofokeng M, Makgato M, Amoo S, Maboka K, du Plooy C, Araya H. Regrowth Response and Nutritional Composition of Moringa oleifera to Cutting Back in Three Agro-Ecological Zones in South Africa. Horticulturae. 2022; 8(10):963. https://doi.org/10.3390/horticulturae8100963
Chicago/Turabian StyleMokgehle, Salmina, Nadia Araya, Motiki Mofokeng, Manaka Makgato, Stephen Amoo, Khomotso Maboka, Christian du Plooy, and Hintsa Araya. 2022. "Regrowth Response and Nutritional Composition of Moringa oleifera to Cutting Back in Three Agro-Ecological Zones in South Africa" Horticulturae 8, no. 10: 963. https://doi.org/10.3390/horticulturae8100963
APA StyleMokgehle, S., Araya, N., Mofokeng, M., Makgato, M., Amoo, S., Maboka, K., du Plooy, C., & Araya, H. (2022). Regrowth Response and Nutritional Composition of Moringa oleifera to Cutting Back in Three Agro-Ecological Zones in South Africa. Horticulturae, 8(10), 963. https://doi.org/10.3390/horticulturae8100963








