Comprehensive Evaluation of 24 Red Raspberry Varieties in Northeast China Based on Nutrition and Taste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Determination of Fruit Length, Diameter, Weight
2.4. Determination of Titratable Acidity (TAC), Soluble Solids Contents (SSC), pH and Sugar-Acid Ratio (SAR)
2.5. Determination of Individual Sugar
2.6. Determination of Organic Acids
2.7. Sensory Characteristics Analysis
2.8. Determination of Ascorbic Acid
2.9. Determination of Total Phenol Contents (TPC)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Length, Diameter, Weight and Appearance Range of Red Raspberry
3.2. Moisture, Total Sugar, Reducing Sugar, Titratable Acidity (TAC), Soluble Solids Contents (SSC), pH and Sugar-Acid Ratio (SAR)
3.3. Individual Sugar and Organic Acid Contents
3.3.1. Individual Sugar Contents
3.3.2. Organic Acid Contents
3.4. Sensory Characteristics Analysis
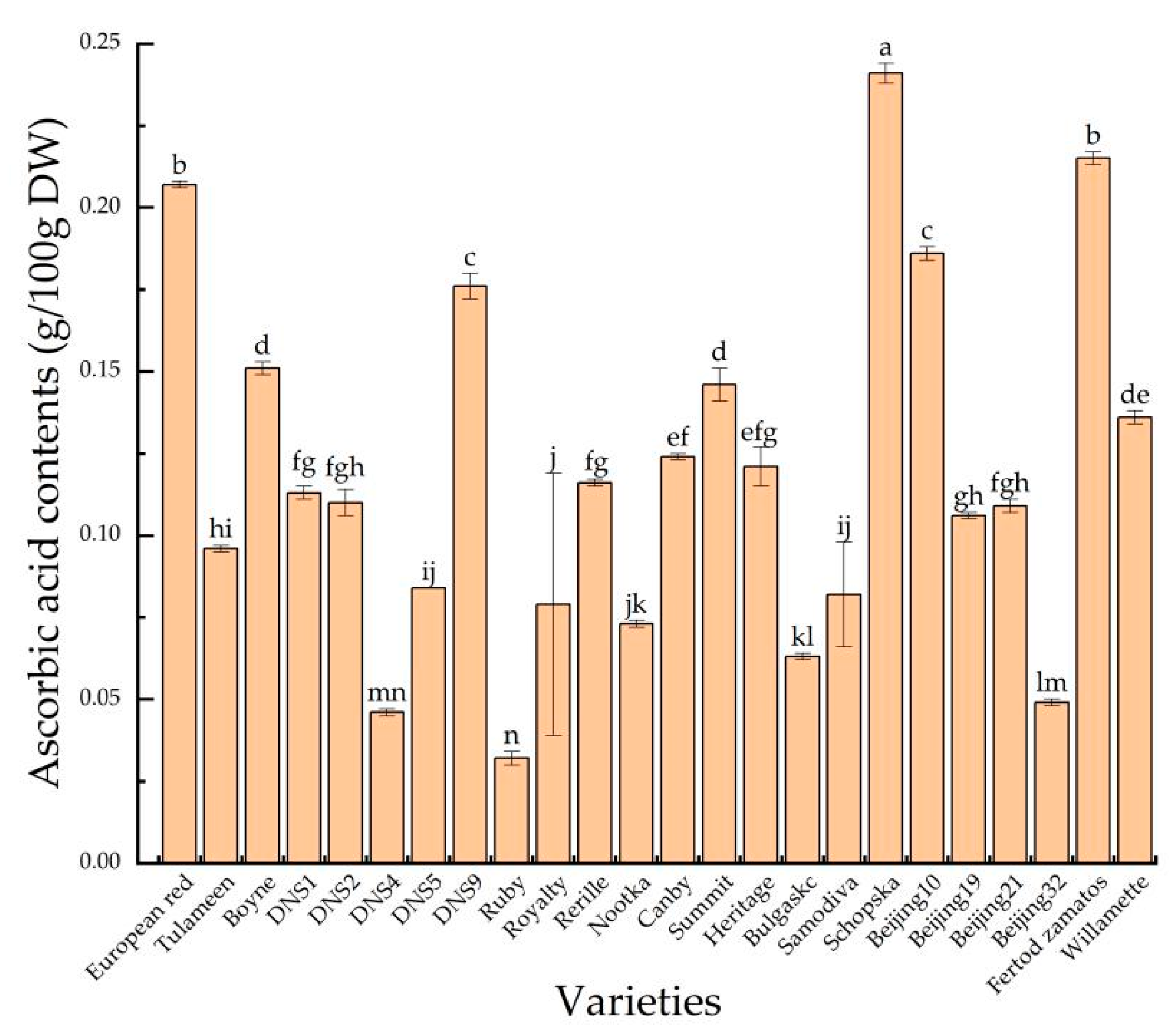
3.5. Ascorbic Acid Contents
3.6. Total Phenol Contents (TPC)
3.7. Principal Component Analysis (PCA)
3.8. Cluster Analysis (CA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veljković, B.; Šoštarić, I.; Dajić, S.Z.; Liber, Z.; Šatović, Z. Genetic structure of wild raspberry populations in the Central Balkans depends on their location and on their relationship to commercial cultivars. Sci. Hortic. 2019, 256, 108–606. [Google Scholar] [CrossRef]
- Xian, L.; Sun, J.H.; Ahuja, J.; Haytowitz, D.B.; Chen, P.; Burton, F.B.; Pehrsson, P.R. Anthocyanins in processed red raspberries on the US market. J. Berry Res. 2019, 9, 603–613. [Google Scholar] [CrossRef]
- Vara, A.L.; Pinela, J.; Dias, M.I.; PetroviÄ, J.; Nogueira, A.; SokoviÄ, M.; Ferreira, I.C.F.R.; Barros, L. Compositional features of the “Kweli” red raspberry and its antioxidant and antimicrobial activities. Foods 2019, 9, 1522. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Choi, I.S. Comparison of the phenolic composition and antioxidant activity of Korean black raspberry, Bokbunja, (Rubus coreanus Miquel) with those of six other berries. CyTA—J. Food 2017, 15, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Toshima, S.; Hirano, T.; Kunitake, H. Comparison of anthocyanins, polyphenols, and antioxidant capacities among raspberry, blackberry, and Japanese wild Rubus species. Sci. Hortic. 2021, 285, 110–204. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katarzyna, K.; Anna, O.; Joanna, Z.W.; Mariola, O. Raspberry (Rubus idaeus L.) fruit extract decreases oxidation markers, improves lipid metabolism and reduces adipose tissue inflammation in hypertrophied 3T3-L1 adipocytes. J. Funct. Foods 2019, 62, 103–568. [Google Scholar] [CrossRef]
- Sara, D.; Cheryl, L.; Gordon, J.M.J.; William, A.; Gema, P.C.; José, M.M.R. Ex vivo fecal fermentation of human ileal fluid collected after raspberry consumption modifies (poly) phenolics and modulates genoprotective effects in colonic epithelial cells. Redox Biol. 2021, 40, 101–862. [Google Scholar] [CrossRef]
- Noratto, G.D.; Chew, B.P.; Atienza, L.M. Red raspberry (Rubus idaeus L.) intake decreases oxidative stress in obese diabetic (db/db) mice. Food Chem. 2017, 227, 305–314. [Google Scholar] [CrossRef]
- Chen, K.; Fijen, T.P.M.; Kleijn, D.; Scheper, J. Insect pollination and soil organic matter improve raspberry production independently of the effects of fertilizers. Agric. Ecosyst. Environ. 2021, 309, 107–270. [Google Scholar] [CrossRef]
- Teng, H.; Fang, T.; Lin, Q.Y.; Song, H.B.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Technol. 2017, 16, 305–337. [Google Scholar] [CrossRef]
- Akimov, M.Y. New breeding and technological evaluation criteria for fruit and berry products for the healthy and dietary food industry. Vopr. Pitan. 2020, 89, 244–254. [Google Scholar] [CrossRef]
- Pauliina, P.; Tuomo, L.; Katriina, M. Floricane yield and berry quality of seven primocane red raspberry (Rubus idaeus L.) cultivars. Sci. Hortic. 2021, 285, 110–201. [Google Scholar] [CrossRef]
- Yang, J.; Cui, J.Y.; Chen, J.X.; Yao, J.Y.; Hao, Y.X.; Fan, Y.L.; Liu, Y.P. Evaluation of physicochemical properties in three raspberries (Rubus idaeus) at five ripening stages in northern China. Sci. Hortic. 2020, 263, 109–146. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, J.Y.; Li, X.B.; Xie, Y.W.; Jin, Z.X.; Wang, X.Y.; Li, Y.L.; Zhong, Y.J.; Lin, J.J.; Yang, W.Q. Bioactive compounds and fruit quality of Chinese raspberry, Rubus chingii Hu varied with genotype and phenological phase. Sci. Hortic. 2021, 281, 109–951. [Google Scholar] [CrossRef]
- Cetin, C.; Mustafa, O. Comparison of antioxidant capacity and phytochemical properties of wild and cultivated red raspberries (Rubus idaeus L.). J. Food Compost. Anal. 2010, 23, 540–544. [Google Scholar] [CrossRef]
- Xu, C.; Yagiz, Y.; Zhao, L.; Simonne, A.; Lu, J.; Marshall, M.R. Fruit quality, nutraceutical and antimicrobial properties of 58 muscadine grape varieties (Vitis rotundifolia Michx.) grown in United States. Food Chem. 2017, 215, 149–156. [Google Scholar] [CrossRef]
- Latimer, G.W.; Horwitz, W. Official Methods of Analysis of AOAC International, 18 th ed.; AOAC International: Rockville, MD, USA, 2006. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Tilahun, S.; Choi, H.R.; Park, D.S.; Lee, Y.M.; Choi, J.H.; Baek, M.W.; Hyok, K.; Park, S.M.; Jeong, C.S. Ripening quality of kiwifruit cultivars is affected by harvest time. Sci. Hortic. 2020, 261, 108–936. [Google Scholar] [CrossRef]
- He, H.Y.; Yan, Y.C.; Dong, D.; Bao, Y.H.; Luo, T.; Chen, Q.; Wang, J.L. Effect of Issatchenkia terricola WJL-G4 on deacidifification characteristics and antioxidant activities of red raspberry wine processing. Fungi 2022, 8, 17. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Mereddy, R.; Webber, D.; Sultanbawa, Y. Biochemical, antioxidant and sensory evaluation of Davidsonia pruriens and Davidsoina jerseyana fruit infusion. Food Chem. 2021, 342, 128349. [Google Scholar] [CrossRef] [PubMed]
- Pantelidis, G.E.; Vasilakakis, M.; Manganaris, G.A.; Diamantidis, G.R. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem. 2007, 102, 777–783. [Google Scholar] [CrossRef]
- Rambaran, T.F.; Bowen-Forbes, C.S. Chemical and sensory characterisation of two Rubus rosifolius (red raspberry) varieties. Int. J. Food Sci. 2020, 2020, 6879460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Liu, S.R.; Hu, C.G. Identifying the genome-wide genetic variation between precocious trifoliate orange and its wild type and developing new markers for genetics research. DNA Res. 2016, 23, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.M.; Gao, X.; Wang, X.P.; Liu, P.Z.; Guan, S.L.; Qi, K.J.; Zhang, S.L.; Gu, C. Transcriptome analysis reveals gene associated with fruit size during fruit development in pear. Sci. Hortic. 2022, 305, 111–367. [Google Scholar] [CrossRef]
- Liu, X.Y.; Li, J.; Huang, M.; Chen, J.Z. Mechanisms for the influence of citrus rootstocks on fruit size. J. Agric. Food Chem. 2015, 63, 2618–2627. [Google Scholar] [CrossRef]
- Stephens, M.J.; Alspach, P.A.; Beatson, R.A.; Winefield, C.; Buck, E.J. Genotype-environmental interaction involving British and Pacific Northwest red raspberry cultivars. J. Am. Soc. Hortic. Sci. 2012, 137, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Birgi, J.; Peri, P.L.; Gargcglione, V. Raspberries and gooseberries in south Patagonia: Production, fruit quality, morphology and phenology in two environmental conditions. Sci. Hortic. 2019, 258, 108–574. [Google Scholar] [CrossRef]
- Harris, J.M.; Kriedemann, P.E.; Possingham, J.V. Anatomical aspects of grape berry development. Vitis 1968, 7, 106–109. [Google Scholar] [CrossRef]
- Tanksley, S.D. The genetic, developmental, and molecular bases of fruit size and shape variation in tomato. Plant Cell 2004, 16, 181–189. [Google Scholar] [CrossRef]
- Stojanov, D.; Milošević, T.; Mašković, P.; Milošević, N.; Glišić, I.; Paunović, G. Influence of organic, organo-mineral and mineral fertilisers on cane traits, productivity and berry quality of red raspberry (Rubus idaeus L.). Sci. Hortic. 2019, 252, 370–378. [Google Scholar] [CrossRef]
- Xie, D.D.; Liu, D.Y.; Guo, W.C. Relationship of the optical properties with soluble solids content and moisture content of strawberry during ripening. Postharvest Biol. Technol. 2021, 179, 111–569. [Google Scholar] [CrossRef]
- Kibar, H.; Kibar, B. Changes in some nutritional, bioactive and morpho-physiological properties of common bean depending on cold storage and seed moisture contents. J. Stored Prod. Res. 2019, 84, 101–531. [Google Scholar] [CrossRef]
- Rodriguez, A.; Bruno, E.; Paola, C.; Campañone, L.; Mascheroni, R.H. Experimental study of dehydration processes of raspberries (Rubus idaeus) with microwave and solar drying. Food Sci. Technol. 2018, 39, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Alibabic, V.; Skender, A.; Bajramovic, M.; Šertovic, E.; Bajric, E. Evaluation of morphological, chemical, and sensory characteristicsof raspberry cultivars grown in Bosnia and Herzegovina. Turk. J. Agric. For. 2018, 42, 67–74. [Google Scholar] [CrossRef]
- Frías-Moreno, M.N.; Olivas, O.G.I.; Gonzalez, A.G.A.; Benitez, E.Y.E.; Paredes, A.A.; Jacobo, C.J.L.; Salas, S.N.A.; Ojeda, B.D.L.; Parra, Q.R.A. Yield, quality and phytochemicals of organic and conventional raspberry cultivated in Chihuahua, Mexico. Not. Bot. Horti Agrobot. 2018, 47, 522. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhang, C.; Huang, Z.; Lyu, L.; Li, J.; Li, W.; Wu, W. The color difference of rubus fruits is closely related to the composition of flavonoids including anthocyanins. LWT 2021, 149, 111–825. [Google Scholar] [CrossRef]
- Anjos, R.; Cosme, F.; Gonçalves, A.; Nunes, F.M.; Vilela, A.; Pinto, T. Effect of agricultural practices, conventional vs. organic, on the phytochemical composition of ‘Kweli’ and ‘Tulameen’ raspberries (Rubus idaeus L.). Food Chem. 2020, 328, 126833. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, R.; Guimarães, F.N.; Santos, V.M.; Resende, J.C.F. Post-harvest quality of the and prata-anã bananas, cultivated in northern Minas Gerais. Rev. Bras. Fruticult. 2013, 32, 42–44. [Google Scholar] [CrossRef]
- Valentinuzzi, F.; Pii, Y.; Mimmo, T.; Savini, G.; Curzel, S.; Cesco, S. Fertilization strategies as a tool to modify the organoleptic properties of raspberry (Rubus idaeus L.) fruits. Sci. Hortic. 2018, 240, 205–212. [Google Scholar] [CrossRef]
- Agredano-De La Garza, C.S.; Balois-Morales, R.; Berumen-Varela, G.; León-Fernández, A.E.; Bautista-Rosales, P.U.; López-Guzmán, G.G.; Pérez-Ramírez, I.F. Physicochemical characterization and dietary fiber of 15 Nance (Byrsonima crassifolia L.) fruits selections from Nayarit. Sci. Hortic. 2021, 289, 110460. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.H.; Zhao, L.; Shen, T.; Sun, J.Y.; Chen, H.W.; Kong, Q.S.; Nawaz, M.A.; Bie, Z.L. Melon fruit sugar and amino acid contents are affected by fruit setting method under protected cultivation. Sci. Hortic. 2017, 214, 288–294. [Google Scholar] [CrossRef]
- Mouillot, T. Taste perception and cerebral activity in the human gustatory cortex induced by glucose, fructose, and sucrose solutions. Chem. Senses 2019, 44, 435–447. [Google Scholar] [CrossRef]
- Stavang, J.A.; Freitag, S.; Foito, A.; Verrall, S.; Heide, O.M.; Stewart, D.; Sønsteby, A. Raspberry fruit quality changes during ripening and storage as assessed by colour, sensory evaluation and chemical analyses. Sci. Hortic. 2015, 195, 216–225. [Google Scholar] [CrossRef]
- Gao, Q.H.; Wang, M. Response to comment on effect of drying of jujubes (Ziziphus jujuba Mill.) on the contents of sugars, organic acids, α-tocopherol, β-carotene, and phenolic compounds. J. Agric. Food Chem. 2013, 61, 4665. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.M.H.; Arzani, K.; Barzegar, M. Analysis of sugars and organic acids contents of date palm (Phoenix dactylifera L.) ‘Barhee’ during fruit development. In Proceedings of the IV International Date Palm Conference, Abu Dhabi, United Arab Emirates, 15–17 March 2010; pp. 793–801. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Compositional and sensory characteristics of three native sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. Agric. Food Chem. 2005, 53, 7586–7591. [Google Scholar] [CrossRef] [PubMed]
- Guan, N.; Liu, L. Microbial response to acid stress: Mechanisms and applications. Appl. Microbiol. Biotechnol. 2019, 104, 51–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lückstädt, C.; Mellor, S. The use of organic acids in animal nutrition, with special focus on dietary potassium diformate under European and Austral-Asian conditions. Recent Adv. Anim. Nutr. 2011, 18, 123–130. [Google Scholar] [CrossRef]
- Seung, K.L.; Adel, A.K. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. J. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Krupa, T.; Latocha, P.; Liwińska, A. Changes of physicochemical quality, phenolics and vitamin C content in hardy kiwifruit (Actinidia arguta and its hybrid) during storage. Sci. Hortic. 2011, 130, 410–417. [Google Scholar] [CrossRef]
- Ma, T.T.; Sun, X.Y.; Zhao, J.M.; You, Y.L.; Lei, Y.S.; Gao, G.T.; Zhan, J.C. Nutrient compositions and antioxidant capacity of kiwifruit (Actinidia) and their relationship with flesh color and commercial value. Food Chem. 2017, 218, 294–304. [Google Scholar] [CrossRef]
- Van de Velde, F.; Grace, M.H.; Esposito, D.; Pirovani, M.E.; Lila, M.A. Quantitative comparison of phytochemical profile, antioxidant, and anti-inflammatory properties of blackberry fruits adapted to Argentina. J. Food Compos. Anal. 2016, 47, 82–91. [Google Scholar] [CrossRef]
- Schulz, M.; Chim, J.F. Nutritional and bioactive value of Rubus berries. Food Biosci. 2019, 31, 100438. [Google Scholar] [CrossRef]
- Vu, K.D.; Carlettini, H.; Bouvet, J.; Côté, J.; Doyon, G.; Sylvain, J.F.; Lacroix, M. Effect of different cranberry extracts and juices during cranberry juice processing on the antiproliferative activity against two colon cancer cell lines. Food Chem. 2012, 132, 959–967. [Google Scholar] [CrossRef]
- Vattem, D.A.; Jang, H.D.; Levin, R.; Shetty, K. Synergism of cranberry phenolics with ellagic acid and rosmarinic acid for antimutagenic and DNA protection functions. Food Biochem. 2006, 30, 98–116. [Google Scholar] [CrossRef]
- Caillet, S.; Côté, J.; Sylvain, J.-F.; Lacroix, M. Antimicrobial effects of fractions from cranberry products on the growth of seven pathogenic bacteria. Food Control 2012, 23, 419–428. [Google Scholar] [CrossRef]
- Caillet, S.; Côté, J.; Doyon, G.; Sylvain, J.-F.; Lacroix, M. Antioxidant and antiradical properties of cranberry juice and extracts. Food Res. Int. 2011, 44, 1408–1413. [Google Scholar] [CrossRef]
- Andreasen, M.F.; Kroon, P.A.; Williamson, G.; Garcia-Conesa, M.T. Esterase activity able to hydrolyze dietary antioxidant hydroxycinnamates is distributed along the intestine of mammals. J. Agric. Food Chem. 2001, 49, 5679–5684. [Google Scholar] [CrossRef]
- Liwicki, S.; Tzimiropoulos, G.; Zafeiriou, S.; Pantic, M. Euler principal component analysis. Int. J. Comput. Vis. 2013, 101, 498–518. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. J. Trends Food Sci. Technol. 2017, 72, 306–362. [Google Scholar] [CrossRef]
- Nie, Z.; Wan, C.; Chen, C.; Chen, J. Comprehensive evaluation of the postharvest antioxidant capacity of Majiayou pomelo harvested at different maturities based on PCA. Antioxidants 2019, 8, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, D.; Delerue-Matos, C.; Rodrigues, F. Bioactivity, phytochemical profile and pro-healthy properties of Actinidia arguta: A review. Food Res. Int. 2020, 136, 109–449. [Google Scholar] [CrossRef] [PubMed]
- Song, M.T.; Xu, H.R.; Xin, G.; Liu, C.J.; Sun, X.R.; Zhi, Y.H.; Li, B.; Shen, Y.X. Comprehensive evaluation of Actinidia argutafruit based on the nutrition and taste: 67 germplasm native to Northeast China. Food Sci. Hum. Well. 2022, 11, 393–404. [Google Scholar] [CrossRef]
- Harker, F.R.; Carr, B.T.; Lenjo, M.; MacRae, E.A.; Wismer, W.V.; Marsh, K.B.; Williams, M.; White, A.; Lund, C.M.; Walker, S.B.; et al. Consumer liking for kiwifruit flavour: A meta-analysis of five studies on fruit quality. Food Qual. Prefer. 2009, 20, 30–41. [Google Scholar] [CrossRef]
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z.; Mbili, N. Postharvest quality and composition of organically and conventionally produced fruits: A review. J. Sci. Hortic. 2017, 216, 148–159. [Google Scholar] [CrossRef]






| Varieties | Pedigree | Length/cm | Diameter/cm | Weight/g |
|---|---|---|---|---|
| European red | Russia | 1.84 ± 0.21 f–i | 1.58 ± 0.21 f,g | 2.38 ± 0.21 f,g |
| Tulameen | Canada | 1.58 ± 0.14 h,i | 1.61 ± 0.21 e–g | 2.16 ± 0.23 g,h |
| Boyne | Canada | 1.86 ± 0.27 f–i | 2.01 ± 0.31 b–d | 2.37 ± 0.11 f,g |
| DNS1 | Northeast Agricultural University | 2.02 ± 0.25 d–f | 2.05 ± 0.11 a–d | 2.94 ± 0.14 d |
| DNS2 | Northeast Agricultural University | 1.77 ± 0.25 f–i | 2.03 ± 0.13 b–d | 2.84 ± 0.17 d,e |
| DNS4 | Northeast Agricultural University | 2.72 ± 0.12 a | 1.97 ± 0.18 b–f | 2.50 ± 0.19 e–g |
| DNS5 | Northeast Agricultural University | 2.60 ± 0.12 a,b | 2.34 ± 0.12 a,b | 4.99 ± 0.21 a |
| DNS9 | Northeast Agricultural University | 1.52 ± 0.11 i | 1.42 ± 0.17 g | 1.54 ± 0.11 i |
| Ruby | The United States | 2.34 ± 0.21 b–d | 1.95 ± 0.11 b–f | 4.02 ± 0.21 b,c |
| Royalty | The United States | 1.98 ± 0.21 e–g | 2.03 ± 0.23 b–d | 2.97 ± 0.26 d |
| Rerille | The United States | 2.54 ± 0.21 a–c | 2.24 ± 0.26 a–c | 3.90 ± 0.41 c |
| Nootka | The United States | 2.44 ± 0.27 a–c | 2.18 ± 0.11 a–d | 3.83 ± 0.29 c |
| Canby | The United States | 1.78 ± 0.31 f–i | 1.86 ± 0.14 c–f | 1.95 ± 0.23 h,i |
| Summit | The United States | 1.92 ± 0.11 e–h | 1.99 ± 0.11 b–e | 2.77 ± 0.25 d–f |
| Heritage | The United States | 2.52 ± 0.11 a–c | 2.43 ± 0.23 a | 4.36 ± 0.31 b |
| Bulgaskc | Bulgaria | 1.76 ± 0.11 f–i | 1.78 ± 0.31 d–g | 1.90 ± 0.22 h,i |
| Samodiva | Bulgaria | 1.78 ± 0.16 f–i | 1.80 ± 0.27d–f | 2.15 ± 0.19 g,h |
| Schopska | Bulgaria | 1.98 ± 0.11 e–g | 2.14 ± 0.21 a–d | 3.10 ± 0.23 d |
| Beijing10 | Beijing Academy of Agriculture and Forestry Sciences | 2.23 ± 0.28 c–e | 2.02 ± 0.20 b–d | 2.85 ± 0.24 d,e |
| Beijing19 | Beijing Academy of Agriculture and Forestry Sciences | 1.54 ± 0.11 i | 1.80 ± 0.20 d–f | 1.91 ± 0.21 h,i |
| Beijing21 | Beijing Academy of Agriculture and Forestry Sciences | 1.62 ± 0.24 g–i | 1.82 ± 0.11 d–f | 1.79 ± 0.18 h,i |
| Beijing32 | Beijing Academy of Agriculture and Forestry Sciences | 2.43 ± 0.12 a–c | 2.05 ± 0.27 a–d | 4.17 ± 0.13 b,c |
| Fertod zamatos | Hungary | 1.63 ± 0.12 g–i | 2.08 ± 0.30 a–d | 1.71 ± 0.21 i |
| Willamette | France | 2.04 ± 0.12 d–f | 2.11 ± 0.11 a–d | 2.75 ± 0.27 d–f |
| Varieties | Moisture (g/100 g FW) | Total Sugar (g/100 g DW) | Reducing Sugar (g/100 g DW) | TAC (g/100 g DW) | SSC (°Brix) | pH | SAR |
|---|---|---|---|---|---|---|---|
| European red | 84.00 ± 0.45 c–h, | 58.05 ± 0.81 h–j | 30.26 ± 0.33 m | 4.90 ± 1.19 l | 9.33 ± 0.58 d,e | 3.19 ± 0.02 a | 11.91 |
| Tulameen | 83.45 ± 1.58 f–h | 71.75 ± 0.18 c,d | 51.87 ± 1.38 b | 10.59 ± 1.20 h,i | 9.33 ± 0.58 d,e | 2.76 ± 0.05 c,d | 5.32 |
| Boyne | 85.38 ± 0.73 a–d | 59.27 ± 0.62 g–i | 43.06 ± 2.18 f–h | 14.93 ± 0.20 b,c | 10.67 ± 0.58 c | 2.62 ± 0.05 d–g | 4.89 |
| DNS1 | 86.15 ± 1.05 a | 71.70 ± 1.30 c,d | 47.77 ± 0.86 c | 11.95 ± 3.10 f–h | 7.33 ± 0.58 g,h | 2.75 ± 0.06c–e | 4.42 |
| DNS2 | 80.73 ± 0.72 i | 76.36 ± 0.06 b | 52.51 ± 0.71 b | 14.65 ± 0.37 c,d | 12.33 ± 0.58 b | 2.61 ± 0.06 d–g | 4.37 |
| DNS4 | 85.16 ± 0.43 a–e | 64.28 ± 0.62 e,f | 55.69 ± 4.18 a | 9.16 ± 0.56 i,j | 6.33 ± 0.58 h | 2.95 ± 0.13 b | 6.46 |
| DNS5 | 86.13 ± 1.43 a | 54.27 ± 2.19 j,k | 45.89 ± 1.68 c–f | 14.84 ± 0.43 c,d | 8.00 ± 0.00 f,g | 2.55 ± 0.10g | 3.89 |
| DNS9 | 83.88 ± 0.86 d–h | 68.74 ± 1.37 d | 36.00 ± 2.26 k,l | 6.47 ± 0.10 k,l | 8.67 ± 0.58 e,f | 3.09 ± 0.09 a,b | 8.34 |
| Ruby | 85.99 ± 0.30 a,b | 64.40 ± 0.29 e,f | 42.00 ± 3.54 g–i | 12.85 ± 2.97 d–f | 7.67 ± 0.58 f,g | 2.62 ± 0.06 d–g | 4.26 |
| Royalty | 84.35 ± 0.30 b–h | 75.54 ± 0.38 b,c | 54.78 ± 0.44 a,b | 14.86 ± 0.19 c,d | 8.33 ± 0.58 e–g | 2.66 ± 0.06 c–g | 3.58 |
| Rerille | 85.67 ± 1.65 a–c | 56.08 ± 0.76 i,j | 39.38 ± 0.14 i,j | 14.36 ± 0.81 c–e | 8.67 ± 0.58 e,f | 2.59 ± 0.09 f,g | 4.22 |
| Nootka | 86.00 ± 0.65 a,b | 72.11 ± 1.56 c,d | 47.91 ± 0.75 c | 14.29 ± 0.52 c–e | 7.33 ± 0.58 g,h | 2.63 ± 0.03 d–g | 3.67 |
| Canby | 83.10 ± 0.81 g,h | 82.64 ± 0.21 a | 43.48 ± 0.82 e–h | 7.42 ± 0.55 j,k | 11.33 ± 0.58 c | 3.16 ± 0.03 a | 9.04 |
| Summit | 83.59 ± 0.95 e–h | 61.56 ± 0.14 f–h | 46.6 ± 3.29 c–e | 15.46 ± 0.70 b,c | 10.67 ± 0.58 c | 2.58 ± 0.12 f,g | 4.21 |
| Heritage | 85.84 ± 0.51 a,b | 67.84 ± 0.70 d,e | 41.64 ± 0.64 g–i | 17.51 ± 0.51 a | 11.33 ± 0.58 c | 2.55 ± 0.10 f,g | 4.57 |
| Bulgaskc | 82.90 ± 0.27 g,h | 74.79 ± 6.57 b,c | 41.30 ± 0.80 h,i | 6.44 ± 0.26 k,l | 10.33 ± 0.58 c,d | 3.18 ± 0.04 a | 9.39 |
| Samodiva | 85.09 ± 0.66 a–f | 82.49 ± 0.87 a | 46.30 ± 1.27 c–f | 10.72 ± 0.29 g–i | 14.33 ± 0.58 a | 2.98 ± 0.18 b | 8.96 |
| Schopska | 82.79 ± 0.46 h | 77.12 ± 7.99 b | 44.83 ± 0.35 c–g | 15.24 ± 1.52 b,c | 9.33 ± 0.58 d,e | 2.61 ± 0.01 d–g | 3.42 |
| Beijing10 | 85.02 ± 0.76 a–f | 63.33 ± 0.53 f,g | 43.78 ± 1.66 d–h | 16.87 ± 0.38 a,b | 7.33 ± 0.58 g,h | 2.53 ± 0.02 g | 2.90 |
| Beijing19 | 84.46 ± 0.68 a–h | 69.56 ± 1.33 d | 55.76 ± 2.66 a | 12.57 ± 0.32 e–g | 13.00 ± 0.00 b | 2.71 ± 0.01 c–f | 6.66 |
| Beijing21 | 84.50 ± 1.21 a–g | 71.96 ± 0.39 c,d | 46.89 ± 0.59 c,d | 10.50 ± 1.05 h,i | 7.33 ± 0.58 g,h | 2.80 ± 0.08 c | 4.51 |
| Beijing32 | 85.99 ± 0.78 a,b | 60.39 ± 1.42 f–h | 38.73 ± 2.12 i–k | 14.35 ± 0.36 c–e | 7.33 ± 0.58 g,h | 2.58 ± 0.13 f,g | 3.65 |
| Fertod zamatos | 83.60 ± 0.94 e–h | 50.78 ± 1.99 k | 36.29 ± 1.21 j–l | 15.23 ± 0.18 b,c | 9.33 ± 0.58 d,e | 2.58 ± 0.01 f,g | 3.73 |
| Willamette | 83.97 ± 0.80 d–h | 55.18 ± 1.34 i,j | 33.78 ± 1.19 l | 14.03 ± 0.27 c–e | 10.33 ± 0.58 c,d | 2.59 ± 0.11 e–g | 4.59 |
| Varieties | l-Rhamnose Monohydrate (g/100 g DW) | Fructose (g/100 g DW) | Glucose (g/100 g DW) | Sucrose (g/100 g DW) | Maltose (g/100 g DW) | d-Trehalose Anhydrous (g/100 g DW) |
|---|---|---|---|---|---|---|
| European red | 0.54 ± 0.02 h,i | 13.79 ± 0.52 l | 10.91 ± 0.89 k | 31.79 ± 0.73 b,c | 0.33 ± 0.20 d–h | 0.19 ± 0.01 b,c |
| Tulameen | 0.84 ± 0.28 c–h | 26.54 ± 1.14 f | 27.11 ± 2.49 c | 0.29 ± 0.03 j | 0.30 ± 0.10 e–i | 0.20 ± 0.00 b,c |
| Boyne | 0.88 ± 0.50 c–h | 19.61 ± 2.53 k | 17.07 ± 2.72 g,h | 2.32 ± 0.35 i,j | 0.32 ± 0.09 d–h | 0.07 ± 0.13 d |
| DNS1 | 1.24 ± 0.33 b,c | 25.83 ± 0.55 f,g | 27.62 ± 0.53 c | 0.33 ± 0.05 j | 0.26 ± 0.21 f–j | 0.26 ± 0.05 b |
| DNS2 | 1.77 ± 0.06 a | 32.98 ± 0.55 c,d | 30.98 ± 0.65 a | 9.89 ± 0.08 f | 0.23 ± 0.07 f–j | 0.18 ± 0.00 b,c |
| DNS4 | 0.32 ± 0.01 i | 23.88 ± 0.04 h,i | 27.24 ± 0.03 c | 2.32 ± 0.00 i,j | 0.05 ± 0.01 i,j | 0.21 ± 0.00 b,c |
| DNS5 | 1.22 ± 0.18 b,c | 23.77 ± 1.20 h,i | 18.22 ± 1.04 e–g | 6.59 ± 0.62 g,h | 0.42 ± 0.18 d–f | 0.07 ± 0.13 d |
| DNS9 | 0.72 ± 0.29 e–h | 14.43 ± 2.89 l | 13.26 ± 3.09 i,j | 38.29 ± 8.55 a | 0.35 ± 0.18 d–h | 0.13 ± 0.12 c,d |
| Ruby | 0.85 ± 0.08 c–h | 21.91 ± 0.07 j | 18.99 ± 0.38 e–g | 0.16 ± 0.04 j | 0.10 ± 0.04 h–j | 0.22 ± 0.01 b,c |
| Royalty | 0.47 ± 0.01 h,i | 29.39 ± 0.91 e | 27.15 ± 1.10 c | 2.17 ± 0.14 i,j | 0.30 ± 0.08 e–h | 0.19 ± 0.00 b,c |
| Rerille | 0.78 ± 0.02 d–h | 22.32 ± 0.52 i,j | 15.30 ± 0.38 h,i | 5.34 ± 0.48 h,i | 0.39 ± 0.02 d–g | 0.22 ± 0.01 b,c |
| Nootka | 0.73 ± 0.00 e–h | 29.42 ± 0.07 e | 12.03 ± 0.58 j,k | 13.59 ± 0.12 e | 0.15 ± 0.02 g–j | 0.22 ± 0.00 b,c |
| Canby | 0.87 ± 0.07 c–h | 24.78 ± 0.33 f–h | 22.86 ± 0.13 d | 33.09 ± 0.32 b | 0.84 ± 0.06 ab | 0.19 ± 0.02 b,c |
| Summit | 1.11 ± 0.03 b–e | 26.51 ± 0.43 f | 23.72 ± 0.40 d | 9.48 ± 1.04 f,g | 0.33 ± 0.27 d–h | 0.21 ± 0.02 b,c |
| Heritage | 0.72 ± 0.01 e–h | 21.16 ± 0.15 j,k | 19.88 ± 0.11 e | 12.17 ± 0.06 e,f | 0.75 ± 0.02 bc | 0.45 ± 0.02 a |
| Bulgaskc | 1.43 ± 0.67 a,b | 25.11 ± 0.50 f–h | 23.13 ± 0.32 d | 20.22 ± 0.05 d | 0.98 ± 0.03 a | 0.20 ± 0.01 b,c |
| Samodiva | 1.21 ± 0.07 b,c | 24.59 ± 0.95 g,h | 22.88 ± 0.92 d | 28.79 ± 2.31 c | 0.55 ± 0.17 c–e | 0.23 ± 0.02 b,c |
| Schopska | 0.98 ± 0.05 c–g | 35.79 ± 0.80 b | 27.87 ± 0.16 b,c | 2.16 ± 0.24 i,j | 0.26 ± 0.12 f–j | 0.19 ± 0.02 b,c |
| Beijing10 | 1.20 ± 0.01 b,c | 25.81 ± 0.06 f,g | 17.55 ± 0.12 f,g | 0.15 ± 0.01 j | 0.03 ± 0.00 j | 0.23 ± 0.00 b,c |
| Beijing19 | 1.17 ± 0.23 b–d | 37.46 ± 0.28 a | 29.85 ± 0.13 a,b | 0.15 ± 0.03 j | 0.24 ± 0.12 f–j | 0.21 ± 0.02 b,c |
| Beijing21 | 1.06 ± 0.01 b–f | 31.52 ± 0.10 d | 28.61 ± 0.05 b,c | 0.35 ± 0.00 j | 0.13 ± 0.00 g–j | 0.22 ± 0.00 b,c |
| Beijing32 | 0.88 ± 0.06 c–h | 20.89 ± 1.25 j,k | 13.96 ± 1.02 i,j | 13.31 ± 0.45 e | 0.57 ± 0.33 c,d | 0.15 ± 0.13 c,d |
| Fertod zamatos | 0.69 ± 0.01 f–i | 19.85 ± 0.08 k | 19.50 ± 0.11 e,f | 2.14 ± 0.03 i,j | 0.19 ± 0.01 f–j | 0.26 ± 0.01 b |
| Willamette | 0.64 ± 0.00 g–i | 34.00 ± 0.05 c | 12.09 ± 2.32 j,k | 2.23 ± 0.19 i,j | 0.18 ± 0.01 f–j | 0.19 ± 0.00 b,c |
| Varieties | Oxalic Acid (g/100 g DW) | Tartaric Acid (g/100 g DW) | Malic Acid (g/100 g DW) | α-Ketoglutaric Acid (g/100 g DW) | Lactic Acid (g/100 g DW) | Citric Acid (g/100 g DW) | Fumaric Acid (g/100 g DW) | Succinic Acid (g/100 g DW) |
|---|---|---|---|---|---|---|---|---|
| European red | 0.13 ± 0.01 g | 0.28 ± 0.02 e–g | 1.06 ± 0.15 i | 0.06 ± 0.01 k,l | n.d. | 2.97 ± 0.02 l | 0.01 ± 0.01 c | n.d. |
| Tulameen | 0.13 ± 0.00 g | 0.29 ± 0.02 e,f | 1.23 ± 0.06 f–i | 0.13 ± 0.01 e | 0.40 ± 0.05 d,e | 7.36 ± 0.41 h,i | 0.01 ± 0.00 b | n.d. |
| Boyne | 0.19 ± 0.04 c,d | n.d. | 1.23 ± 0.14 f–i | 0.06 ± 0.00 j,k | 0.20 ± 0.02 h,i | 11.69 ± 0.11 c,d | 0.01 ± 0.00 b | n.d. |
| DNS1 | 0.19 ± 0.00 c,d | 0.26 ± 0.02 f,g | 1.26 ± 0.12 f–i | 0.14 ± 0.01 e | 0.21 ± 0.01 h,i | 9.21 ± 0.22 f,g | 0.01 ± 0.00 b | n.d. |
| DNS2 | 0.21 ± 0.01 b | n.d. | 1.72 ± 0.02 c | 0.08 ± 0.02 g–j | 0.17 ± 0.02 i | 12.27 ± 0.22 c | 0.02 ± 0.00 a | n.d. |
| DNS4 | 0.14 ± 0.01 f,g | 0.19 ± 0.01 h | 0.31 ± 0.02 k | 0.04 ± 0.00 l | 0.27 ± 0.01 g,h | 4.63 ± 0.19 j,k | 0.01 ± 0.00 b | 0.33 ± 0.06 a |
| DNS5 | 0.21 ± 0.01 b,c | 0.37 ± 0.01 c,d | 1.62 ± 0.28 c–e | 0.21 ± 0.02 c | 0.49 ± 0.01 c | 9.86 ± 1.18 e,f | 0.01 ± 0.00 b | n.d. |
| DNS9 | 0.14 ± 0.01 f,g | 0.19 ± 0.01 h | 0.71 ± 0.07 j | 0.25 ± 0.01 b | 0.74 ± 0.02 a | 3.81 ± 0.33 k | n.d. | n.d. |
| Ruby | 0.19 ± 0.00 c,d | 0.27 ± 0.01 f,g | 1.22 ± 0.00 g–i | 0.06 ± 0.00 j,k | n.d. | 9.43 ± 0.01 f | 0.01 ± 0.00 b | n.d. |
| Royalty | 0.17 ± 0.01 d,e | 0.35 ± 0.06 d | 1.51 ± 0.23 c–g | 0.05 ± 0.02 k,l | 0.31 ± 0.19 f,g | 11.13 ± 1.31 c,d | 0.01 ± 0.00 b | n.d. |
| Rerille | 0.29 ± 0.01 a | 0.41 ± 0.07 c | 1.56 ± 0.18 c–e | 0.06 ± 0.02 i–k | 0.33 ± 0.11 e–g | 10.61 ± 0.65 d,e | 0.01 ± 0.00 b | 0.08 ± 0.04 c |
| Nootka | 0.22 ± 0.01 b | n.d. | 1.81 ± 0.01 b,c | 0.09 ± 0.01 f,g | 0.35 ± 0.03 d–g | 11.33 ± 0.02 c,d | n.d. | n.d. |
| Canby | 0.16 ± 0.01 e | n.d. | 1.34 ± 0.01 e–i | 0.07 ± 0.01 i–k | 0.28 ± 0.01 g,h | 5.12 ± 0.26 j | 0.01 ± 0.00 b | n.d. |
| Summit | 0.22 ± 0.01 b | 0.47 ± 0.01 b | 1.73 ± 0.09 c | 0.20 ± 0.01 c | 0.38 ± 0.04 d–f | 11.89 ± 0.03 c,d | n.d. | 0.06 ± 0.00 c |
| Heritage | 0.13 ± 0.01 g | n.d. | 2.37 ± 0.26 a | 0.09 ± 0.02 f–h | 0.58 ± 0.05 b | 13.85 ± 2.38 a | 0.02 ± 0.00 a | n.d. |
| Bulgaskc | 0.19 ± 0.01 c,d | 0.19 ± 0.00 h | 1.59 ± 0.06 c–e | 0.16 ± 0.00 d | 0.76 ± 0.07 a | 2.95 ± 0.19 l | 0.01 ± 0.00 b | 0.02 ± 0.00 d |
| Samodiva | 0.28 ± 0.01 a | 0.30 ± 0.01 e | 2.02 ± 0.07 b | 0.17 ± 0.01 d | 0.82 ± 0.02 a | 6.28 ± 0.12 i | 0.01 ± 0.00 b | n.d. |
| Schopska | 0.16 ± 0.01 e,f | 0.20 ± 0.01 h | 2.06 ± 0.04 b | 0.07 ± 0.00 h–k | 0.28 ± 0.03 g,h | 12.41 ± 0.31 b,c | 0.01 ± 0.00 b | n.d. |
| Beijing10 | 0.22 ± 0.03 b | 0.61 ± 0.01 a | 1.67 ± 0.40 c,d | 0.10 ± 0.02 f | n.d. | 13.47 ± 0.49 a,b | 0.01 ± 0.00 b | n.d. |
| Beijing19 | 0.19 ± 0.00 c,d | 0.39 ± 0.00 c | 1.39 ± 0.02 d–h | 0.07 ± 0.01 i–k | 0.05 ± 0.01 j | 9.15 ± 0.91 f,g | 0.01 ± 0.00 b | n.d. |
| Beijing21 | 0.08 ± 0.01 h | n.d. | 1.11 ± 0.36 h,i | 0.07 ± 0.00 h–k | 0.43 ± 0.01 c,d | 8.08 ± 0.02 g,h | 0.01 ± 0.00 b | n.d. |
| Beijing32 | 0.06 ± 0.00 i | n.d. | 1.23 ± 0.04 f–i | 0.07 ± 0.01 i–k | 0.42 ± 0.02 c–e | 11.37 ± 0.05 c,d | n.d. | 0.22 ± 0.01 b |
| Fertod zamatos | 0.16 ± 0.01 e | 0.25 ± 0.01 g | 1.53 ± 0.06 c–f | 0.26 ± 0.01 a | n.d. | 11.67 ± 0.08 c,d | 0.01 ± 0.00 b | n.d. |
| Willamette | 0.17 ± 0.01 d,e | 0.09 ± 0.01 i | 1.22 ± 0.09 g–i | 0.08 ± 0.00 f–i | 0.06 ± 0.01 j | 11.75 ± 0.27 c,d | 0.02 ± 0.00 a | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Yang, G.; Sun, L.; Song, X.; Bao, Y.; Luo, T.; Wang, J. Comprehensive Evaluation of 24 Red Raspberry Varieties in Northeast China Based on Nutrition and Taste. Foods 2022, 11, 3232. https://doi.org/10.3390/foods11203232
Yu Y, Yang G, Sun L, Song X, Bao Y, Luo T, Wang J. Comprehensive Evaluation of 24 Red Raspberry Varieties in Northeast China Based on Nutrition and Taste. Foods. 2022; 11(20):3232. https://doi.org/10.3390/foods11203232
Chicago/Turabian StyleYu, Yiping, Guang Yang, Lanying Sun, Xingshun Song, Yihong Bao, Ting Luo, and Jinling Wang. 2022. "Comprehensive Evaluation of 24 Red Raspberry Varieties in Northeast China Based on Nutrition and Taste" Foods 11, no. 20: 3232. https://doi.org/10.3390/foods11203232
APA StyleYu, Y., Yang, G., Sun, L., Song, X., Bao, Y., Luo, T., & Wang, J. (2022). Comprehensive Evaluation of 24 Red Raspberry Varieties in Northeast China Based on Nutrition and Taste. Foods, 11(20), 3232. https://doi.org/10.3390/foods11203232