Valorization of Pineapple Leaves Waste for the Production of Bioethanol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganism and Culture Conditions
2.3. Hydrothermal Pretreatment of Pineapple Leaves Waste Biomass
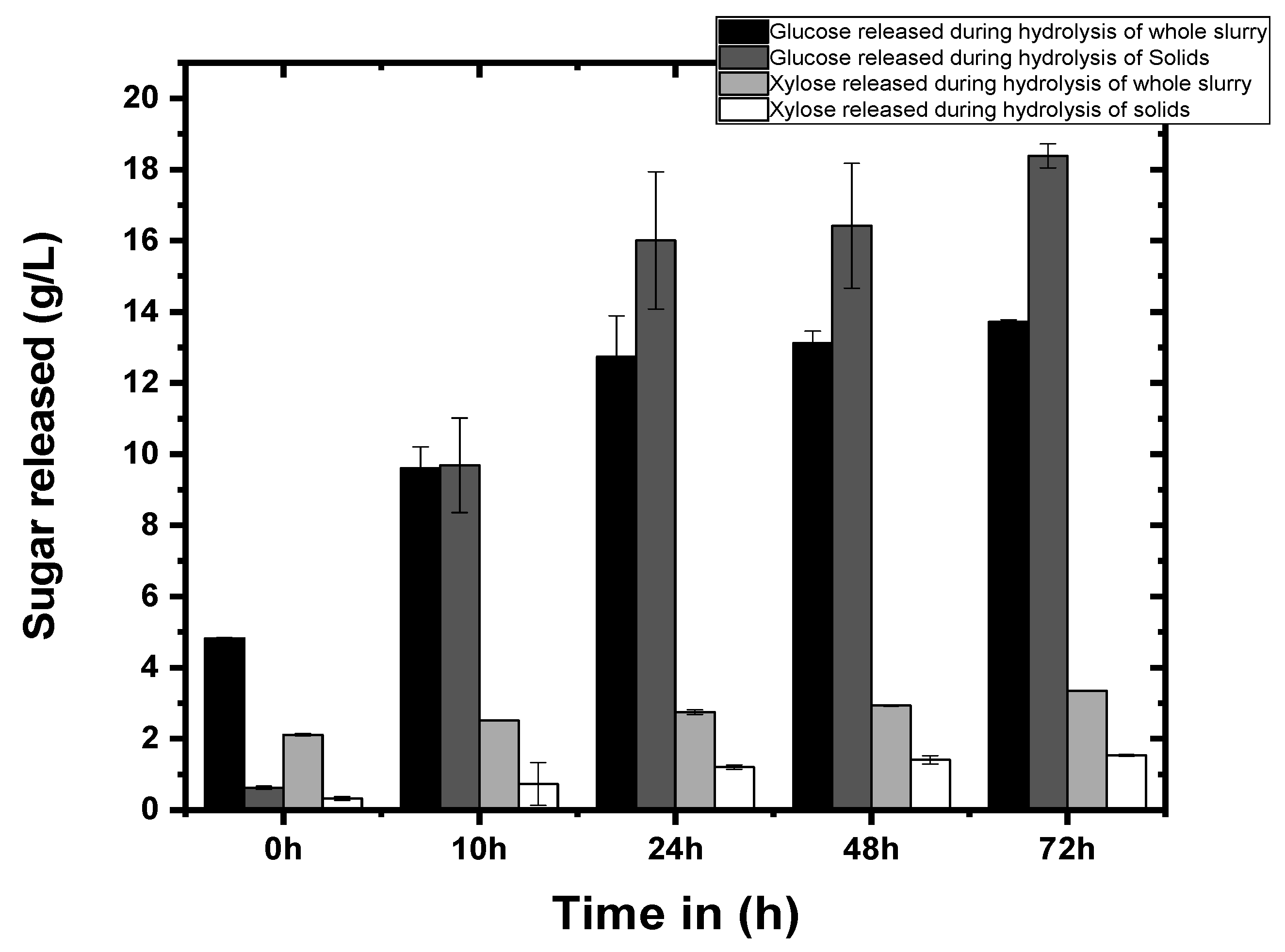
2.4. Enzymatic Hydrolysis of Pretreated Biomass
2.5. Separate Hydrolysis and Fermentation
2.6. Analytical Methods
2.6.1. Chemical Characterization of Biomass
2.6.2. HPLC Analysis
2.6.3. Calculations
3. Results and Discussion
3.1. Hydrothermal Pretreatment of PL Waste and Biomass Composition
3.2. Enzymatic Hydrolysis of Pineapple Leaves Waste
3.3. Fermentation of Pre-Treated Slurry and Cellulose-Rich Solid Fraction Hydrolysate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Lee, H. Local firms step up for pineapples. Taipei Times, (Retrieved 20 July 2022). 28 February 2021. [Google Scholar]
- Lin, C.; Chang, C. Pineapple Production and Industry in Taiwan. Acta Hortic. 2000, 529, 93–98. [Google Scholar] [CrossRef]
- Rabiu, Z.; Maigari, F.U.; Lawan, U.; Mukhtar, Z.G. Pineapple waste utilization as a sustainable means of waste management. In Sustainable Technologies for the Management of Agricultural Wastes; Springer: Singapore, 2018; pp. 143–154. [Google Scholar]
- Singhania, R.R.; Patel, A.K.; Raj, T.; Chen, C.-W.; Ponnusamy, V.K.; Tahir, N.; Kim, S.-H.; Dong, C.-D. Lignin valorisation via enzymes: A sustainable approach. Fuel 2022, 311, 122608. [Google Scholar] [CrossRef]
- Bertrand, E.; Pradel, M.; Dussap, C.G. Economic and environmental aspects of biofuels. In Green Fuels Technology; Springer: Cham, Switzerland, 2016; pp. 525–555. [Google Scholar]
- Sharma, B.; Larroche, C.; Dussap, C.G. Comprehensive assessment of 2G bioethanol production. Bioresour. Technol. 2020, 313, 123630. [Google Scholar] [CrossRef]
- Singhania, R.R.; Ruiz, H.A.; Awasthi, M.K.; Dong, C.D.; Chen, C.W.; Patel, A.K. Challenges in cellulase bioprocess for biofuel applications. Ren. Sus. Energ. Rev. 2021, 151, 111622. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, B.; Luo, L.; Zhang, F.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Wang, X.; Lü, X. A review on recycling techniques for bioethanol production from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2021, 149, 111370. [Google Scholar] [CrossRef]
- Panahi, H.K.S.; Dehhaghi, M.; Guillemin, G.J.; Gupta, V.K.; Lam, S.S.; Aghbashlo, M.; Tabatabaei, M. Bioethanol production from food wastes rich in carbohydrates. Curr. Opin. Food Sci. 2022, 43, 71–81. [Google Scholar] [CrossRef]
- Saini, R.; Patel, A.K.; Saini, J.K.; Chen, C.W.; Varjani, S.; Singhania, R.R.; Di Dong, C. Recent advancements in prebiotic oligomers synthesis via enzymatic hydrolysis of lignocellulosic biomass. Bioengineered 2022, 13, 2139–2172. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, R.; Kaur, A.; Saini, J.K.; Patel, A.K.; Varjani, S.; Chen, C.W.; Singhania, R.R.; Dong, C.D. Trends in Lignin Biotransformations for Bio-Based Products and Energy Applications. BioEnergy Res. 2022. [CrossRef]
- Singhania, R.R.; Saini, J.K.; Saini, R.; Adsul, M.; Mathur, A.; Gupta, R.; Tuli, D.K. Bioethanol production from wheat straw via enzymatic route employing Penicillium janthinellum cellulases. Bioresour. Technol. 2014, 169, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Cybulska, I.; Julson, J. Hydrothermal pretreatment of lignocellulosic biomass and kinetics. J. Sustain. Bioenergy Syst. 2013, 3, 250. [Google Scholar] [CrossRef] [Green Version]
- Nashiruddin, N.I.; Mansor, A.F.; Rahman, R.A.; Ilias, R.M.; Yussof, H.W. Process parameter optimization of pretreated pineapple leaves fiber for enhancement of sugar recovery. Ind. Crops Prod. 2020, 152, 112514. [Google Scholar] [CrossRef]
- Imman, S.; Kreetachat, T.; Khongchamnan, P.; Laosiripojana, N.; Champreda, V.; Suwannahong, K.; Sakulthaew, C.; Chokejaroenrat, C.; Suriyachai, N. Optimization of sugar recovery from pineapple leaves by acid-catalyzed liquid hot water pretreatment for bioethanol production. Energy Rep. 2021, 7, 6945–6954. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Pinheiro, T.; Coelho, E.; Romaní, A.; Domingues, L. Intensifying ethanol production from brewer’s spent grain waste: Use of whole slurry at high solid loadings. New Biotechnol. 2019, 53, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Azelee, N.I.W.; Adnan, S.A.M.; Manas, N.H.A.; Dailin, D.J.; Ramli, A.N.M.; Illias, R.M. Assessment of microwave-assisted pretreatments for enhancing pineapple waste delignification. In Proceedings of the AIP Conference Proceedings, Leuven, Belgium, 8–10 September 2019; Volume 2155, p. 020003. [Google Scholar]
- Nordin, N.; Illias, R.M.; Manas, N.H.A.; Ramli, A.N.M.; Azelee, N.I.W. Efficient Delignification of Pineapple Waste by Low-Pressure Steam Heating Pre-Treatment. In Proceedings of the Third International Conference on Separation Technology 2020 (ICoST 2020), Senai, Malaysia, 15–16 December 2020; pp. 10–16. [Google Scholar]
- García-Aparicio, M.P.; Ballesteros, I.; González, A.; Oliva, J.M.; Ballesteros, M.; Negro, M.J. Effect of inhibitors released during steam-explosion pretreatment of barley straw on enzymatic hydrolysis. Appl. Biochem. Biotechnol. 2006, 129, 278–288. [Google Scholar] [CrossRef]
- Nielsen, F.; Galbe, M.; Zacchi, G.; Wallberg, O. The effect of mixed agricultural feedstocks on steam pretreatment, enzymatic hydrolysis, and cofermentation in the lignocellulose-to-ethanol process. Biomass Convers. Biorefinery 2020, 10, 253–266. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.J.; Cao, S.L.; Lin, L.; Luo, X.L.; Hu, H.C.; Chen, L.H.; Huang, L.L. Hydrothermal pretreatment of bamboo and cellulose degradation. Bioresour. Technol. 2013, 148, 408–413. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, W.J.; Pang, B.; Sun, Z.; Lam, S.S.; Sonne, C.; Yuan, T.Q. Ultrastructural change in lignocellulosic biomass during hydrothermal pretreatment. Bioresour. Technol. 2021, 341, 125807. [Google Scholar] [CrossRef]
- Qing, Q.; Yang, B.; Wyman, C.E. Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour. Technol. 2010, 101, 9624–9630. [Google Scholar] [CrossRef]
- Kim, Y.; Ximenes, E.; Mosier, N.S.; Ladisch, M.R. Soluble inhibitors/de activators of cellulase enzymes from lignocellulosic biomass. Enzym. Microb. Technol. 2011, 48, 408–415. [Google Scholar] [CrossRef]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Deactivation of cellulases by phenols. Enzym. Microb. Technol. 2011, 48, 54–60. [Google Scholar] [CrossRef] [PubMed]
- del Río, P.G.; Gullón, P.; Rebelo, F.R.; Romaní, A.; Garrote, G.; Gullón, B. A whole-slurry fermentation approach to high-solid loading for bioethanol production from corn stover. Agronomy 2020, 10, 1790. [Google Scholar] [CrossRef]
- Martínez-Patiño, J.C.; Ruiz, E.; Cara, C.; Romero, I.; Castro, E. Advanced bioethanol production from olive tree biomass using different bioconversion schemes. Biochem. Eng. J. 2018, 137, 172–181. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saini, R.; Chen, C.-W.; Patel, A.K.; Saini, J.K.; Dong, C.-D.; Singhania, R.R. Valorization of Pineapple Leaves Waste for the Production of Bioethanol. Bioengineering 2022, 9, 557. https://doi.org/10.3390/bioengineering9100557
Saini R, Chen C-W, Patel AK, Saini JK, Dong C-D, Singhania RR. Valorization of Pineapple Leaves Waste for the Production of Bioethanol. Bioengineering. 2022; 9(10):557. https://doi.org/10.3390/bioengineering9100557
Chicago/Turabian StyleSaini, Reetu, Chiu-Wen Chen, Anil Kumar Patel, Jitendra Kumar Saini, Cheng-Di Dong, and Reeta Rani Singhania. 2022. "Valorization of Pineapple Leaves Waste for the Production of Bioethanol" Bioengineering 9, no. 10: 557. https://doi.org/10.3390/bioengineering9100557
APA StyleSaini, R., Chen, C.-W., Patel, A. K., Saini, J. K., Dong, C.-D., & Singhania, R. R. (2022). Valorization of Pineapple Leaves Waste for the Production of Bioethanol. Bioengineering, 9(10), 557. https://doi.org/10.3390/bioengineering9100557










