Schistosomiasis Mansoni-Recruited Eosinophils: An Overview in the Granuloma Context
Abstract
:1. Introduction
2. Immunopathology of Schistosoma mansoni Infection
2.1. The Host Immune Response
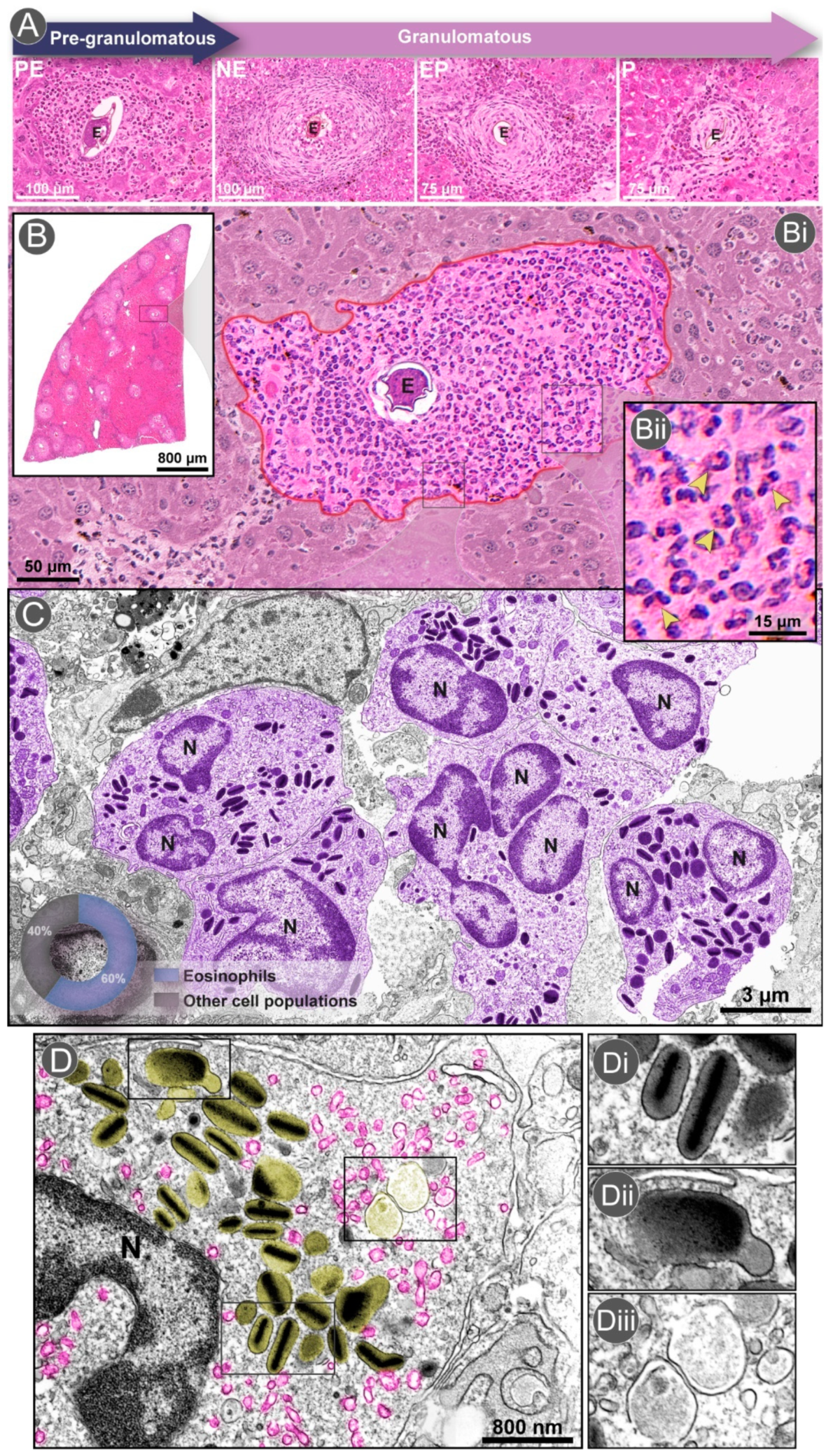
2.2. The Granuloma Architecture
3. Eosinophil Dynamics within the Granuloma
3.1. Eosinophil Recruitment and Accumulation
3.2. Differential Distribution of Eosinophils within the Granuloma
3.3. Are Eosinophils Produced Locally in Schistosoma mansoni Granulomas?
3.4. Eosinophil Degranulation Mechanisms
3.5. Eosinophil Interaction with Other Cell Populations
4. Eosinophils in Natural Models of Schistosomiasis
5. Eosinophils in Schistosoma mansoni Infection: Effector or Immunomodulatory Cells?
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations:
| ΔdblGATA | Lineage-ablated delta bone marrow progenitors |
| BALB/C | Albin Laboratory-Mouse |
| Bregs | Lymphocyte B Regulatory |
| CCL2 | C-C Motif Chemokine Ligand 2 |
| CCL3 | C-C Motif Chemokine Ligand 3 |
| CCL4 | C-C Motif Chemokine Ligand 4 |
| CCL5 | C-C Motif Chemokine Ligand 5 |
| CCL7 | C-C Motif Chemokine Ligand 7 |
| CCL11 | C-C Motif Chemokine Ligand 11 |
| CCL17 | C-C Motif Chemokine Ligand 17 |
| CCL22 | C-C Motif Chemokine Ligand 22 |
| CCL24 | C-C Motif Chemokine Ligand 24 |
| CCL26 | C-C Motif Chemokine Ligand 26 |
| CD11c | Marker for dendritic cells |
| CD4+ T | Lymphocyte T Cluster of Differentiation 4 |
| ECM | Extracellular Matrix |
| ECP | Eosinophil Cationic Protein |
| EDN | Eosinophil-Derived Neurotoxin |
| EP | Exudative-Productive stage of granuloma development |
| EPO | Eosinophil Peroxidase |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| IL | Interleukin |
| IL-1 | Interleukin Type 1 |
| IL-2 | Interleukin Type 2 |
| IL-4 | Interleukin Type 4 |
| IL-5 | Interleukin Type 5 |
| IL-6 | Interleukin Type 6 |
| IL-10 | Interleukin Type 10 |
| IL-12 | Interleukin Type 12 |
| IL-13 | Interleukin Type 13 |
| IL-17 | Interleukin Type 17 |
| IL-33 | Interleukin Type 33 |
| IL-4Rα | Interleukin Type 4 Receptor-Alpha |
| IL-5Rα | Interleukin 5 Receptor Alpha |
| IFN-γ | Interferon Gamma |
| MBP | Major Basic Protein |
| MIF | Migration inhibitory factor |
| MMP9 | Matrix Metallopeptidase 9 |
| NE | Necrotic-Exudative stage of granuloma development |
| P | Productive stage of granuloma development |
| PE | Pre-Granulomatous Exudative stage of granuloma development |
| PMD | Piecemeal Degranulation |
| RELM-α | Resistin-Like Molecule-alpha |
| SEA | Soluble Egg Antigens |
| TEM | Transmission Electron Microscopy |
| TGF-α | Transforming Growth Factor alpha |
| TGF-β | Transforming Growth Factor beta |
| TgPHIL | Transgenic mice that lack eosinophils |
| Th1 | Lymphocyte T Helper Type 1 |
| Th2 | Lymphocyte T Helper Type 2 |
| TNF-α | Tumor Necrosis Factor Alpha |
| Tregs | Lymphocyte T Regulatory |
| WSI | Whole Slide Image |
| WT | Wild-Type |
References
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Prim. 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, A.E. Immunological aspects of human schistosomiasis. Brit. Med. Bull. 1998, 54, 357–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sher, A.; Coffman, R.L.; Hieny, S.; Scott, P.; Cheever, A.W. Interleukin 5 is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with Schistosoma mansoni. Proc. Natl. Acad. Sci. USA 1990, 87, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Gazzinelli, G.; Lambertucci, J.R.; Katz, N.; Rocha, R.S.; Lima, M.S.; Colley, D.G. Immune responses during human Schistosomiasis mansoni. XI. Immunologic status of patients with acute infections and after treatment. J. Immunol. 1985, 135, 2121–2127. [Google Scholar]
- Ariyaratne, A.; Finney, C.A. Eosinophils and Macrophages within the Th2-Induced Granuloma: Balancing Killing and Healing in a Tight Space. Infect. Immun. 2019, 87, e00127-19. [Google Scholar] [CrossRef] [Green Version]
- Malta, K.K.; Silva, T.P.; Palazzi, C.; Neves, V.H.; Carmo, L.A.S.; Cardoso, S.J.; Melo, R.C.N. Changing our view of the Schistosoma granuloma to an ecological standpoint. Biol. Rev. 2021, 96, 1404–1420. [Google Scholar] [CrossRef]
- Huang, L.; Appleton, J.A. Eosinophils in helminth infection: Defenders and dupes. Trends Parasitol. 2016, 32, 798–807. [Google Scholar] [CrossRef] [Green Version]
- Behm, C.A.; Ovington, K.S. The role of eosinophils in parasitic helminth infections: Insights from genetically modified mice. Parasitol. Today 2000, 16, 202–209. [Google Scholar] [CrossRef]
- de Jesus, A.R.; Silva, A.; Santana, L.B.; Magalhães, A.; de Jesus, A.A.; de Almeida, R.P.; Rêgo, M.A.; Burattini, M.N.; Pearce, E.J.; Carvalho, E.M. Clinical and immunologic evaluation of 31 patients with acute schistosomiasis mansoni. J. Infect. Dis. 2002, 185, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Fallon, P.G.; Smith, P.; Dune, D.W. Type 1 and type 2 cytokine-producing mouse CD4+ and CD8+ T cells in acute Schistosoma mansoni infection. Eur. J. Immunol. 1998, 28, 1408–1416. [Google Scholar] [CrossRef]
- Stadecker, M.J.; Hernandez, H.J. The immune response and immunopathology in infection with Schistosoma mansoni: A key role of major egg antigen Sm-p40. Parasite Immunol. 1998, 20, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Jauréguiberry, S.; Paris, L.; Caumes, E. Acute schistosomiasis, a diagnostic and therapeutic challenge. Clin. Microbiol. Inf. 2010, 16, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel Aziz, N.; Musaigwa, F.; Mosala, P.; Berkiks, I.; Brombacher, F. Type 2 immunity: A two-edged sword in schistosomiasis immunopathology. Trends Immunol. 2022, 43, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Grzych, J.M.; Pearce, E.J.; Cheever, A.W.; Caulada, Z.A.; Caspar, P.; Heiny, S.; Lewis, F.; Sher, A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J. Immunol. 1991, 146, 1322–1327. [Google Scholar] [PubMed]
- Fallon, P.G. Immunopathology of schistosomiasis: A cautionary tale of mice and men. Immunol. Today 2000, 21, 29–35. [Google Scholar] [CrossRef]
- Schwartz, C.; Fallon, P.G. Schistosoma “Eggs-Iting” the Host: Granuloma Formation and Egg Excretion. Front. Immunol. 2018, 9, 2492. [Google Scholar] [CrossRef] [Green Version]
- Gobbi, F.; Martelli, G.; Attard, L.; Buonfrate, D.; Angheben, A.; Marchese, V.; Bortesi, L.; Gobbo, M.; Vanino, E.; Viale, P.; et al. Schistosoma mansoni Eggs in Spleen and Lungs, Mimicking Other Diseases. PLoS Negl. Trop. Dis. 2015, 9, e0003860. [Google Scholar] [CrossRef] [Green Version]
- Lopes De Faria, J. Cor pulmonale in Manson’s schistosomiasis. I. Frequency in necropsy material; pulmonary vascular changes caused by schistosome ova. Am. J. Pathol. 1954, 30, 167–193. [Google Scholar]
- Lima, C.W.R.; Oliveira, N.M.C.; Silva, S.V.D.; Duarte, M.E.L.; Barbosa, A.P.F. Ectopic forms of schistosomiasis mansoni in the second macroregion of Alagoas: Case series report and review of the literature. Rev. Soc. Bras. Med. Trop. 2017, 50, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Dastoli, P.A.; Leite, A.L.; da Costa, M.D.S.; Nicácio, J.M.; Pinho, R.S.; Ferrarini, M.A.G.; Cavalheiro, S. Medullary neuroschistosomiasis in adolescence: Case report and literature review. Childs. Nerv. Syst. 2021, 37, 2735–2741. [Google Scholar] [CrossRef] [PubMed]
- Artal, F.J.C.; Mesquita, H.M.; de A Gepp, R.; Antunes, J.S.; Kalil, R.K. Brain involvement in a Schistosoma mansoni myelopathy patient. J. Neur. Neurosur. Psych. 2006, 77, 512. [Google Scholar] [CrossRef] [PubMed]
- van der Vlugt, L.E.P.M.; Labuda, L.A.; Ozir-Fazalalikhan, A.; Lievers, E.; Gloudemans, A.K.; Liu, K.Y.; Barr, T.A.; Sparwasser, T.; Boon, L.; Ngoa, U.A. Schistosomes induce regulatory features in human and mouse CD1dhi B cells: Inhibition of allergic inflammation by IL-10 and regulatory T cells. PLoS ONE 2012, 7, e30883. [Google Scholar] [CrossRef]
- van der Vlugt, L.E.P.M.; Zinsou, J.F.; Ozir-Fazalalikhan, A.; Kremsner, P.G.; Yazdanbakhsh, M.; Adegnika, A.A.; Smits, H.H. Interleukin 10 (IL-10)–producing CD1dhi regulatory B cells from Schistosoma haematobium–infected individuals induce IL-10–positive T cells and suppress effector T-cell cytokines. J. Infect. Dis. 2014, 210, 1207–1216. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.D.; Jenkins, G.R.; Hogg, K.G.; Aynsley, S.A.; Paveley, R.A.; Cook, P.C.; Coles, M.C.; Mountford, A.P. CD4+ CD25+ regulatory cells contribute to the regulation of colonic Th2 granulomatous pathology caused by schistosome infection. PLoS Negl. Trop. Dis. 2011, 5, e1269. [Google Scholar] [CrossRef]
- Hesse, M.; Piccirillo, C.A.; Belkaid, Y.; Prufer, J.; Mentink-Kane, M.; Leusink, M.; Cheever, A.W.; Shevach, E.M.; Wynn, T.A. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J. Immunol. 2004, 172, 3157–3166. [Google Scholar] [CrossRef] [Green Version]
- Chuah, C.; Jones, M.K.; Burke, M.L.; McManus, D.P.; Gobert, G.N. Cellular and chemokine-mediated regulation in schistosome-induced hepatic pathology. Trends Parasitol. 2014, 30, 141–150. [Google Scholar] [CrossRef]
- Amaral, K.B.; Silva, T.P.; Dias, F.F.; Malta, K.K.; Rosa, F.M.; Costa-Neto, S.F.; Gentile, R.; Melo, R.C.N. Histological assessment of granulomas in natural and experimental Schistosoma mansoni infections using whole slide imaging. PLoS ONE 2017, 12, e0184696. [Google Scholar] [CrossRef] [Green Version]
- Hams, E.; Aviello, G.; Fallon, P.G. The schistosoma granuloma: Friend or foe? Front. Immunol. 2013, 4, 89. [Google Scholar] [CrossRef] [Green Version]
- Pagán, A.J.; Ramakrishnan, L. The formation and function of granulomas. Annu. Rev. Immunol. 2018, 36, 639–665. [Google Scholar] [CrossRef]
- Llanwarne, F.; Helmby, H. Granuloma formation and tissue pathology in Schistosoma japonicum versus Schistosoma mansoni infections. Parasite Immunol. 2021, 43, e12778. [Google Scholar] [CrossRef]
- Lenzi, H.L.; Kimmel, E.; Schechtman, H.; Pelajo-Machado, M.; Romanha, W.S.; Pacheco, R.G.; Mariano, M.; Lenzi, J.A. Histoarchitecture of schistosomal granuloma development and involution: Morphogenetic and biomechanical approaches. Mem. Inst. Oswaldo Cruz 1998, 93, 141–151. [Google Scholar] [CrossRef]
- Lenzi, H.L.; Romanha, W.S.; Santos, R.M.; Rosas, A.; Mota, E.M.; Manso, P.P.A.; Caputo, L.F.G.; Pelajo-Machado, M. Four whole-istic aspects of schistosome granuloma biology: Fractal arrangement, internal regulation, autopoietic component and closure. Mem. Inst. Oswaldo Cruz 2006, 101, 219–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, J.; Correia Dacal, A.R.; Vergetti Siqueira, J.G.; Poggensee, G.; Mannsmann, U.; Deelder, A.; Feldmeier, H. Sonographic prediction of variceal bleeding in patients with liver fibrosis due to Schistosoma mansoni. Trop. Med. Inter. Health 1998, 3, 728–735. [Google Scholar] [CrossRef]
- Andrade, Z.A. Schistosomiasis and liver fibrosis. Parasite Immunol. 2009, 31, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.F.; Amaral, K.B.; Malta, K.K.; Silva, T.P.; Rodrigues, G.S.C.; Rosa, F.M.; Rodrigues, G.O.L.; Costa, V.V.; Chiarini-Garcia, H.; Weller, P.F.; et al. Identification of piecemeal degranulation and vesicular transport of MBP-1 in liver-infiltrating mouse eosinophils during acute experimental Schistosoma mansoni infection. Front. Immunol. 2018, 9, 3019. [Google Scholar] [CrossRef] [Green Version]
- Caldas, I.R.; Campi-Azevedo, A.C.; Oliveira, L.F.A.; Silveira, A.M.S.; Oliveira, R.C.; Gazzinelli, G. Human schistosomiasis mansoni: Immune responses during acute and chronic phases of the infection. Acta Tropi. 2008, 108, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Lambertucci, J.R. Acute schistosomiasis mansoni: Revisited and reconsidered. Mem. Inst. Oswaldo Cruz 2010, 105, 422–435. [Google Scholar] [CrossRef]
- Mitre, E.; Klion, A.D. Eosinophils and helminth infection: Protective or pathogenic? Springer Berlin Heidelberg. Semminars Immunopathol. 2021, 43, 363–381. [Google Scholar] [CrossRef]
- Mahmoud, A.A.F.; Warren, K.S.; Graham Jr, R.C. Antieosinophil serum and the kinetics of eosinophilia in Schistosomiasis mansoni. J. Exp. Med. 1975, 142, 560–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knopf, P.M. Schistosoma mansoni: Peripheral and tissue eosinophilia in infected rats. Exp. Parasitol. 1979, 47, 232–245. [Google Scholar] [CrossRef]
- Coffman, R.L.; Seymour, B.W.P.; Hudak, S.; Jackson, J.; Rennick, D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science 1989, 245, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Dent, L.A.; Strath, M.; Mellor, A.L.; Sanderson, C.J. Eosinophilia in transgenic mice expressing interleukin 5. J. Exp. Med. 1990, 172, 1425–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopf, M.; Brombacher, F.; Hodgkin, P.D.; Ramsay, A.J.; Milbourne, E.A.; Dai, W.J.; Ovington, K.S.; Behm, C.A.; Köhler, G.; Young, I.G. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 1996, 4, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.A.F. The ecology of eosinophils in schistosomiasis. J. Infect. Dis. 1982, 145, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Borojevic, R.; Stocker, S.; Grimaud, J.A. Hepatic eosinophil granulocytopoiesis in murine experimental Schistosomiasis mansoni. Brit. J. Exp. Pathol. 1981, 62, 480. [Google Scholar]
- Lenzi, H.L.; Pacheco, R.G.; Pelajo-Machado, M.; Panasco, M.S.; Romanha, W.S.; Lenzi, J.A. Immunological system and Schistosoma mansoni: Co-evolutionary immunobiology. What is the eosinophil role in parasite-host relationship? Mem. Inst. Oswaldo Cruz 1997, 92, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Swartz, J.M.; Dyer, K.D.; Cheever, A.W.; Ramalingam, T.; Pesnicak, L.; Domachowske, J.B.; Lee, J.J.; Lee, N.A.; Foster, P.S.; Wynn, T.A. Schistosoma mansoni infection in eosinophil lineage–ablated mice. Blood 2006, 108, 2420–2427. [Google Scholar] [CrossRef] [Green Version]
- Costain, A.H.; Phythian-Adams, A.T.; Colombo, S.A.P.; Marley, A.K.; Owusu, C.; Cook, P.C.; Brown, S.L.; Webb, L.M.; Lundie, R.J.; Smits, H.H. Dynamics of host immune response development during Schistosoma mansoni infection. Front. Immunol. 2022, 13, 906338. [Google Scholar] [CrossRef]
- Dyer, K.D.; Moser, J.M.; Czapiga, M.; Siegel, S.J.; Percopo, C.M.; Rosenberg, H.F. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J. Immunol. 2008, 181, 4004–4009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonjour, K.; Palazzi, C.; Silva, T.P.; Malta, K.K.; Neves, V.H.; Oliveira-Barros, E.G.; Neves, I.; Kersten, V.A.; Fortuna, B.T.; Samarasinghe, A.E.; et al. Mitochondrial Population in Mouse Eosinophils: Ultrastructural Dynamics in Cell Differentiation and Inflammatory Diseases. Front. Cell Dev. Bio. 2022, 10, 836755. [Google Scholar] [CrossRef] [PubMed]
- Tamarozzi, F.; Ursini, T.; Hoekstra, P.T.; Silva, R.; Costa, C.; Gobbi, F.; Monteiro, G.B.; Motta, L.; van Dam, G.J.; Corstjens, P.L.; et al. Evaluation of microscopy, serology, circulating anodic antigen (CAA), and eosinophil counts for the follow-up of migrants with chronic schistosomiasis: A prospective cohort study. Parasit. Vectors 2021, 14, 149. [Google Scholar] [CrossRef]
- Paran, Y.; Ben-Ami, R.; Orlev, B.; Halutz, O.; Elalouf, O.; Wasserman, A.; Zimmerman, O.; Nahmani, I.; Rabinowich, L.; Finn, T. Chronic schistosomiasis in African immigrants in Israel: Lessons for the non-endemic setting. Medicine 2019, 98, e18481. [Google Scholar] [CrossRef]
- Moore, D.L.; Grove, D.I.; Warren, K.S. The Schistosoma mansoni egg granuloma: Quantitation of cell populations. J. Pathol. 1977, 121, 41–50. [Google Scholar] [CrossRef]
- Melo, R.C.N.; Dvorak, A.M.; Weller, P.F. Eosinophil-associated diseases (EADs). In Eosinophil Ultrastructure: Atlas of Eosinophil Cell Biology and Pathology; Academic Press: Cambridge, MA, USA, 2022; pp. 289–394. [Google Scholar]
- Kephart, G.M.; Andrade, Z.A.; Gleich, G.J. Localization of eosinophil major basic protein onto eggs of Schistosoma mansoni in human pathologic tissue. Am. J. Pathol. 1988, 133, 389. [Google Scholar] [PubMed]
- Thorne, K.J.; Mazza, G. Eosinophilia, activated eosinophils and human schistosomiasis. J. Cell. Sci. 1991. [Google Scholar] [CrossRef]
- Hussein, M.R. Mucocutaneous Splendore-Hoeppli phenomenon. J. Cutane. Pathol. 2008, 35, 979–988. [Google Scholar] [CrossRef]
- White, J.R.; Imburgia, C.; Dul, E.; Appelbaum, E.; O’Donnell, K.; O’Shannessy, D.J.; Brawner, M.; Fornwald, J.; Adamou, J.; Elshourbagy, N.A. Cloning and functional characterization of a novel human CC chemokine that binds to the CCR3 receptor and activates human eosinophils. J. Leukoc. Biol. 1997, 62, 667–675. [Google Scholar] [CrossRef]
- Souza, P.R.S.; Souza, A.L.S.; Negrao-Correa, D.; Teixeira, A.L.; Teixeira, M.M. The role of chemokines in controlling granulomatous inflammation in Schistosoma mansoni infection. Acta Tropi. 2008, 108, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Jakubzick, C.; Wen, H.; Matsukawa, A.; Keller, M.; Kunkel, S.L.; Hogaboam, C.M. Role of CCR4 ligands, CCL17 and CCL22, during Schistosoma mansoni egg-induced pulmonary granuloma formation in mice. Am. J. Pathol. 2004, 165, 1211–1221. [Google Scholar] [CrossRef] [Green Version]
- Sousa-Pereira, S.R.; Teixeira, A.L.; Silva, L.C.S.; Souza, A.L.S.; Antunes, C.M.; Teixeira, M.M.; Lambertucci, J.R. Serum and cerebral spinal fluid levels of chemokines and Th2 cytokines in Schistosoma mansoni myeloradiculopathy. Parasite Immunol. 2006, 28, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Silveira-Lemos, D.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Souza-Soares, A.L.; Castro-Silva, P.; Costa-Silva, M.F.; Guimarães, P.H.G.; Ferraz, H.B.; Oliveira-Fraga, L.A.; Teixeira, M.M. Seric chemokines and chemokine receptors in eosinophils during acute human schistosomiasis mansoni. Mem. Ins. Oswaldo Cruz 2010, 105, 380–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, P.F.; Spencer, L.A. Functions of tissue-resident eosinophils. Nat. Rev. Immunol. 2017, 17, 746–760. [Google Scholar] [CrossRef]
- Capron, M.; Torpier, G.; Capron, A. In vitro killing of S. mansoni schistosomula by eosinophils from infected rats: Role of cytophilic antibodies. J. Immunol. 1979, 123, 2220–2230. [Google Scholar]
- Butterworth, A.E.; Vadas, M.A.; Wassom, D.L.; Dessein, A.; Hogan, M.; Sherry, B.; Gleich, G.J. Interactions between human eosinophils and schistosomula of Schistosoma mansoni. II. The mechanism of irreversible eosinophil adherence. J. Exp. Med. 1979, 150, 1456–1471. [Google Scholar] [CrossRef] [Green Version]
- Adachi, T.; Alam, R. The mechanism of IL-5 signal transduction. Am. J. Physio. Cell. Physio. 1998, 275, C623–C633. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Talvani, A.; Tafuri, W.L.; Lukacs, N.W.; Hellewell, P.G. Eosinophil recruitment into sites of delayed-type hypersensitivity reactions in mice. J. Leukoc. Biol. 2001, 69, 353–360. [Google Scholar] [CrossRef]
- Shin, M.H.; Lee, Y.A.; Min, D.-Y. Eosinophil-mediated tissue inflammatory responses in helminth infection. Kor. J. Parasitol. 2009, 47, S125. [Google Scholar] [CrossRef]
- Yi, S.; Zhai, J.; Niu, R.; Zhu, G.; Wang, M.; Liu, J.; Huang, H.; Wang, Y.; Jing, X.; Kang, L. Eosinophil recruitment is dynamically regulated by interplay among lung dendritic cell subsets after allergen challenge. Nat. Commun. 2018, 9, 3879. [Google Scholar] [CrossRef] [Green Version]
- Lins, R.A.B.; Chaves, M.E.C.; de Melo-Júnior, M.R.; Araújo-Filho, J.L.S.; de Lima Cavalcanti, C.B. A distribuição dos eosinófilos nas diferentes fases de evolução do granuloma hepático em camundongos infectados pelo Schistosoma mansoni. Rev. Soc. Bras. Med. Trop. 2008, 41, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Lenzi, H.L.; Romanha, W.S.; Pelajo-Machado, M.; Mota, E.M.; Lenzi, J.A. Patologia experimental com enfoque no granuloma esquistossomótico. In Schistosoma Mansoni & Esquistossomose: Uma Visão Multidisciplinary; Carvalho, O.S., Coelho, P.M.Z., Lenzi, H., Eds.; L. Fiocruz: Rio de Janeiro, Brazil, 2008; pp. 569–654. [Google Scholar]
- Geuskens, M.; Borojevic, R.; Van Gansen, P. Eosinophil granulocytopoiesis in hepatic periovular granulomas during the chronic phase of experimental murine Schistosomiasis mansoni. Biol. Cell 1991, 71, 89–96. [Google Scholar] [CrossRef]
- Lenzi, H.L.; Lenzi, J.A.; Rosman, F.C.; Pelajo-Machado, M.; Mota, E.M.; Panasco, M.S.; Oliveira, D.N. Extramedullary hematopoiesis in murine schistosomiasis mansoni. Mem. Inst. Oswaldo Cruz 1995, 90, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francisco, J.S.; Terra, M.A.B.L.; Klein, G.C.T.; Dias de Oliveira, B.C.E.P.; Pelajo Machado, M. The Hepatic Extramedullary Hematopoiesis during Experimental Murine Schistosomiasis. Front. Immunol. 2022, 4861. [Google Scholar] [CrossRef]
- Dutra, H.S.; Rossi, M.I.D.; Azevedo, S.P.; El-Cheikh, M.C.; Borojevic, R. Haematopoietic capacity of colony-forming cells mobilized in hepatic inflammatory reactions as compared to that of normal bone marrow cells. Res. Immunol. 1997, 148, 437–444. [Google Scholar] [CrossRef]
- Rossi, M.I.D.; Dutra, H.S.; El-Cheikh, M.C.; Bonomo, A.; Borojevic, R. Extramedullar B lymphopoiesis in liver schistosomal granulomas: Presence of the early stages and inhibition of the full B cell differentiation. Int. Immuol. 1999, 11, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, R.C.N.; Dvorak, A.M.; Weller, P.F. Eosinophil Ultrastructure: Atlas of Eosinophil Cell Biology and Pathology; Academic Press: Cambridge, MA, USA, 2022; p. 518. [Google Scholar]
- Lukacs, N.W.; Kunkel, S.L.; Strieter, R.M.; Warmington, K.; Chensue, S.W. The role of macrophage inflammatory protein 1 alpha in Schistosoma mansoni egg-induced granulomatous inflammation. J. Exp. Med. 1993, 177, 1551–1559. [Google Scholar] [CrossRef] [Green Version]
- Souza, A.L.S.; Roffê, E.; Pinho, V.; Souza, D.G.; Silva, A.F.; Russo, R.C.; Guabiraba, R.; Pereira, C.A.J.; Carvalho, F.M.; Barsante, M.M. Potential role of the chemokine macrophage inflammatory protein 1α in human and experimental schistosomiasis. Inf. Immunity 2005, 73, 2515–2523. [Google Scholar] [CrossRef] [Green Version]
- Magalhaes, E.S.; Paiva, C.N.; Souza, H.S.P.; Pyrrho, A.S.; Mourao-Sá, D.; Figueiredo, R.T.; Vieira-de-Abreu, A.; Dutra, H.S.; Silveira, M.S.; Gaspar-Elsas, M.I.C. Macrophage migration inhibitory factor is critical to interleukin-5-driven eosinophilopoiesis and tissue eosinophilia triggered by Schistosoma mansoni infection. FASEB J. 2009, 23, 1262–1271. [Google Scholar] [CrossRef]
- da Paz, V.R.F.; Figueiredo-Vanzan, D.; dos Santos Pyrrho, A. Interaction and involvement of cellular adhesion molecules in the pathogenesis of Schistosomiasis mansoni. Immunol. Lett. 2019, 206, 11–18. [Google Scholar] [CrossRef]
- Spencer, L.A.; Bonjour, K.; Melo, R.C.N.; Weller, P.F. Eosinophil secretion of granule-derived cytokines. Front. Immunol. 2014, 5, 496. [Google Scholar] [CrossRef] [Green Version]
- Melo, R.C.N.; Weller, P.F. Piecemeal degranulation in human eosinophils: A distinct secretion mechanism underlying inflammatory responses. Histo. Hitopathol. 2010, 25, 1341. [Google Scholar] [CrossRef]
- Melo, R.C.N.; Weller, P.F. Contemporary understanding of the secretory granules in human eosinophils. J. Leukoc. Biol. 2018, 104, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.C.N.; Spencer, L.A.; Dvorak, A.M.; Weller, P.F. Mechanisms of eosinophil secretion: Large vesiculotubular carriers mediate transport and release of granule-derived cytokines and other proteins. J. Leukoc. Biol. 2008, 83, 229–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, R.C.N.; Weller, P.F. Vesicular trafficking of immune mediators in human eosinophils revealed by immunoelectron microscopy. Exp. Cell. Res. 2016, 347, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, H.L.; Lenzi, J.A.; Sobral, A.C.L. Eosinophils favor the passage of eggs to the intestinal lumen in schistosomiasis. Braz. J. Med. 1987, 20, 433–435. [Google Scholar]
- Metwali, A.; Elliott, D.; Blum, A.M.; Weinstock, J.V. Granuloma eosinophils enhance IL-5 production by lymphocytes from mice infected with Schistosoma mansoni. J. Immunol. 1993, 151, 7048–7056. [Google Scholar]
- Rumbley, C.A.; Sugaya, H.; Zekavat, S.A.; El Refaei, M.; Perrin, P.J.; Phillips, S.M. Activated eosinophils are the major source of Th2-associated cytokines in the schistosome granuloma. J. Immunol. 1999, 162, 1003–1009. [Google Scholar]
- Souza, C.O.S.; Gardinassi, L.G.; Rodrigues, V.; Faccioli, L.H. Monocyte and macrophage-mediated pathology and protective immunity during schistosomiasis. Front. Microbiol. 2020, 11, 1973. [Google Scholar] [CrossRef]
- Reiman, R.M.; Thompson, R.W.; Feng, C.G.; Hari, D.; Knight, R.; Cheever, A.W.; Rosenberg, H.F.; Wynn, T.A. Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect. Immun. 2006, 74, 1471–1479. [Google Scholar] [CrossRef] [Green Version]
- Lenzi, H.L.; Sobral, A.C.L.; Lenzi, J.A. In vivo kinetics of eosinophils and mast cells in experimental murine schistosomiasis. Mem. Inst. Oswaldo Cruz 1987, 82, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Capron, M.; Rousseaux, J.; Mazingue, C.; Bazin, H.; Carpon, A. Rat mast cell-eosinophil interaction in antibody-dependent eosinophil cytotoxicity to Schistosoma mansoni schistosomula. J. Immunol. 1978, 121, 2518–2525. [Google Scholar] [PubMed]
- Capron, M.; Capron, A. Schistosomes and eosinophils. Trans. Roy. Soc. Trop. Med. Hygie. 1980, 74, 44–50. [Google Scholar] [CrossRef]
- Sanches, R.C.O.; Mambelli, F.; Oliveira, S.C. Neutrophils and schistosomiasis: A missing piece in pathology. Parasite Immunol. 2022, 44, e12916. [Google Scholar] [CrossRef] [PubMed]
- Tweyongyere, R.; Namanya, H.; Naniima, P.; Cose, S.; Tukahebwa, E.M.; Elliott, A.M.; Dunne, D.W.; Wilson, S. Human eosinophils modulate peripheral blood mononuclear cell response to Schistosoma mansoni adult worm antigen in vitro. Parasite Immunol. 2016, 38, 516–522. [Google Scholar] [CrossRef] [Green Version]
- Paiva, L.A.; Brand, C.; Bandeira-Melo, C.; Bozza, P.T.; El-Cheikh, M.C.; Silva, P.M.; Borojevic, R.; Perez, S.A.C. Hepatic myofibroblasts derived from Schistosoma mansoni-infected mice are a source of IL-5 and eotaxin: Controls of eosinophil populations in vitro. Parasite Vectors 2015, 8, 577. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.-E.; Morrison, P.J.; Wilhelm, C.; Wilson, M.; Ahlfors, H.; Renauld, J.-C.; Panzer, U.; Helmby, H.; Stockinger, B. IL-9–mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J. Exp. Med. 2013, 210, 2951–2965. [Google Scholar] [CrossRef] [Green Version]
- Shamri, R.; Xenakis, J.J.; Spencer, L.A. Eosinophils in innate immunity: An evolving story. Cell Tissue Res. 2011, 343, 57–83. [Google Scholar] [CrossRef]
- Chu, V.T.; Fröhlich, A.; Steinhauser, G.; Scheel, T.; Roch, T.; Fillatreau, S.; Lee, J.J.; Löhning, M.; Berek, C. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 2011, 12, 151–159. [Google Scholar] [CrossRef]
- Goh, Y.P.S.; Henderson, N.C.; Heredia, J.E.; Red Eagle, A.; Odegaard, J.I.; Lehwald, N.; Nguyen, K.D.; Sheppard, D.; Mukundan, L.; Locksley, R.M. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 9914–9919. [Google Scholar] [CrossRef] [Green Version]
- James, S.L.; Colley, D.G. Eosinophil-mediated destruction of Schistosoma mansoni eggs. J. Reticulo. Soc. 1976, 20, 359–374. [Google Scholar]
- Duplantier, J.-M.; Sene, M. Rodents as reservoir hosts in the transmission of Schistosoma mansoni in Richard-Toll, Senegal, West Africa. J. Helminthol. 2000, 74, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Catalano, S.; Sène, M.; Diouf, N.D.; Fall, C.B.; Borlase, A.; Léger, E.; Bâ, K.; Webster, J.P. Rodents as natural hosts of zoonotic Schistosoma species and hybrids: An epidemiological and evolutionary perspective from West Africa. J. Infect. Dis. 2018, 218, 429–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, L.; Erko, B.; Ponpetch, K.; Ryan, S.J.; Liang, S. Assessing the nonhuman primate reservoir of Schistosoma mansoni in Africa: A systematic review. Infect. Dis. Pov. 2019, 8, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Rey, L. Non-human vertebrate hosts of Schistosoma mansoni and schistosomiasis transmission in Brazil. Res. Rev. Parasitol. 1993, 53, 13–25. [Google Scholar]
- Wilson, R.A.; Li, X.-H.; Castro-Borges, W. Do schistosome vaccine trials in mice have an intrinsic flaw that generates spurious protection data? Parasite Vectors 2016, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Bastos, O.d.C.; Sadigursky, M.; Nascimento, M.d.D.S.B.d.; Brazil, R.P.; Holanda, J.C. Holochilus brasiliensis nanus Thomas, 1897: As an experimental model for filariasis, leishmaniasis and schistosomiasis. Rev. Inst. Med. Trop. 1984, 26, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Duplantier, J.-M.; Sene, M. Rodents as definitive hosts of Schistosoma, with special reference to S. mansoni transmission. In Micromammals and Macroparasites; Morand, S., Krasnov, B.R., Poulin, R., Eds.; Springer: Tokyo, Japan, 2006; pp. 527–543. [Google Scholar]
- Théron, A.; Pointier, J.P.; Morand, S.; Imbert-Establet, D.; Borel, G. Long-term dynamics of natural populations of Schistosoma mansoni among Rattus rattus in patchy environment. Parasitology 1992, 104, 291–298. [Google Scholar] [CrossRef]
- de Noya, B.A.; Pointier, J.; Colmenares, C.; Théron, A.; Balzan, C.; Cesari, I.; González, S.; Noya, O. Natural Schistosoma mansoni infection in wild rats from Guadeloupe: Parasitological and immunological aspects. Acta Tropi. 1997, 68, 11–21. [Google Scholar] [CrossRef]
- Miranda, G.S.; Miranda, B.S.; Rodrigues, J.G.M.; Lira, M.G.S.; Nogueira, R.A.; Viegas-Melo, D.; Silva-Souza, N. Research Note. The wild water-rats and their relevance in the context of schistosomiasis mansoni in Brazil: What we know and recommendations for further research. Helminthologia 2017, 54, 165–169. [Google Scholar] [CrossRef]
- Picot, H. Holochilus brasiliensis and Nectomys squamipes (Rodentia-Cricetidae) natural hosts of Schistosoma mansoni. Mem. Inst. Oswaldo Cruz 1992, 87, 255–260. [Google Scholar] [CrossRef]
- Gentile, R.; Soares, M.S.; Barreto, M.G.M.; Gonçalves, M.M.L.; D’Andrea, P.S. The Role of Wild Rodents in the Transmission of Schistosoma mansoni in Brazil. In Schistosomiasis; Rokni, M.B., Ed.; IntechOpen: London, UK, 2012; pp. 231–254. [Google Scholar]
- do Carmo-Silva, C.F.; Teles-Reis, A.; Silva-Soares, R.F.; Rodrigues, J.G.M.; Lira, M.G.S.; Nogueira, R.A.; Viegas-Melo, D.; Cardoso, D.T.; Miranda, G.S.; Silva-Souza, N. Spatial and seasonal distribution of Holochilus sciureus with Schistosoma mansoni infection in an endemic area for schistosomiasis in Brazil. Acta Parasit. 2019, 64, 932–937. [Google Scholar] [CrossRef]
- Abdala-Valencia, H.; Coden, M.E.; Chiarella, S.E.; Jacobsen, E.A.; Bochner, B.S.; Lee, J.J.; Berdnikovs, S. Shaping eosinophil identity in the tissue contexts of development, homeostasis, and disease. J. Leukoc. Biol. 2018, 104, 95–108. [Google Scholar] [CrossRef]
- Rodrigues, J.G.M.; Lira, M.G.S.; Nogueira, R.A.; Gomes, G.C.C.; Licá, I.C.L.; Silva, J.K.A.d.O.; Miranda, G.S.; Silva-Souza, N. Alterations in blood glucose concentration in wild rodents, Holochilus sciureus, naturally infected with Schistosoma mansoni. Rev. Braz. Parasitol. Vet. 2022, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Miranda, G.S.; Rodrigues, J.G.M.; Lira, M.G.S.; Nogueira, R.A.; Gomes, G.C.C.; Silva-Souza, N. Schistosoma mansoni infection in Holochilus sciureus shows sex-related differences in parasitological patterns. Open J. Anim. Sci. 2019, 9, 173. [Google Scholar] [CrossRef] [Green Version]
- Costa Neto, S.F.d.; Alves, V.M.T.; Alves, V.M.T.; Garcia, J.S.; Santos, M.A.J.d.; Nogueira, V.d.A.; Brito, M.d.F.; Gentile, R.; Pinheiro, J. Biochemical and histological changes in liver of Nectomys squamipes naturally infected by Schistosoma mansoni. Rev. Braz. Parasitol. Vet. 2013, 22, 519–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Souza, N.d.; Silva, A.P.d.C.d.; Oliveira, R.M.d.; Lira, M.G.S.; Nogueira, R.A.; Penha-Silva, T.A.d.; Melo, S.d.A.; Andrade, F.H.E.d.; Santos-Ribeiro, L.S.d.; Carvalho, A.V.d. Parasitological and histological aspects of Holochilus sciureus naturally infected by Schistosoma mansoni. Rev. Braz. Parasitol. Vet. 2019, 28, 769–772. [Google Scholar] [CrossRef]
- Costa-Silva, M.; Rodrigues-Silva, R.; Hulstijn, M.; Neves, R.H.; Panasco, M.d.S.; Lenzi, H.L.; Machado-Silva, J.R. Natural Schistosoma mansoni infection in Nectomys squamipes: Histopathological and morphometric analysis in comparison to experimentally infected N. squamipes and C3H/He mice. Mem. Inst. Oswaldo Cruz 2002, 97, 129–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenzi, J.A.; Mota, E.M.; Machado, M.P.; Paiva, R.A.; Lenzi, H.L. Calomys callosus: An alternative model to study fibrosis in Schistosomiasis mansoni: The pathology of the acute phase. Mem. Inst. Oswaldo Cruz 1995, 90, 311–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, R.R.; Silva, J.R.M.; Faerstein, N.F.; Lenzi, H.L.; Rey, L. Natural infection of wild rodents by Schistosoma mansoni parasitological aspects. Mem. Inst. Oswaldo Cruz 1992, 87, 271–276. [Google Scholar] [CrossRef]
- D’andrea, P.S.; Fernandes, F.A.; Cerqueira, R.; Rey, L. Experimental evidence and ecological perspectives for the adaptation of Schistosoma mansoni Sambon, 1907 (Digenea: Schistosomatidae) to a wild host, the water-rat, Nectomys squamipes Brants, 1827 (Rodentia: Sigmodontinae). Mem. Inst. Oswaldo Cruz 2002, 97, 11–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsü, S.L.; Hsü, H.F.; Mitros, F.A.; Helms, C.M.; Solomon, R.I. Eosinophils as effector cells in the destruction of Schistosoma mansoni eggs in granulomas. Ann. Trop. Med. Parasitol. 1980, 74, 179–183. [Google Scholar] [CrossRef]
- Khalife, J.; Dunne, D.W.; Richardson, B.A.; Mazza, G.; Thorne, K.J.; Capron, A.; Butterworth, A.E. Functional role of human IgG subclasses in eosinophil-mediated killing of schistosomula of Schistosoma mansoni. J. Immunol. 1989, 142, 4422–4427. [Google Scholar] [PubMed]
- Ramalho-Pinto, F.J.; McLaren, D.J.; Smithers, S.R. Complement-mediated killing of schistosomula of Schistosoma mansoni by rat eosinophils in vitro. J. Exp. Med. 1978, 147, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Klion, A.D.; Ackerman, S.J.; Bochner, B.S. Contributions of eosinophils to human health and disease. Ann. Rev. Pathol. 2020, 15, 179. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, E.A.; Taranova, A.G.; Lee, N.A.; Lee, J.J. Eosinophils: Singularly destructive effector cells or purveyors of immunoregulation? J. Alle. Clin. Immunol. 2007, 119, 1313–1320. [Google Scholar] [CrossRef]
- Rosenberg, H.F.; Dyer, K.D.; Foster, P.S. Eosinophils: Changing perspectives in health and disease. Nat. Rev. 2013, 13, 9. [Google Scholar] [CrossRef]
- de Oliveira, V.G.; Rodrigues, V.F.; Moreira, J.M.P.; Rodrigues, J.L.; Maggi, L.; Resende, S.D.; Negrão-Corrêa, D. Eosinophils participate in modulation of liver immune response and tissue damage induced by Schistosoma mansoni infection in mice. Cytokine 2022, 149, 155701. [Google Scholar] [CrossRef] [PubMed]
- Coakley, G.; Wright, M.D.; Borger, J.G. Schistosoma mansoni-derived lipids in extracellular vesicles: Potential agonists for eosinophillic tissue repair. Front. Immunol. 2019, 10, 1010. [Google Scholar] [CrossRef] [Green Version]
- Masterson, J.C.; Menard-Katcher, C.; Larsen, L.D.; Furuta, G.T.; Spencer, L.A. Heterogeneity of intestinal tissue eosinophils: Potential considerations for next-generation eosinophil-targeting strategies. Cells 2021, 10, 426. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malta, K.K.; Palazzi, C.; Neves, V.H.; Aguiar, Y.; Silva, T.P.; Melo, R.C.N. Schistosomiasis Mansoni-Recruited Eosinophils: An Overview in the Granuloma Context. Microorganisms 2022, 10, 2022. https://doi.org/10.3390/microorganisms10102022
Malta KK, Palazzi C, Neves VH, Aguiar Y, Silva TP, Melo RCN. Schistosomiasis Mansoni-Recruited Eosinophils: An Overview in the Granuloma Context. Microorganisms. 2022; 10(10):2022. https://doi.org/10.3390/microorganisms10102022
Chicago/Turabian StyleMalta, Kássia K., Cinthia Palazzi, Vitor H. Neves, Yasmin Aguiar, Thiago P. Silva, and Rossana C. N. Melo. 2022. "Schistosomiasis Mansoni-Recruited Eosinophils: An Overview in the Granuloma Context" Microorganisms 10, no. 10: 2022. https://doi.org/10.3390/microorganisms10102022
APA StyleMalta, K. K., Palazzi, C., Neves, V. H., Aguiar, Y., Silva, T. P., & Melo, R. C. N. (2022). Schistosomiasis Mansoni-Recruited Eosinophils: An Overview in the Granuloma Context. Microorganisms, 10(10), 2022. https://doi.org/10.3390/microorganisms10102022








