Motivations and Barriers to Participation in a Randomized Trial on Melanoma Genomic Risk: A Mixed-Methods Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Quantitative Data Collection and Analysis
2.3. Qualitative Data Collection and Analysis
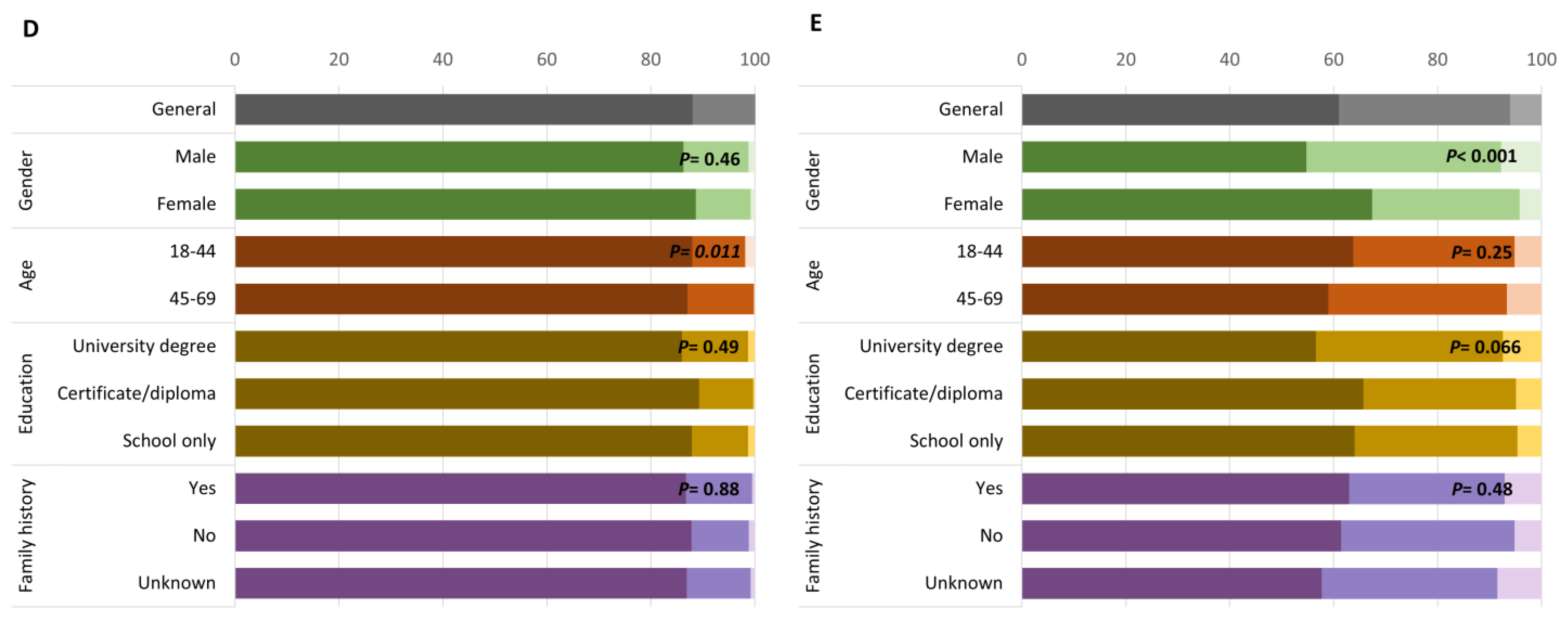
3. Results
3.1. Motivations and Perceived Benefit for Participation
3.2. Barriers to Participation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torkamani, A.; Wineinger, N.E.; Topol, E.J. The personal and clinical utility of polygenic risk scores. Nat. Rev. Genet. 2018, 19, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.J.; Iademarco, M.F.; Riley, W.T. Precision Public Health for the Era of Precision Medicine. Am. J. Prev. Med. 2016, 50, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Smith-Uffen, M.; Bartley, N.; Davies, G.; Best, M. Motivations and barriers to pursue cancer genomic testing: A systematic review. Patient Educ. Couns. 2021, 104, 1325–1334. [Google Scholar] [CrossRef]
- Sanderson, S.C.; Linderman, M.D.; Suckiel, S.A.; Diaz, G.A.; Zinberg, R.E.; Ferryman, K.; Wasserstein, M.; Kasarskis, A.; Schadt, E.E. Motivations, concerns and preferences of personal genome sequencing research participants: Baseline findings from the HealthSeq project. Eur. J. Hum. Genet. EJHG 2016, 24, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.L.; Meyer White, K.; Sussman, A.; Kaphingst, K.; Guest, D.; Schofield, E.; Dailey, Y.T.; Robers, E.; Schwartz, M.R.; Zielaskowski, K.; et al. Psychosocial and Cultural Determinants of Interest and Uptake of Skin Cancer Genetic Testing in Diverse Primary Care. Public Health Genom. 2019, 22, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Shen, E.C.; Srinivasan, S.; Passero, L.E.; Allen, C.G.; Dixon, M.; Foss, K.; Halliburton, B.; Milko, L.V.; Smit, A.K.; Carlson, R.; et al. Barriers and Facilitators for Population Genetic Screening in Healthy Populations: A Systematic Review. Front. Genet. 2022, 13, 865384. [Google Scholar] [CrossRef]
- Smit, A.K.; Newson, A.J.; Morton, R.L.; Kimlin, M.; Keogh, L.; Law, M.H.; Kirk, J.; Dobbinson, S.; Kanetsky, P.A.; Fenton, G.; et al. The melanoma genomics managing your risk study: A protocol for a randomized controlled trial evaluating the impact of personal genomic risk information on skin cancer prevention behaviors. Contemp. Clin. Trials 2018, 70, 106–116. [Google Scholar] [CrossRef]
- Lo, S.N.; Smit, A.K.; Espinoza, D.; Cust, A.E.; Managing Your Risk Study, G. The Melanoma Genomics Managing Your Risk Study randomised controlled trial: Statistical analysis plan. Trials 2020, 21, 594. [Google Scholar] [CrossRef]
- Branstrom, R.; Kasparian, N.A.; Affleck, P.; Tibben, A.; Chang, Y.M.; Azizi, E.; Baron-Epel, O.; Bergman, W.; Chan, M.; Davies, J.; et al. Perceptions of genetic research and testing among members of families with an increased risk of malignant melanoma. Eur. J. Cancer 2012, 48, 3052–3062. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.L.; Zielaskowski, K.; Meyer White, K.; Kaphingst, K.; Robers, E.; Guest, D.; Sussman, A.; Talamantes, Y.; Schwartz, M.; Rodriguez, V.M.; et al. Interest and Uptake of MC1R Testing for Melanoma Risk in a Diverse Primary Care Population: A Randomized Clinical Trial. JAMA Dermatol. 2018, 154, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.K.; Espinoza, D.; Newson, A.J.; Morton, R.L.; Fenton, G.; Freeman, L.; Dunlop, K.; Butow, P.N.; Law, M.H.; Kimlin, M.G.; et al. A Pilot Randomized Controlled Trial of the Feasibility, Acceptability, and Impact of Giving Information on Personalized Genomic Risk of Melanoma to the Public. Cancer Epidemiol. Biomark. Prev. 2017, 26, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Clarke, V. Successful Qualitative Research; Sage: London, UK, 2013. [Google Scholar]
- Pluye, P.; Hong, Q.N. Combining the power of stories and the power of numbers: Mixed methods research and mixed studies reviews. Annu. Rev. Public Health 2014, 35, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Goodman, D.; Johnson, C.; Bowen, D.; Wenzel, L.; Edwards, K. Factors that Motivate Participation in Observational Genetic Cancer Research Studies. Open J. Epidemiol. 2019, 9, 156–172. [Google Scholar] [CrossRef]
- Facio, F.M.; Brooks, S.; Loewenstein, J.; Green, S.; Biesecker, L.G.; Biesecker, B.B. Motivators for participation in a whole-genome sequencing study: Implications for translational genomics research. Eur. J. Hum. Genet. EJHG 2011, 19, 1213–1217. [Google Scholar] [CrossRef]
- Jeffrey, C.; Johnny, S.; Andrew, T.; Aaron, M.; Christina, T.; Nail, A.; Subhasis, M. Gender-Based Differences and Barriers in Skin Protection Behaviors in Melanoma Survivors. J. Ski. Cancer 2016, 2016, 3874572–3874574. [Google Scholar] [CrossRef]
- Lee, A.; Lee, A.; Garbutcheon-Singh, K.B.; Garbutcheon-Singh, K.B.; Dixit, S.; Dixit, S.; Brown, P.; Brown, P.; Smith, S.D.; Smith, S.D. The Influence of Age and Gender in Knowledge, Behaviors and Attitudes Towards Sun Protection: A Cross-Sectional Survey of Australian Outpatient Clinic Attendees. Am. J. Clin. Dermatol. 2015, 16, 47–54. [Google Scholar] [CrossRef]
- Haluza, D.; Moshammer, H.; Kundi, M.; Cervinka, R. Public (Skin) Health perspectives of gender differences in tanning habits and sun protective behaviour: A cross-sectional questionnaire survey. Wien. Klin. Wochenschr. 2015, 127, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.K.; Allen, M.; Beswick, B.; Butow, P.; Dawkins, H.; Dobbinson, S.J.; Dunlop, K.L.; Espinoza, D.; Fenton, G.; Kanetsky, P.A.; et al. Impact of personal genomic risk information on melanoma prevention behaviors and psychological outcomes: A randomized controlled trial. Genet. Med. 2021, 23, 2394–2403. [Google Scholar] [CrossRef]
- Volkov, A.; Dobbinson, S.; Wakefield, M.; Slevin, T. Seven-year trends in sun protection and sunburn among Australian adolescents and adults. Aust. N. Z. J. Public Health 2013, 37, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Perez, D.; Kite, J.; Dunlop, S.M.; Cust, A.E.; Goumas, C.; Cotter, T.; Walsberger, S.C.; Dessaix, A.; Bauman, A. Exposure to the ‘Dark Side of Tanning’ skin cancer prevention mass media campaign and its association with tanning attitudes in New South Wales, Australia. Health Educ. Res. 2015, 30, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, S.; Ben-Sinai, R.; Keinan, G. Effects of Controllability, Predictability, and Information-Seeking Style on Interest in Predictive Genetic Testing. Personal. Soc. Psychol. Bull. 1999, 25, 1187–1195. [Google Scholar] [CrossRef]
- Keogh, L.A.; Niven, H.; Rutstein, A.; Flander, L.; Gaff, C.; Jenkins, M. Choosing not to undergo predictive genetic testing for hereditary colorectal cancer syndromes: Expanding our understanding of decliners and declining. J. Behav. Med. 2017, 40, 583–594. [Google Scholar] [CrossRef]
- Tiller, J.M.; Keogh, L.A.; McInerney-Leo, A.M.; Belcher, A.; Barlow-Stewart, K.; Boughtwood, T.; Gleeson, P.; Dowling, G.; Prince, A.; Bombard, Y.; et al. A step forward, but still inadequate: Australian health professionals’ views on the genetics and life insurance moratorium. J. Med. Genet. 2022, 59, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Newson, A.J.; Ayres, S.; Boyle, J.; Gabbett, M.T.; Nisselle, A. Human Genetics Society of Australasia Position Statement: Genetic Testing and Personal Insurance Products in Australia. Twin Res. Hum. Genet. 2018, 21, 533–537. [Google Scholar] [CrossRef]
- Milne, R.; Raven-Adams, M.; Nicol, D.; The Public Attitudes for Genomic Policy Subgroup. Public Attitudes for Genomic Policy Brief: Trust and Trustworthiness. Global Alliance for Genomics and Health, Blog. 2022. Available online: https://www.ga4gh.org/news/public-attitudes-for-genomic-policy-brief-trust-and-trustworthiness (accessed on 30 May 2022).
- World Health Organization. Accelerating Access to Genomics for Global Health: Promotion, Implementation, Collaboration, and Ethical, Legal, and Social Issues. A Report of the WHO Science Council; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Yancey, A.K.; Ortega, A.N.; Kumanyika, S.K. Effective recruitment and retention of minority research participants. Annu. Rev. Public Health 2006, 27, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Corbie-Smith, G.; Moody-Ayers, S.; Thrasher, A.D. Closing the circle between minority inclusion in research and health disparities. Arch. Intern. Med. 2004, 164, 1362–1364. [Google Scholar] [CrossRef]
- Rubin, E. Striving for Diversity in Research Studies. N. Engl. J. Med. 2021, 385, 1429–1430. [Google Scholar] [CrossRef]
- Rodriguez, V.M.; Robers, E.; Zielaskowski, K.; Javier Gonzalez, C.; Hunley, K.; Kaphingst, K.A.; Guest, D.D.; Sussman, A.; Meyer White, K.A.; Schwartz, M.R.; et al. Translation and adaptation of skin cancer genomic risk education materials for implementation in primary care. J. Community Genet. 2017, 8, 53–63. [Google Scholar] [CrossRef]

| Characteristics | Participants Who Completed the Baseline Questionnaire (N = 1024 1) | Interviewed Participants (N = 40 2) |
|---|---|---|
| N (%) | N (%) | |
| Gender | ||
| Female | 522 (51.0%) | 24 (60.0%) |
| Male | 502 (49.0%) | 16 (40.0%) |
| Age group | ||
| 18-44 years | 483 (47.2%) | 15 (37.5%) |
| 45-69 years | 541 (52.8%) | 25 (62.5%) |
| Education | ||
| University degree | 458 (44.7%) | 20 (50.0%) |
| Certificate/diploma | 327 (31.9%) | 11 (27.5%) |
| School level (or equivalent) | 239 (23.4%) | 9 (22.5%) |
| Family history of melanoma | ||
| Yes | 197 (19.2%) | 7 (17.5%) |
| No | 697 (68.1%) | 28 (70.0%) |
| Unknown | 130 (12.7%) | 5 (12.5%) |
| State of residence | ||
| NSW | 289 (28.2%) | 10 (25.0%) |
| QLD | 210 (20.5%) | 6 (15.0%) |
| WA | 106 (10.4%) | 7 (17.5%) |
| NT | 6 (0.6%) | 0 |
| TAS | 45 (4.4%) | 2 (5.0%) |
| VIC | 293 (28.6%) | 12 (30.0%) |
| SA | 56 (5.5%) | 3 (7.5%) |
| ACT | 19 (1.9%) | 0 |
| Genomic risk category (intervention arm only) | N = 509 | N = 20 |
| Lower than average | 107 (21.0%) | 10 (50%) |
| Average | 267 (52.5%) | 3 (15%) |
| Higher than average | 135 (26.5%) | 7 (35%) |
| Reason | N (%) (N = 87) | Description | Example Quote |
|---|---|---|---|
| Eligibility | |||
| Diagnosed with melanoma | 19 (22) | Participants had a prior history of melanoma and/or had a melanoma excised | “I would like to take part in this study but am unsuitable as I have had a melanoma.” |
| Overseas or a different state | 21 (24) | Participants are currently living abroad or in a different state | “Received letter advising that addressee now lives overseas and is unable to participate.” |
| Age | 3 (3) | Participants are above the age cut off for the research | “Called to notify that he had just recently turned 70 years old, so now not eligible.” |
| No European ancestry | 5 (6) | Participants disclosed they have no European ancestry | “I cannot take part in this study because I do not have any European ancestry.” |
| Other Reasons | |||
| Insurance concerns | 5 (6) | Participants do not want to participate because they are worried their genetic risk will be used by insurance companies | “I have declined due to having to report genetic results to insurance bodies. This would not only impact me, but also my children.” |
| Time constraints | 5 (6) | Participants express that they are too busy to dedicate time to the research | “Sorry but I run a business and don’t have time for this research." |
| Distrust in genetic data collection/handling | 3 (3) | Participants are worried their genetic information wouldn’t be protected/safe | “I’m simply not keen on having my genetic material collected and stored. Despite best intentions, accidents and breaches can still occur.” |
| Other health conditions | 3 (3) | Participants disclosed that they are currently undergoing other health problems and cannot take on other commitments | “I do not wish to do this due to me suffering renal carcinoma.” |
| Disability | 4 (5) | Participant and/or carer informed that they have a disability that prevents them from participating | “Our son has an intellectual and partly physical disability and cannot participate in your study.” |
| No interest | 2 (2) | Participant disclosed no interest in taking part in the research | “Not interested in taking part in study.” |
| Other | 8 (9) | Reason disclosed does not fall under previous categories | “I am not in a position to pay any fees.” |
| “English not good.” | |||
| “Declining due to not being able to wear that wristband to work due to being a mechanic.” | |||
| Declined but no reason given | 4 (5) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercado, G.; Newson, A.J.; Espinoza, D.; The Managing Your Risk Study Group; Cust, A.E.; Smit, A.K. Motivations and Barriers to Participation in a Randomized Trial on Melanoma Genomic Risk: A Mixed-Methods Analysis. J. Pers. Med. 2022, 12, 1704. https://doi.org/10.3390/jpm12101704
Mercado G, Newson AJ, Espinoza D, The Managing Your Risk Study Group, Cust AE, Smit AK. Motivations and Barriers to Participation in a Randomized Trial on Melanoma Genomic Risk: A Mixed-Methods Analysis. Journal of Personalized Medicine. 2022; 12(10):1704. https://doi.org/10.3390/jpm12101704
Chicago/Turabian StyleMercado, Gabriela, Ainsley J. Newson, David Espinoza, The Managing Your Risk Study Group, Anne E. Cust, and Amelia K. Smit. 2022. "Motivations and Barriers to Participation in a Randomized Trial on Melanoma Genomic Risk: A Mixed-Methods Analysis" Journal of Personalized Medicine 12, no. 10: 1704. https://doi.org/10.3390/jpm12101704
APA StyleMercado, G., Newson, A. J., Espinoza, D., The Managing Your Risk Study Group, Cust, A. E., & Smit, A. K. (2022). Motivations and Barriers to Participation in a Randomized Trial on Melanoma Genomic Risk: A Mixed-Methods Analysis. Journal of Personalized Medicine, 12(10), 1704. https://doi.org/10.3390/jpm12101704






