Quality by Design Guided Development of Polymeric Nanospheres of Terbinafine Hydrochloride for Topical Treatment of Onychomycosis Using a Nano-Gel Formulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
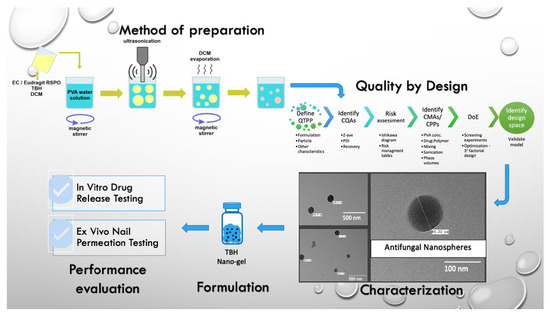
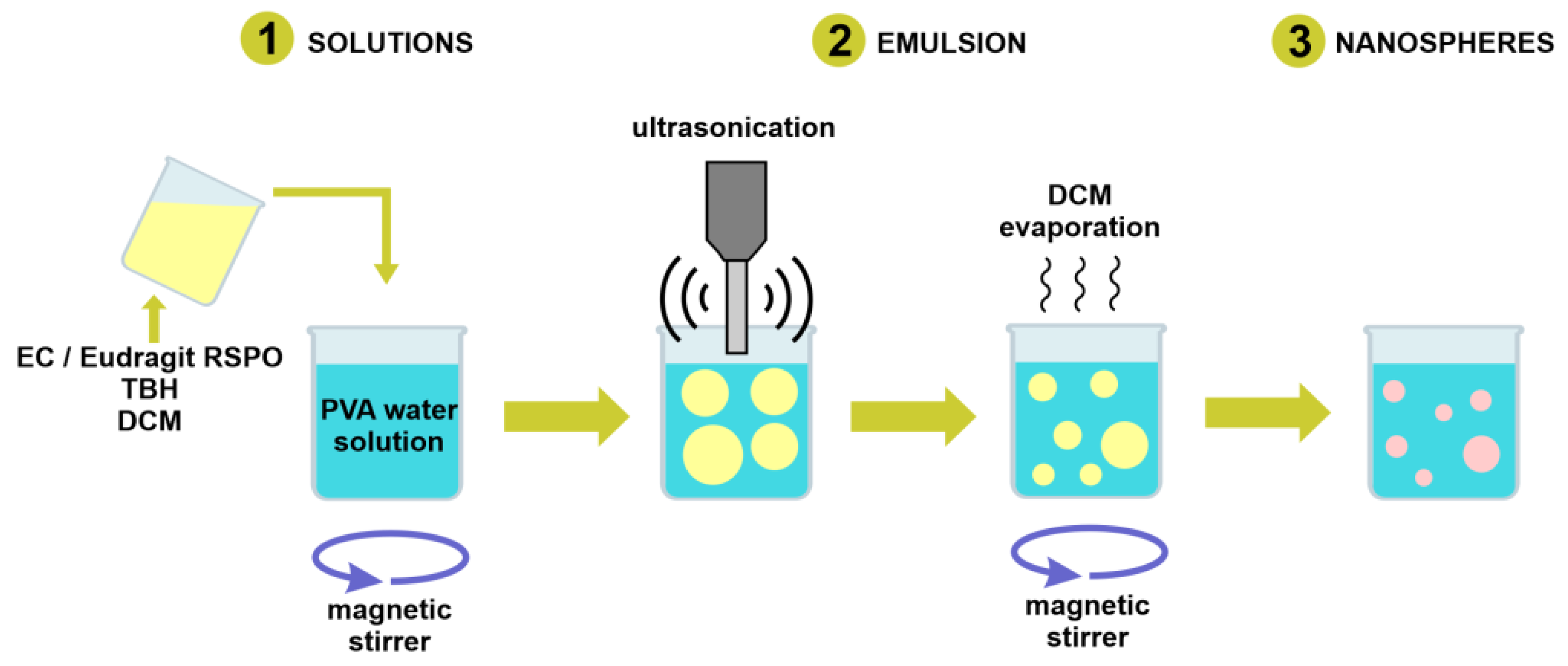
2.2. Preparation of TBH Nanospheres
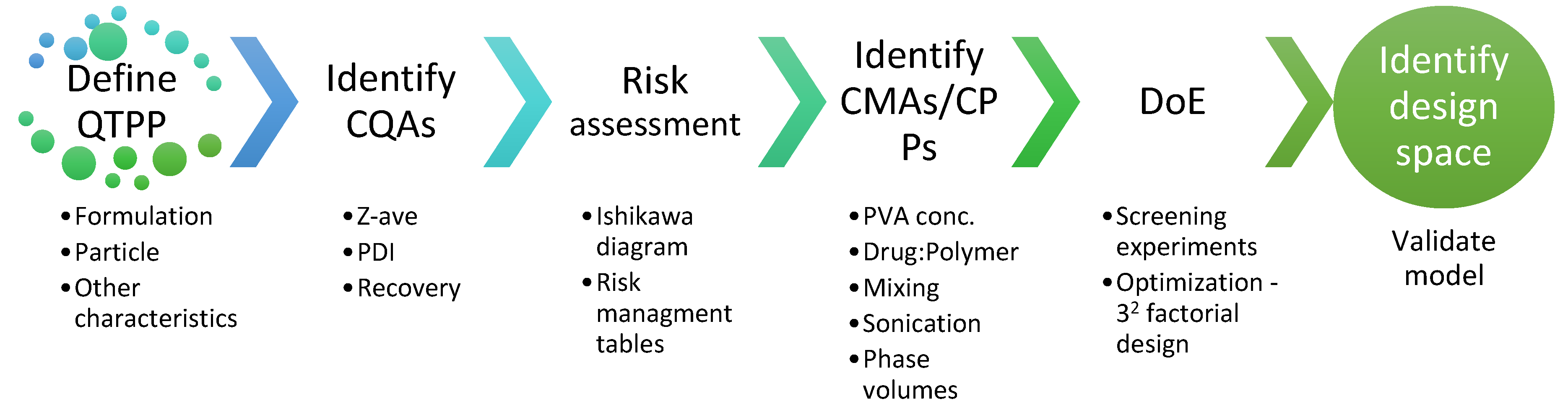
2.3. Quality by Design (QbD) Steps
2.3.1. Quality Target Product Profile (QTPP)
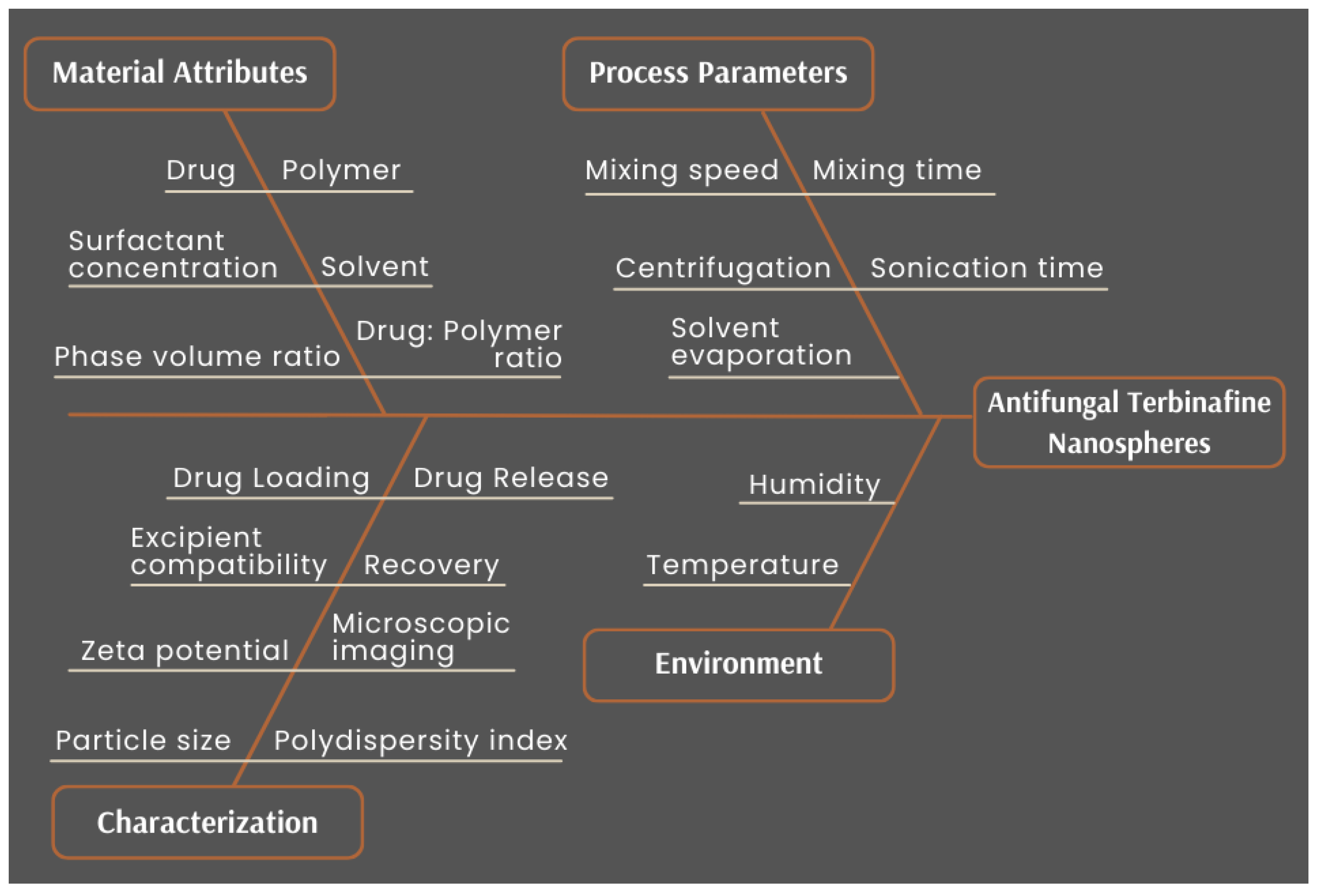
2.3.2. Risk Assessment
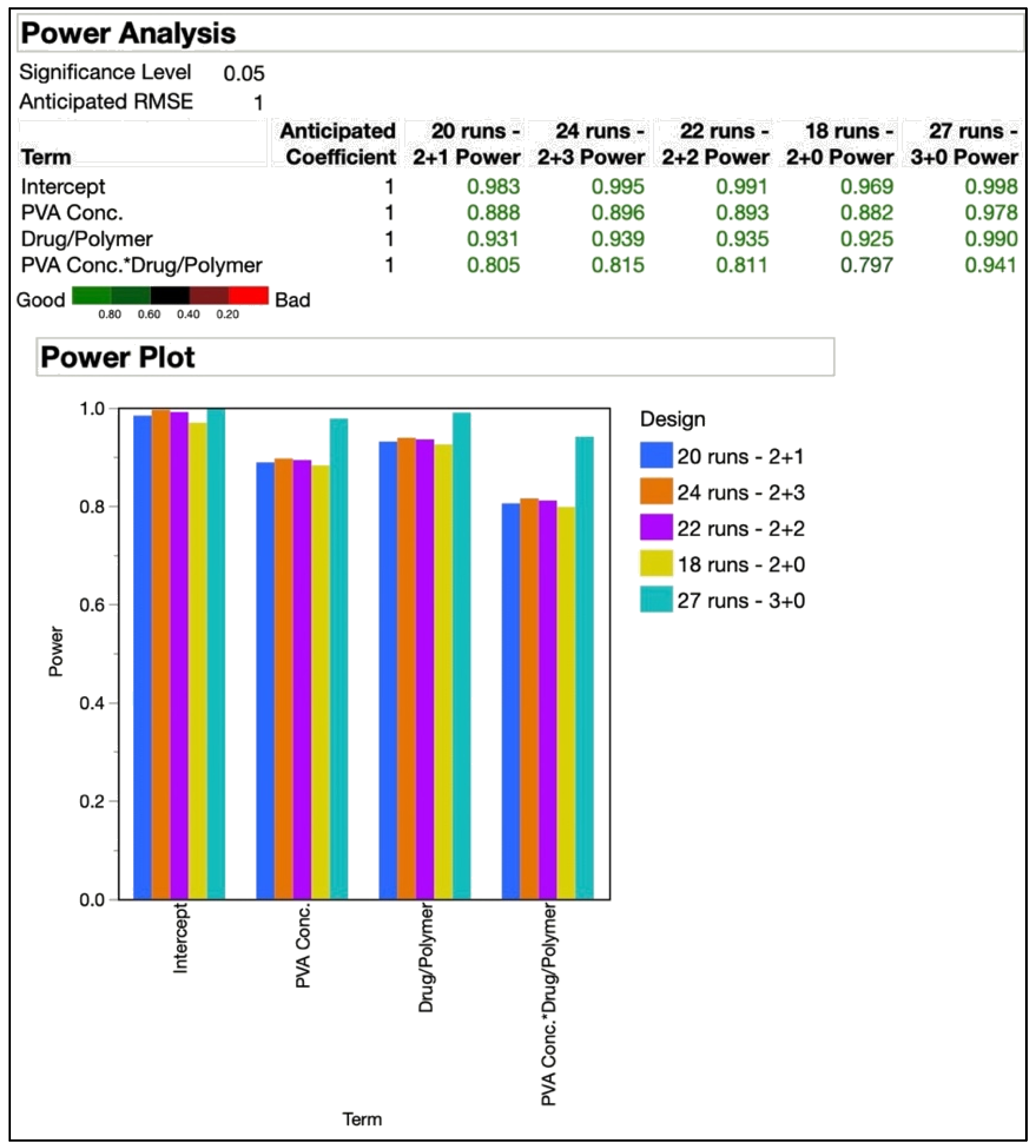
2.3.3. Design of Experiments (DoE)
2.4. Particle Size Analysis
2.5. Estimation of Recovery
2.6. Zeta Potential (ZP) Analysis
2.7. Differential Scanning Calorimetry (DSC)
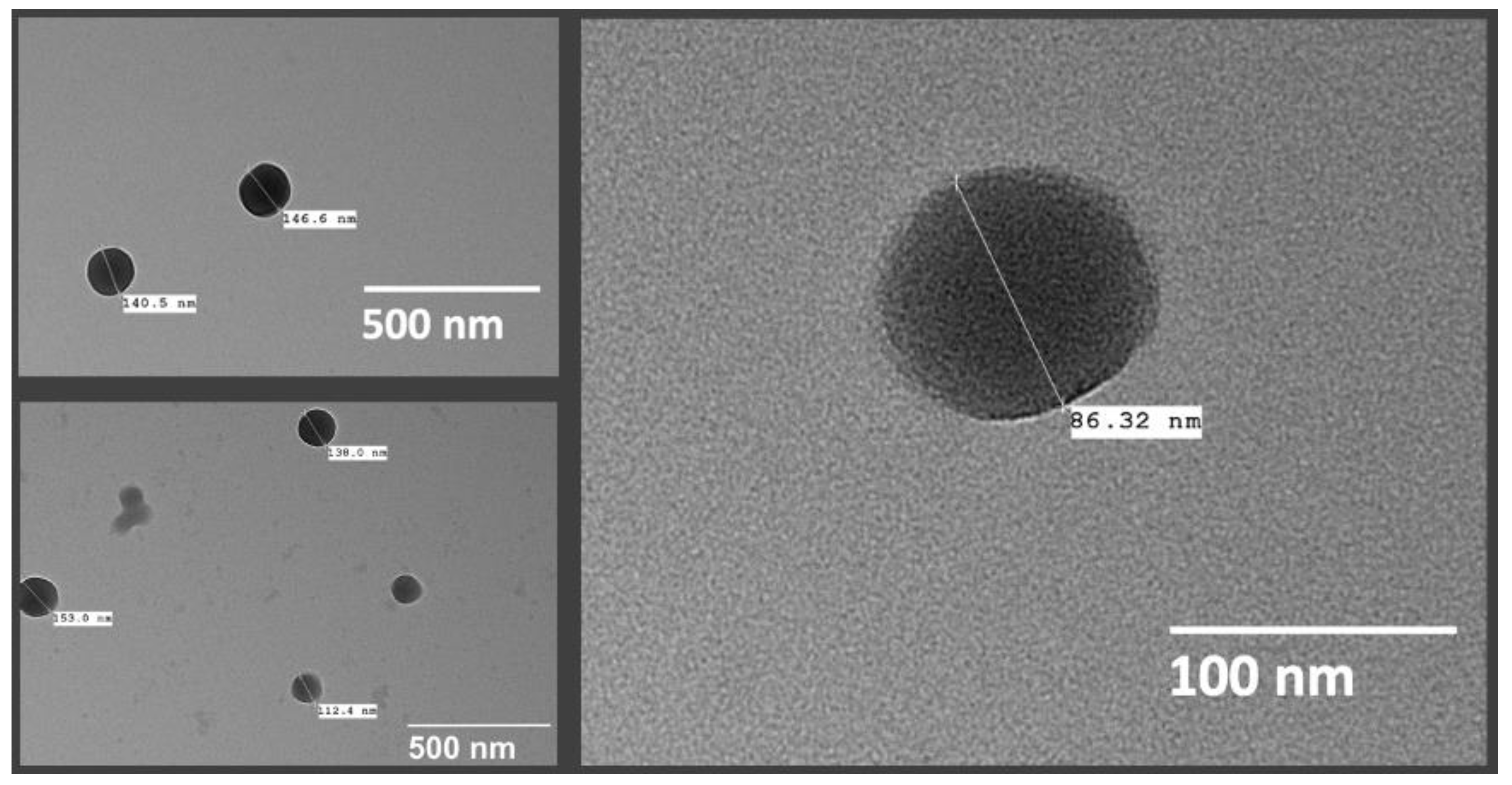
2.8. Transmission Electron Microscopy (TEM)
2.9. Preparation of TBH Nano-Gel Formulation
2.10. Characterization of TBH Nano-Gel Formulation
2.11. In Vitro Drug Release Study
2.12. Ex Vivo Nail Permeation Study
2.12.1. Preparation of Nails
2.12.2. Pre-Treatment of Nails
2.12.3. Nail Permeation Study
2.13. Analytical Method for Drug Determination
2.14. Statistical Analysis
3. Results and Discussion
3.1. Identification of QTPP, CQAs, CMAs and CPPs
3.2. Preliminary and Secondary Screening DoE
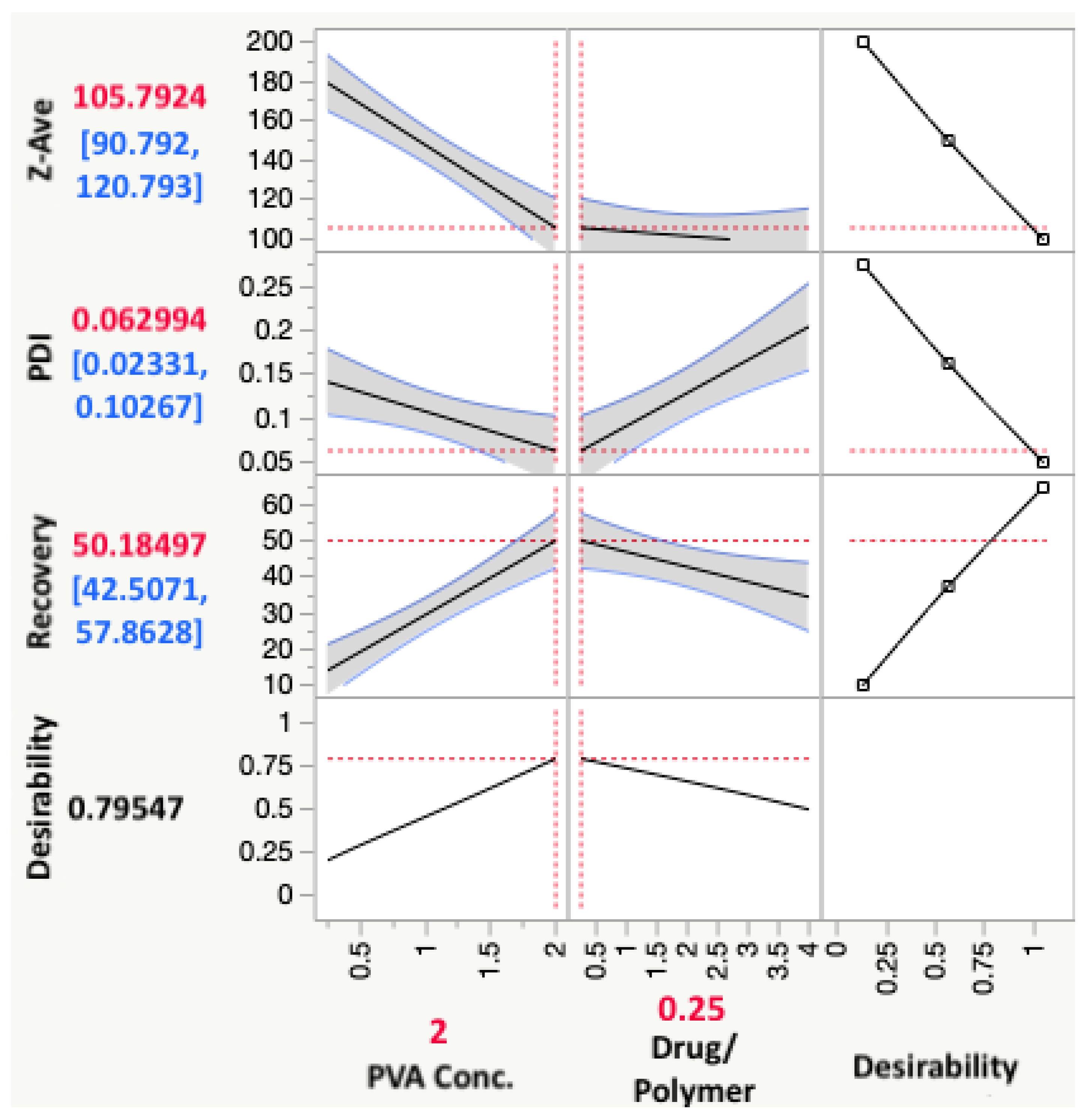
3.3. Final Experimental Design
3.4. Response Analysis
3.4.1. Effect on Particle Size
3.4.2. Effect on PDI
3.4.3. Effect on Recovery
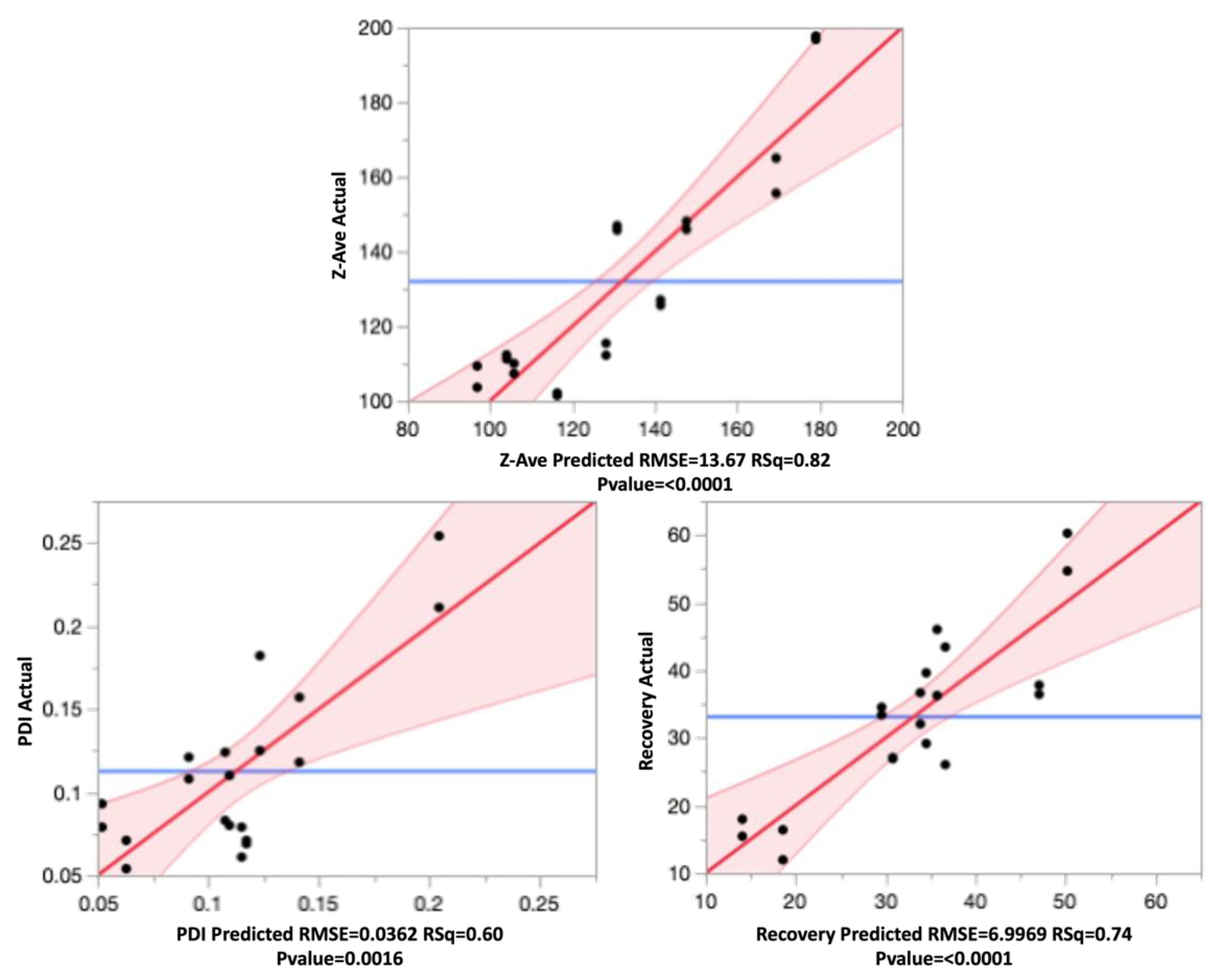
3.5. Model Validation
3.6. ZP of TBH Nanospheres
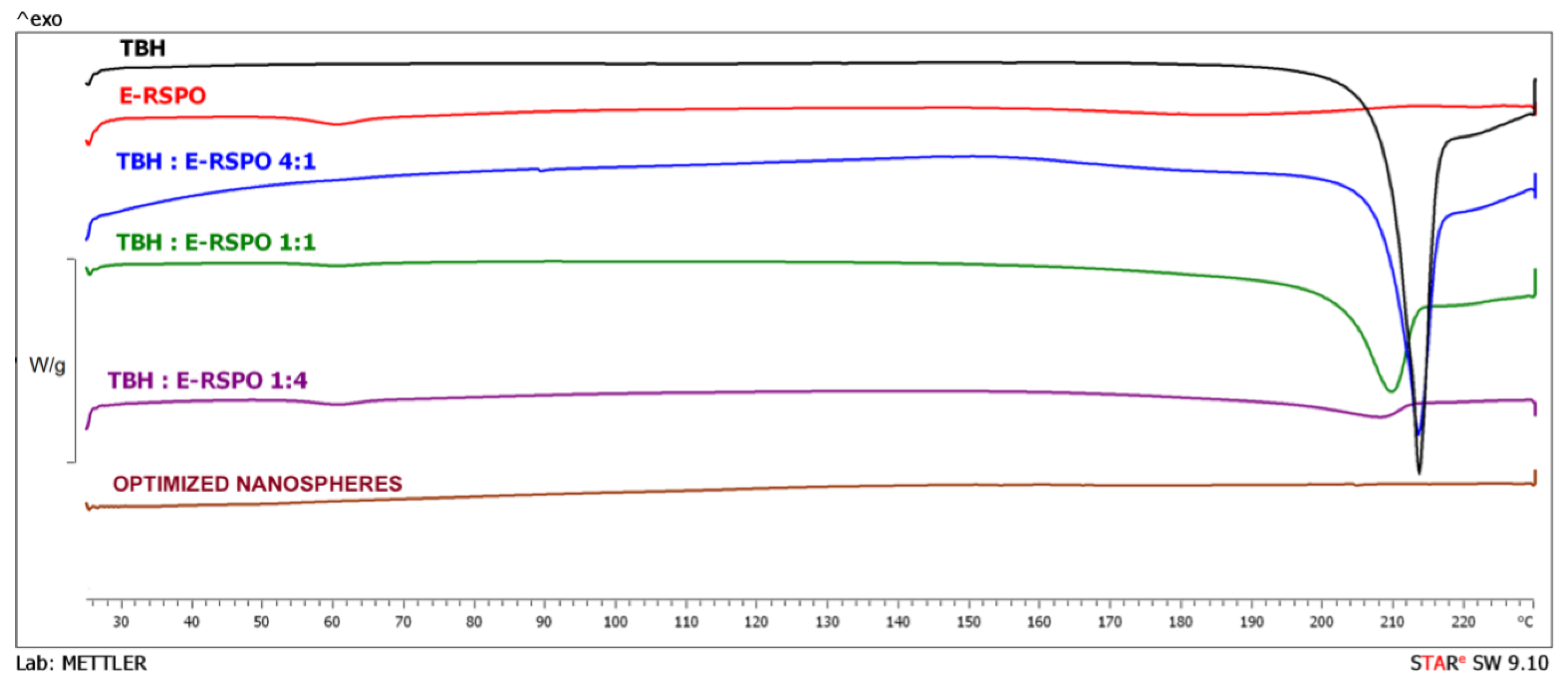
3.7. DSC Analysis
3.8. Nanosphere Morphology
3.9. Characterization of TBH Nano-Gel Formulation
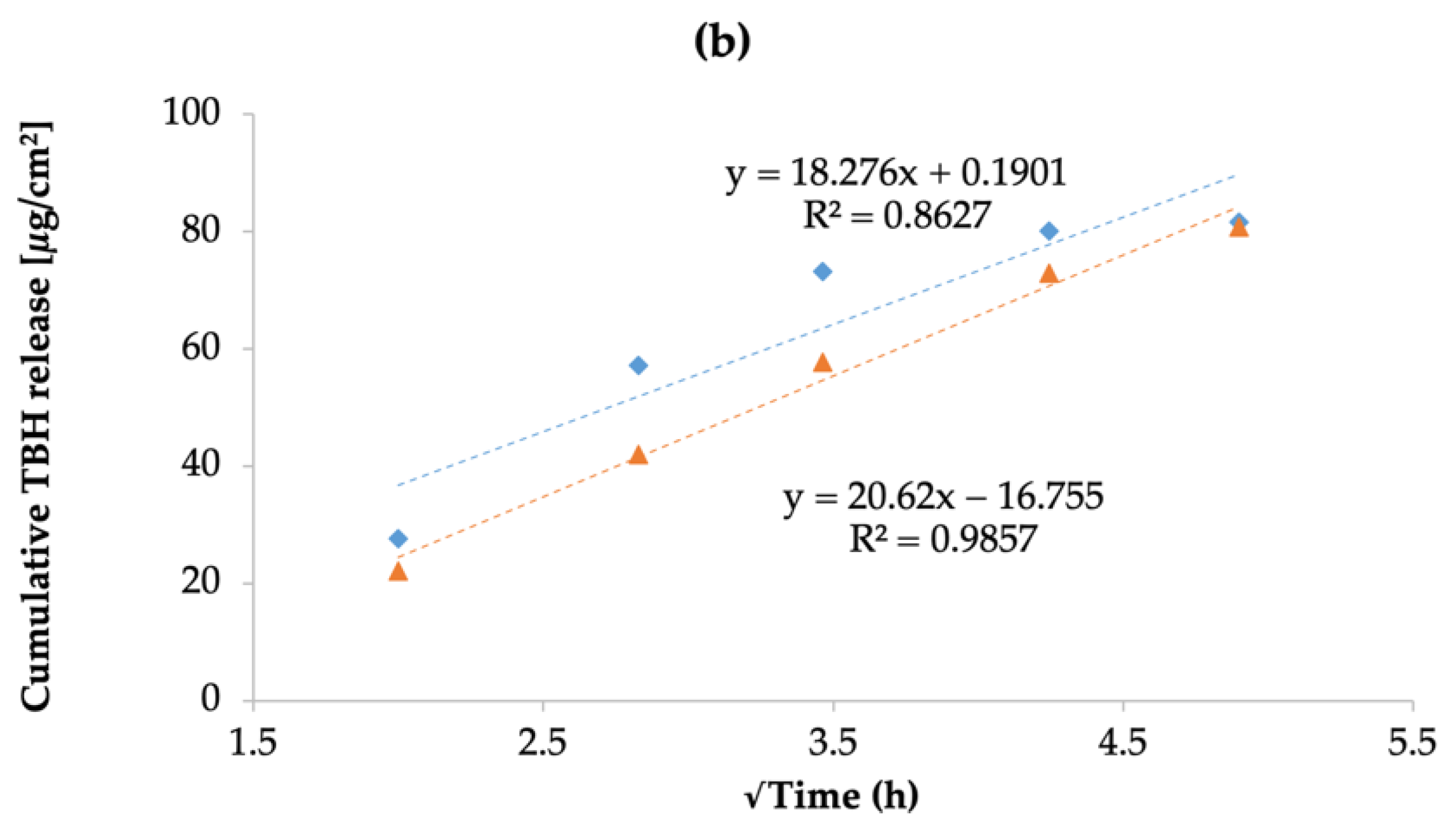
3.10. In Vitro Drug Release Study
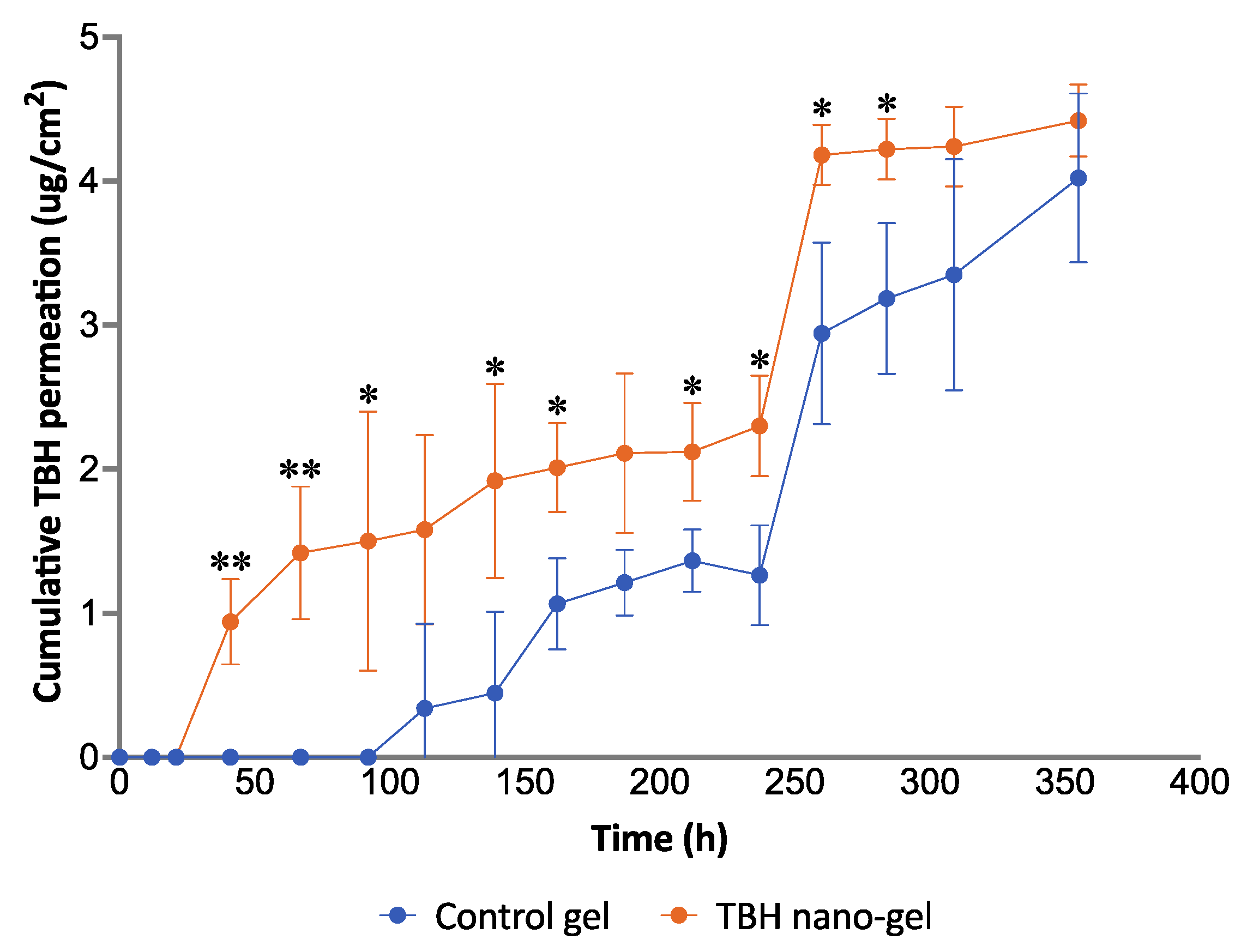
3.11. Ex Vivo Nail Permeation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QbD | Quality by design |
| TBH | Terbinafine hydrochloride |
| EC | Ethyl cellulose |
| E-RSPO | Eudragit® RSPO |
| QTPP | Quality target product profile |
| CQA | Critical quality attributes |
| CMA | Critical material attributes |
| CPP | Critical process parameters |
| DoE | Design of experiments |
| Z-ave | Average particle size |
| PDI | Polydispersity index |
| PVA | Polyvinyl alcohol |
| DCM | Dichloromethane |
| IPV | Internal phase volume |
| EPV | External phase volume |
| RSM | Response surface methodology |
| DSC | Differential scanning calorimetry |
| TEM | Transmission electron microscopy |
| HPLC | High-performance liquid chromatography |
| DLS | Dynamic light scattering |
| ANOVA | Analysis of variance |
| OFAT | One factor at a time |
| IVRT | In vitro release testing |
References
- Hossain, A.S.M.M.A.; Sil, B.C.; Iliopoulos, F.; Lever, R.; Hadgraft, J.; Lane, M.E. Preparation, Characterisation, and Topical Delivery of Terbinafine. Pharmaceutics 2019, 11, 548. [Google Scholar] [CrossRef]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef]
- Lipner, S.R.; Scher, R.K. Onychomycosis: Treatment and prevention of recurrence. J. Am. Acad. Dermatol. 2019, 80, 853–867. [Google Scholar] [CrossRef]
- Welsh, O.; Vera-Cabrera, L.; Welsh, E. Onychomycosis. Clin. Dermatol. 2010, 28, 151–159. [Google Scholar] [CrossRef]
- Ghannoum, M.; Isham, N. Fungal nail infections (onychomycosis): A never-ending story? PLoS Pathog. 2014, 10, e1004105. [Google Scholar] [CrossRef]
- Waghule, T.; Sankar, S.; Rapalli, V.K.; Gorantla, S.; Dubey, S.K.; Chellappan, D.K.; Dua, K.; Singhvi, G. Emerging role of nanocarriers based topical delivery of anti-fungal agents in combating growing fungal infections. Dermatol. Ther. 2020, 33, e13905. [Google Scholar] [CrossRef]
- Garber, G. An overview of fungal infections. Drugs 2001, 61, 1–12. [Google Scholar] [CrossRef]
- Aishwarya; Goel, A.; Nijhawan, R. A Deep Learning Approach for Classification of Onychomycosis Nail Disease. In Proceedings of ICETIT 2019; Springer: Cham, Switzerland, 2020; pp. 1112–1118. [Google Scholar]
- Szepietowski, J.C.; Reich, A.; Garlowska, E.; Kulig, M.; Baran, E.; Onychomycosis Epidemiology Study Group. Factors Influencing Coexistence of Toenail Onychomycosis with Tinea Pedis and Other Dermatomycoses: A Survey of 2761 Patients. Arch. Dermatol. 2006, 142, 1279–1284. [Google Scholar] [CrossRef]
- Chacon, A.; Franca, K.; Fernandez, A.; Nouri, K. Psychosocial impact of onychomycosis: A review. Int. J. Dermatol. 2013, 52, 1300–1307. [Google Scholar] [CrossRef]
- Crawford, F.; Hollis, S. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Database Syst. Rev. 2007, 2007, CD001434. [Google Scholar] [CrossRef]
- Soliman, G.M. Nanoparticles as safe and effective delivery systems of antifungal agents: Achievements and challenges. Int. J. Pharm. 2017, 523, 15–32. [Google Scholar] [CrossRef]
- Baran, R.; Kaoukhov, A. Topical antifungal drugs for the treatment of onychomycosis: An overview of current strategies for monotherapy and combination therapy. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 21–29. [Google Scholar] [CrossRef]
- Avner, S.; Nir, N.; Henri, T. Combination of oral terbinafine and topical ciclopirox compared to oral terbinafine for the treatment of onychomycosis. J. Dermatolog. Treat 2005, 16, 327–330. [Google Scholar] [CrossRef]
- Polak, A. The past, present and future of antimycotic combination therapy. Mycoses 1999, 42, 355–370. [Google Scholar] [CrossRef]
- Hay, R. Therapy of Skin, Hair and Nail Fungal Infections. J. Fungi 2018, 4, 99. [Google Scholar] [CrossRef]
- Tiwary, A.K.; Sapra, B. High failure rate of transungal drug delivery: Need for new strategies. Ther. Deliv. 2017, 8, 239–242. [Google Scholar] [CrossRef]
- Hirschowitz, B.I. Zollinger-Ellison syndrome: Pathogenesis, diagnosis, and management. Am. J. Gastroenterol. 1997, 92, 44S–50S. [Google Scholar]
- Angelo, T.; Borgheti-Cardoso, L.N.; Gelfuso, G.M.; Taveira, S.F.; Gratieri, T. Chemical and physical strategies in onychomycosis topical treatment: A review. Med. Mycol. 2017, 55, 461–475. [Google Scholar] [CrossRef]
- Gunt, H.B.; Kasting, G.B. Effect of hydration on the permeation of ketoconazole through human nail plate in vitro. Eur. J. Pharm. Sci. 2007, 32, 254–260. [Google Scholar] [CrossRef]
- Vikas, A.; Rashmin, P.; Mrunali, P.; Chavan, R.B.; Kaushik, T. Mechanistic Insights of Formulation Approaches for the Treatment of Nail Infection: Conventional and Novel Drug Delivery Approaches. AAPS PharmSciTech 2020, 21, 67. [Google Scholar] [CrossRef]
- Thapa, R.K.; Choi, J.Y.; Han, S.D.; Lee, G.H.; Yong, C.S.; Jun, J.-H.; Kim, J.O. Therapeutic effects of a novel DA5505 formulation on a guinea pig model of tinea pedis. Dermatol. Sin. 2017, 35, 59–65. [Google Scholar] [CrossRef]
- Sigurgeirsson, B.; Hay, R.J. The Antifungal Drugs Used in Skin Disease. In Antibiotic and Antifungal Therapies in Dermatology; Ólafsson, J.H., Hay, R.J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 141–156. [Google Scholar]
- Balfour, J.A.; Faulds, D. Terbinafine. Drugs 1992, 43, 259–284. [Google Scholar] [CrossRef]
- RYDER, N.S. The mechanism of action of terbinafine. Clin. Exp. Dermatol. 1989, 14, 98–100. [Google Scholar] [CrossRef]
- Haugh, M.; Helou, S.; Boissel, J.P.; Cribier, B.J. Terbinafine in fungal infections of the nails: A meta-analysis of randomized clinical trials. Br. J. Dermatol. 2002, 147, 118–121. [Google Scholar] [CrossRef]
- Thatai, P.; Sapra, B. Transungual Gel of Terbinafine Hydrochloride for the Management of Onychomycosis: Formulation, Optimization, and Evaluation. AAPS PharmSciTech 2017, 18, 2316–2328. [Google Scholar] [CrossRef]
- Tuncay Tanrıverdi, S.; Hilmioğlu Polat, S.; Yeşim Metin, D.; Kandiloğlu, G.; Özer, Ö. Terbinafine hydrochloride loaded liposome film formulation for treatment of onychomycosis: In vitro and in vivo evaluation. J. Liposome Res. 2016, 26, 163–173. [Google Scholar] [CrossRef]
- Shivakumar, H.; Vaka, S.R.K.; Madhav, N.S.; Chandra, H.; Murthy, S.N. Bilayered nail lacquer of terbinafine hydrochloride for treatment of onychomycosis. J. Pharm. Sci. 2010, 99, 4267–4276. [Google Scholar] [CrossRef]
- Tanrıverdi, S.T.; Özer, Ö. Novel topical formulations of Terbinafine-HCl for treatment of onychomycosis. Eur. J. Pharm. Sci. 2013, 48, 628–636. [Google Scholar] [CrossRef]
- Kaity, S.; Maiti, S.; Ghosh, A.K.; Pal, D.; Ghosh, A.; Banerjee, S. Microsponges: A novel strategy for drug delivery system. J. Adv. Pharm. Technol. Res. 2010, 1, 283–290. [Google Scholar] [CrossRef]
- Sharma, R.; Pathak, K. Polymeric nanosponges as an alternative carrier for improved retention of econazole nitrate onto the skin through topical hydrogel formulation. Pharm. Dev. Technol. 2011, 16, 367–376. [Google Scholar] [CrossRef]
- Waghule, T.; Rapalli, V.K.; Singhvi, G.; Manchanda, P.; Hans, N.; Dubey, S.K.; Hasnain, M.S.; Nayak, A.K. Voriconazole loaded nanostructured lipid carriers based topical delivery system: QbD based designing, characterization, in-vitro and ex-vivo evaluation. J. Drug Deliv. Sci. Technol. 2019, 52, 303–315. [Google Scholar] [CrossRef]
- Yu, L.X. Pharmaceutical Quality by Design: Product and Process Development, Understanding, and Control. Pharm. Res. 2008, 25, 781–791. [Google Scholar] [CrossRef]
- Politis, S.N.; Colombo, P.; Colombo, G.; M. Rekkas, D. Design of experiments (DoE) in pharmaceutical development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef]
- Arora, D.; Khurana, B.; Nanda, S. DoE directed optimization, development and evaluation of resveratrol loaded ultradeformable vesicular cream for topical antioxidant benefits. Drug Dev. Ind. Pharm. 2020, 46, 227–235. [Google Scholar] [CrossRef]
- Marto, J.; Gouveia, L.F.; Gonçalves, L.M.; Gaspar, D.P.; Pinto, P.; Carvalho, F.A.; Oliveira, E.; Ribeiro, H.M.; Almeida, A.J. A Quality by design (QbD) approach on starch-based nanocapsules: A promising platform for topical drug delivery. Colloids Surf. B Biointerfaces 2016, 143, 177–185. [Google Scholar] [CrossRef]
- Beg, S.; Hasnain, M.S.; Rahman, M.; Swain, S. Chapter 1—Introduction to Quality by Design (QbD): Fundamentals, Principles, and Applications. In Pharmaceutical Quality by Design; Beg, S., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–7. [Google Scholar]
- Group, I.E.W. ICH Harmonized Tripartite Guideline. Pharmaceutical Development Q8 (R2); International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use: Geneva, Switzerland, 2009. [Google Scholar]
- Guideline, I.H.T. Quality risk management. Q9 Curr. Step 2005, 4, 408. [Google Scholar]
- Durakovic, B. Design of experiments application, concepts, examples: State of the art. Period. Eng. Nat. Sci. 2017, 5, 421–439. [Google Scholar] [CrossRef]
- Baswan, S.M.; Li, S.K.; LaCount, T.D.; Kasting, G.B. Size and Charge Dependence of Ion Transport in Human Nail Plate. J. Pharm. Sci. 2016, 105, 1201–1208. [Google Scholar] [CrossRef]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and nanofibers for topical drug delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef]
- Božič, M.; Elschner, T.; Tkaučič, D.; Bračič, M.; Hribernik, S.; Stana Kleinschek, K.; Kargl, R. Effect of different surface active polysaccharide derivatives on the formation of ethyl cellulose particles by the emulsion-solvent evaporation method. Cellulose 2018, 25, 6901–6922. [Google Scholar] [CrossRef]
- Lubrizol. Pharmaceutical Bulletin 5—Neutralization Procedures; Lubrizol: Wickliffe, OH, USA, 2011. [Google Scholar]
- Lubrizol. Technical Data Sheet 237—Neutralizing Carbopol® and Pemulen™ Polymers in Aqueous and Hydroalcoholic Systems; Lubrizol: Wickliffe, OH, USA, 2009. [Google Scholar]
- Brown, M.B.; Khengar, R.H.; Turner, R.B.; Forbes, B.; Traynor, M.J.; Evans, C.R.G.; Jones, S.A. Overcoming the nail barrier: A systematic investigation of ungual chemical penetration enhancement. Int. J. Pharm. 2009, 370, 61–67. [Google Scholar] [CrossRef]
- Krawczyk-Santos, A.P.; da Rocha, P.B.R.; Kloppel, L.L.; Souza, B.d.S.; Anjos, J.L.V.; Alonso, A.; de Faria, D.L.A.; Gil, O.M.; Gratieri, T.; Marreto, R.N.; et al. Enhanced nail delivery of voriconazole-loaded nanomicelles by thioglycolic acid pretreatment: A study of protein dynamics and disulfide bond rupture. Int. J. Pharm. 2021, 602, 120597. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Khosa, A.; Singhvi, G.; Girdhar, V.; Jain, R.; Dubey, S.K. Chapter 13—Application of QbD Principles in Nanocarrier-Based Drug Delivery Systems. In Pharmaceutical Quality by Design; Beg, S., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 255–296. [Google Scholar]
- Rupinder, K.D.; Harvinder, P.; Gupta, M. Novel Drug Delivery Strategies for the Treatment of Onychomycosis. Pharm. Nanotechnol. 2019, 7, 24–38. [Google Scholar] [CrossRef]
- Aggarwal, R.; Targhotra, M.; Sahoo, P.K.; Chauhan, M.K. Onychomycosis: Novel strategies for treatment. J. Drug Deliv. Sci. Technol. 2020, 57, 101774. [Google Scholar] [CrossRef]
- Dhat, S.; Pund, S.; Kokare, C.; Sharma, P.; Shrivastava, B. Risk management and statistical multivariate analysis approach for design and optimization of satranidazole nanoparticles. Eur. J. Pharm. Sci. 2017, 96, 273–283. [Google Scholar] [CrossRef]
- Alam, M.S.; Javed, M.N.; Pottoo, F.H.; Waziri, A.; Almalki, F.A.; Hasnain, M.S.; Garg, A.; Saifullah, M.K. QbD approached comparison of reaction mechanism in microwave synthesized gold nanoparticles and their superior catalytic role against hazardous nirto-dye. Appl. Organomet. Chem. 2019, 33, e5071. [Google Scholar] [CrossRef]
- Kumar, P.M.; Ghosh, A. Development and evaluation of silver sulfadiazine loaded microsponge based gel for partial thickness (second degree) burn wounds. Eur. J. Pharm. Sci. 2017, 96, 243–254. [Google Scholar] [CrossRef]
- Raina, H.; Kaur, S.; Jindal, A.B. Development of efavirenz loaded solid lipid nanoparticles: Risk assessment, quality-by-design (QbD) based optimisation and physicochemical characterisation. J. Drug Deliv. Sci. Technol. 2017, 39, 180–191. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Saudagar, R. Formulation, development and evaluation of film-forming gel for prolonged dermal delivery of terbinafine hydrochloride. Int. J. Pharm. Sci. Res. 2014, 5, 537–554. [Google Scholar]
- Mahaparale, P.; NIkam, S.; Chavan, M. Development and evaluation of terbinafine hydrochloride polymeric microsponges for topical drug delivery. Indian J. Pharm. Sci. 2018, 80, 1086–1092. [Google Scholar] [CrossRef]
- R®πttimann, B.G.; Wegener, K. The Power of DOE: How to Increase Experimental Design Success and Avoid Pitfalls. J. Serv. Sci. Manag. 2015, 8, 9. [Google Scholar] [CrossRef][Green Version]
- Ridge, E.; Kudenko, D. Tuning an Algorithm Using Design of Experiments. In Experimental Methods for the Analysis of Optimization Algorithms; Bartz-Beielstein, T., Chiarandini, M., Paquete, L., Preuss, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 265–286. [Google Scholar]
- Shah, P.; Goodyear, B.; Haq, A.; Puri, V.; Michniak-Kohn, B. Quality by design: A systematic approach using an advanced statistical tool to optimization of niosomes preparation for the topical delivery. Therapy 2020, 6, 7. [Google Scholar]
- Galindo-Rodriguez, S.; Allemann, E.; Fessi, H.; Doelker, E. Physicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-diffusion, and nanoprecipitation methods. Pharm. Res. 2004, 21, 1428–1439. [Google Scholar] [CrossRef]
- Shah, P.; Goodyear, B.; Haq, A.; Puri, V.; Michniak-Kohn, B. Evaluations of quality by design (QbD) elements impact for developing niosomes as a promising topical drug delivery platform. Pharmaceutics 2020, 12, 246. [Google Scholar] [CrossRef]
- Elsherif, N.I.; Shamma, R.N.; Abdelbary, G. Terbinafine Hydrochloride Trans-ungual Delivery via Nanovesicular Systems: In Vitro Characterization and Ex Vivo Evaluation. AAPS PharmSciTech 2017, 18, 551–562. [Google Scholar] [CrossRef]
- Vaghasiya, H.; Kumar, A.; Sawant, K. Development of solid lipid nanoparticles based controlled release system for topical delivery of terbinafine hydrochloride. Eur. J. Pharm. Sci. 2013, 49, 311–322. [Google Scholar] [CrossRef]
- Kuminek, G.; Rauber, G.S.; Riekes, M.K.; Campos, C.E.M.d.; Monti, G.A.; Bortoluzzi, A.J.; Cuffini, S.L.; Cardoso, S.G. Single crystal structure, solid state characterization and dissolution rate of terbinafine hydrochloride. J. Pharm. Biomed. Anal. 2013, 78, 105–111. [Google Scholar] [CrossRef]
- Fujimori, J.; Yoshihashi, Y.; Yonemochi, E.; Terada, K. Application of Eudragit RS to thermo-sensitive drug delivery systems: II. Effect of temperature on drug permeability through membrane consisting of Eudragit RS/PEG 400 blend polymers. J. Control. Release 2005, 102, 49–57. [Google Scholar] [CrossRef]
- Van den Mooter, G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today: Technol. 2012, 9, e79–e85. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Naumann, S.; Meyer, J.-P.; Kiesow, A.; Mrestani, Y.; Wohlrab, J.; Neubert, R.H.H. Controlled nail delivery of a novel lipophilic antifungal agent using various modern drug carrier systems as well as in vitro and ex vivo model systems. J. Control. Release 2014, 180, 60–70. [Google Scholar] [CrossRef]
- Shirwaikar, A.A.; Thomas, T.; Shirwaikar, A.; Lobo, R.; Prabhu, K.S. Treatment of onychomycosis: An update. Indian J. Pharm. Sci. 2008, 70, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Mertin, D.; LIPPOLD, B.C. In-vitro permeability of the human nail and of a keratin membrane from bovine hooves: Prediction of the penetration rate of antimycotics through the nail plate and their efficacy. J. Pharm. Pharmacol. 1997, 49, 866–872. [Google Scholar] [CrossRef]
- Salah, S.; Awad, G.E.A.; Makhlouf, A.I.A. Improved vaginal retention and enhanced antifungal activity of miconazole microsponges gel: Formulation development and in vivo therapeutic efficacy in rats. Eur. J. Pharm. Sci. 2018, 114, 255–266. [Google Scholar] [CrossRef]
- Moin, A.; Deb, T.K.; Osmani, R.A.M.; Bhosale, R.R.; Hani, U. Fabrication, characterization, and evaluation of microsponge delivery system for facilitated fungal therapy. J. Basic Clin. Pharm. 2016, 7, 39–48. [Google Scholar] [CrossRef]
- Yang, Y.; Ou, R.; Guan, S.; Ye, X.; Hu, B.; Zhang, Y.; Lu, S.; Zhou, Y.; Yuan, Z.; Zhang, J.; et al. A novel drug delivery gel of terbinafine hydrochloride with high penetration for external use. Drug Deliv. 2015, 22, 1086–1093. [Google Scholar] [CrossRef]
- Şen, M.; Uzun, C.; Güven, O. Controlled release of terbinafine hydrochloride from pH sensitive poly (acrylamide/maleic acid) hydrogels. Int. J. Pharm. 2000, 203, 149–157. [Google Scholar] [CrossRef]
- Chen, K.; Puri, V.; Michniak-Kohn, B. Iontophoresis to Overcome the Challenge of Nail Permeation: Considerations and Optimizations for Successful Ungual Drug Delivery. AAPS J. 2021, 23, 1–15. [Google Scholar] [CrossRef]
- Kevin Li, S.; Hao, J.; Liddell, M. Chapter 11—Electrotransport across Membranes in Biological Media: Electrokinetic Theories and Applications in Drug Delivery. In Transport in Biological Media; Becker, S.M., Kuznetsov, A.V., Eds.; Elsevier: Boston, MA, USA, 2013; pp. 417–454. [Google Scholar]
- Hao, J.; Smith, K.A.; Li, S.K. Time-dependent electrical properties of human nail upon hydration in vivo. J. Pharm. Sci. 2010, 99, 107–118. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Goodyear, B.; Dholaria, N.; Puri, V.; Michniak-Kohn, B. Nanostructured Non-Ionic Surfactant Carrier-Based Gel for Topical Delivery of Desoximetasone. Int. J. Mol. Sci. 2021, 22, 1535. [Google Scholar] [CrossRef] [PubMed]
- Šveikauskaitė, I.; Pockevičius, A.; Briedis, V. Potential of Chemical and Physical Enhancers for Transungual Delivery of Amorolfine Hydrochloride. Materials 2019, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- B Nair, A.; Chakraborty, B.; Narasimha Murthy, S. Effect of polyethylene glycols on the trans-ungual delivery of terbinafine. Curr. Drug Deliv. 2010, 7, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.N.; Vaka, S.R.K.; Sammeta, S.M.; Nair, A.B. TranScreen-N™: Method for rapid screening of trans-ungual drug delivery enhancers. J. Pharm. Sci. 2009, 98, 4264–4271. [Google Scholar] [CrossRef]
- Šveikauskaitė, I.; Briedis, V. Potential of Naftifine Application for Transungual Delivery. Molecules 2020, 25, 3043. [Google Scholar] [CrossRef]

 ) and orange triangles (
) and orange triangles ( ) represent the control and nano-gel, respectively.
) represent the control and nano-gel, respectively.
 ) and orange triangles (
) and orange triangles ( ) represent the control and nano-gel, respectively.
) represent the control and nano-gel, respectively.
| Independent Variables | Low (−) | High (+) | |
|---|---|---|---|
| CMAs | PVA concentration (% w/v) | 0.25 | 1 |
| Drug/polymer | 1:1 | 1:2 | |
| CPPs | Mixing time (min) | 0 | 10 |
| Sonication time (min) | 5 | 15 | |
| Independent Variables | Low (−1) | Medium (0) | High (+1) |
|---|---|---|---|
| PVA concentration (% w/v) | 0.25 | 0.5 | 1 |
| Drug/polymer | 1:1 | 1:4 | 4:1 |
| EPV/IPV | 10:4 | 10:2 | 10:6 |
| Independent Variables | Low (−1) | Med. (0) | High (+1) | Exp. Runs | Replicates (N) | Center Points |
|---|---|---|---|---|---|---|
| PVA concentration (% w/v) (X1) | 0.25 | 1 | 2 | 18 | 2 | 0 |
| Drug/polymer (X2) | 1:4 | 1:1 | 4:1 | 20 | 2 | 1 |
| Responses | 22 | 2 | 2 | |||
| Z-ave (nm) (Y1) | PDI (Y2) | Recovery (%) (Y3) | 24 | 2 | 3 | |
| 27 | 3 | 0 | ||||
| (a) | (b) | |||||
| Parameter | Amount |
|---|---|
| Drug and polymer total weight | 100 mg |
| Internal phase volume | 2 mL |
| External phase volume | 10 mL |
| Mixing time | 10 min |
| Mixing speed | 500 rpm |
| Sonication time | 0.7 s ON/0.3 s OFF, for 15 min ON time |
| Sonication power | 70% |
| Vial | 20 mL glass scintillation |
| QTPP Elements | Target | CQA * | Justification |
|---|---|---|---|
| Dosage form | Polymeric nanospheres | To improve permeability, stability, and efficacy of API | |
| Route of administration | Topical | Self-administrable, local drug delivery while avoiding systemic adverse effects | |
| Particle size (Z-ave) | <250 nm | ✓ | Suitable for effective permeability |
| Polydispersity index (PDI) | <0.3 | ✓ | Impacts physical stability and drug uniformity |
| Recovery | Maximum possible | ✓ | Ensures formulation efficiency and supports the desired drug release |
| Zeta potential (ZP) | >40 mV | ✓ | Helps with dispersion stability and particle uniformity |
| Particle aggregation | No visible signs of aggregation | Impacts permeability and stability | |
| Particle shape | Spherical | Supports particle uniformity and allows penetration through narrow channels |
| CQAs | Material Attributes | |||||
|---|---|---|---|---|---|---|
| Drug Type | Polymer Type | Drug/Polymer Ratio | Surfactant Type | Surfactant Conc. | Phase Volume Ratio | |
| Average particle size | Low | Medium | High | Medium | High | Medium |
| Polydispersity index | Low | Medium | High | Medium | High | Medium |
| Recovery | Medium | Medium | High | Medium | High | Medium |
| Zeta potential | Low | High | High | Medium | Low | Low |
| CQAs | Process Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Solvent Evaporation | Mixing Speed | Mixing Time | Sonication Time | Sonication Power | Centrifugation Speed | Centrifugation Time | Centrifugation Temperature | |
| Average particle size | Low | Medium | Low | Medium | Medium | Low | Low | Low |
| Polydispersity index | Low | Medium | Low | Medium | Medium | Low | Low | Low |
| Recovery | Low | Low | Low | Medium | Medium | Low | Low | Low |
| Zeta potential | Low | Low | Low | Low | Low | Low | Low | Low |
| Pattern | Independent Variables | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| EC | E-RSPO | |||||||
| PVA Conc. (% w/v) | Mixing Time (min) | Drug/ Polymer | Sonics (min) | Z-Ave (nm) | PDI | Z-Ave (nm) | PDI | |
| − + + − | 0.25 | 10 | 1:2 | 5 | 210.5 | 0.083 | 214.8 | 0.157 |
| + − + − | 1 | 0 | 1:2 | 5 | 229.8 | 0.09 | 233.4 | 0.118 |
| − + + + | 0.25 | 10 | 1:2 | 15 | 363.6 | 0.245 | 320.2 | 0.334 |
| − − + + | 0.25 | 0 | 1:2 | 15 | 409.6 | 0.211 | 229.9 | 0.237 |
| − + − − | 0.25 | 10 | 1:1 | 5 | 353.5 | 0.218 | 403.3 | 0.635 |
| + + − + | 1 | 10 | 1:1 | 15 | 193.5 | 0.072 | 241.6 | 0.168 |
| + − − − | 1 | 0 | 1:1 | 5 | 208.4 | 0.108 | 243.2 | 0.141 |
| − − − + | 0.25 | 0 | 1:1 | 15 | 375 | 0.19 | 258.1 | 0.259 |
| + − + + | 1 | 0 | 1:2 | 15 | 238.7 | 0.091 | 247.3 | 0.176 |
| + + − − | 1 | 10 | 1:1 | 5 | 208.3 | 0.099 | 293.4 | 0.242 |
| − − − − | 0.25 | 0 | 1:1 | 5 | 460.9 | 0.201 | 230.3 | 0.221 |
| + + + + | 1 | 10 | 1:2 | 15 | 235.6 | 0.118 | 249.1 | 0.178 |
| − − + − | 0.25 | 0 | 1:2 | 5 | 379.3 | 0.245 | 250.7 | 0.282 |
| − + − + | 0.25 | 10 | 1:1 | 15 | 365.2 | 0.216 | 245.1 | 0.266 |
| + + + − | 1 | 10 | 1:2 | 5 | 229.8 | 0.117 | 242.5 | 0.133 |
| + − − + | 1 | 0 | 1:1 | 15 | 198.5 | 0.096 | 237.3 | 0.18 |
| Formulation ID | Independent Variables | Responses—EC | Responses—E-RSPO | ||||
|---|---|---|---|---|---|---|---|
| PVA conc. (% w/v) | Drug/ Polymer | EPV/ IPV | Z-Ave (nm) * | PDI | Z-Ave (nm) | PDI | |
| F-1 | 0.5 | 2:1 | 10:4 | NM | NM | 1000 | >0.5 |
| F-2 | 1 | 1:1 | 10:2 | 214.3 | 0.197 | 118.3 | 0.083 |
| F-3 | 0.25 | 3:1 | 10:2 | 450.9 | 0.423 | 169.8 | 0.253 |
| F-4 | 1 | 1:1 | 10:4 | 289.6 | 0.371 | 132.7 | 0.085 |
| F-5 | 0.25 | 3:1 | 10:4 | NM | NM | 187.3 | 0.147 |
| F-6 | 1 | 1:1 | 10:4 | 178 | 0.168 | 130.9 | 0.123 |
| F-7 | 0.25 | 1:1 | 10:2 | 365.6 | 0.395 | 152.8 | 0.088 |
| F-9 | 0.5 | 2:1 | 10:4 | 171.2 | 0.149 | 1000 | >0.5 |
| F-8 | 1 | 3:1 | 10:6 | NM | NM | 144.3 | 0.115 |
| F-10 | 0.25 | 1:1 | 10:6 | NM | NM | 286.2 | 0.277 |
| F-11 | 1 | 3:1 | 10:2 | 142.7 | 0.106 | 682.1 | 0.291 |
| F-12 | 1 | 3:1 | 10:2 | 145.2 | 0.071 | 116.7 | 0.138 |
| F-13 | 0.5 | 2:1 | 10:4 | 191.5 | 0.209 | 140.5 | 0.108 |
| Formulation | Independent Variables | Responses | ||||
|---|---|---|---|---|---|---|
| Pattern * | F | PVA Conc. (% w/v) | Drug/ Polymer | Z-Ave (nm) | PdI | Recovery (%) |
| 12 | F1 | 0.25 | 1:1 | 155.6 | 0.125 | 11.97 |
| 21 | F2 | 1 | 1:4 | 145.9 | 0.083 | 34.52 |
| 32 | F3 | 2 | 1:1 | 112.3 | 0.108 | 36.43 |
| 23 | F4 | 1 | 4:1 | 101.4 | 0.069 | 36.25 |
| 11 | F5 | 0.25 | 1:4 | 196.8 | 0.157 | 15.45 |
| 22 | F6 | 1 | 1:1 | 127 | 0.11 | 26.92 |
| 23 | F7 | 1 | 4:1 | 102.1 | 0.071 | 46.01 |
| 22 | F8 | 1 | 1:1 | 125.6 | 0.08 | 27.06 |
| 00 | F9 | 1.125 | 17:8 | 112.2 | 0.061 | 32.05 |
| 32 | F10 | 2 | 1:1 | 111.2 | 0.121 | 37.78 |
| 13 | F11 | 0.25 | 4:1 | 145.7 | 0.093 | 26.03 |
| 21 | F12 | 1 | 1:4 | 148.1 | 0.124 | 33.37 |
| 00 | F13 | 1.125 | 17/8 | 115.4 | 0.079 | 36.66 |
| 33 | F14 | 2 | 4:1 | 109.3 | 0.254 | 39.6 |
| 11 | F15 | 0.25 | 1:4 | 197.7 | 0.118 | 17.97 |
| 12 | F16 | 0.25 | 1:1 | 165 | 0.182 | 16.4 |
| 33 | F17 | 2 | 4:1 | 103.6 | 0.211 | 29.15 |
| 31 | F18 | 2 | 1:4 | 110 | 0.071 | 60.22 |
| 13 | F19 | 0.25 | 4:1 | 146.9 | 0.079 | 43.41 |
| 31 | F20 | 2 | 1:4 | 107.3 | 0.054 | 54.65 |
| Source | LogWorth | p-Value | |
|---|---|---|---|
| PVA Conc. (0.25, 2) | 5.248 |  | 0.00001 |
| PVA Conc.*Drug/Polymer | 3.695 |  | 0.00020 |
| Drug/Polymer (0.25, 4) | 2.835 |  | 0.00146 |
| CQAs | Mean Predicted Values | Mean Optimized Formulation Values | p-Value |
|---|---|---|---|
| Z-Ave (nm) | 105.8 ± 3.6 | 108.7 ± 1.7 | 0.2756 |
| PDI | 0.063 ± 0.013 | 0.063 ± 0.014 | 1.000 |
| % Recovery | 50.19 ± 8.00 | 57.43 ± 3.94 | 0.2324 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puri, V.; Froelich, A.; Shah, P.; Pringle, S.; Chen, K.; Michniak-Kohn, B. Quality by Design Guided Development of Polymeric Nanospheres of Terbinafine Hydrochloride for Topical Treatment of Onychomycosis Using a Nano-Gel Formulation. Pharmaceutics 2022, 14, 2170. https://doi.org/10.3390/pharmaceutics14102170
Puri V, Froelich A, Shah P, Pringle S, Chen K, Michniak-Kohn B. Quality by Design Guided Development of Polymeric Nanospheres of Terbinafine Hydrochloride for Topical Treatment of Onychomycosis Using a Nano-Gel Formulation. Pharmaceutics. 2022; 14(10):2170. https://doi.org/10.3390/pharmaceutics14102170
Chicago/Turabian StylePuri, Vinam, Anna Froelich, Parinbhai Shah, Shernelle Pringle, Kevin Chen, and Bozena Michniak-Kohn. 2022. "Quality by Design Guided Development of Polymeric Nanospheres of Terbinafine Hydrochloride for Topical Treatment of Onychomycosis Using a Nano-Gel Formulation" Pharmaceutics 14, no. 10: 2170. https://doi.org/10.3390/pharmaceutics14102170
APA StylePuri, V., Froelich, A., Shah, P., Pringle, S., Chen, K., & Michniak-Kohn, B. (2022). Quality by Design Guided Development of Polymeric Nanospheres of Terbinafine Hydrochloride for Topical Treatment of Onychomycosis Using a Nano-Gel Formulation. Pharmaceutics, 14(10), 2170. https://doi.org/10.3390/pharmaceutics14102170