Fabrication of Polysaccharide-Based Halochromic Nanofibers via Needle-Less Electrospinning and Their Characterization: A Study of the Leaching Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hybrid Solution Preparation
2.2. Rheology and Conductivity Measurements of Spinning Solutions
2.3. Electrospinning
2.4. Morphology Analysis
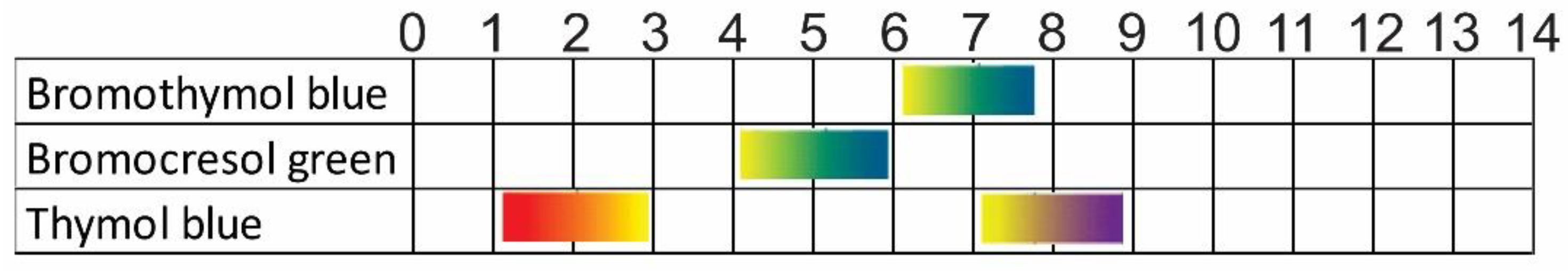
2.5. Characterizations of the Dyes
2.6. Responsivity Measurements
2.7. Analysis of Dye Leaching
3. Results and Discussion
3.1. Optimization of Hybrid Solutions
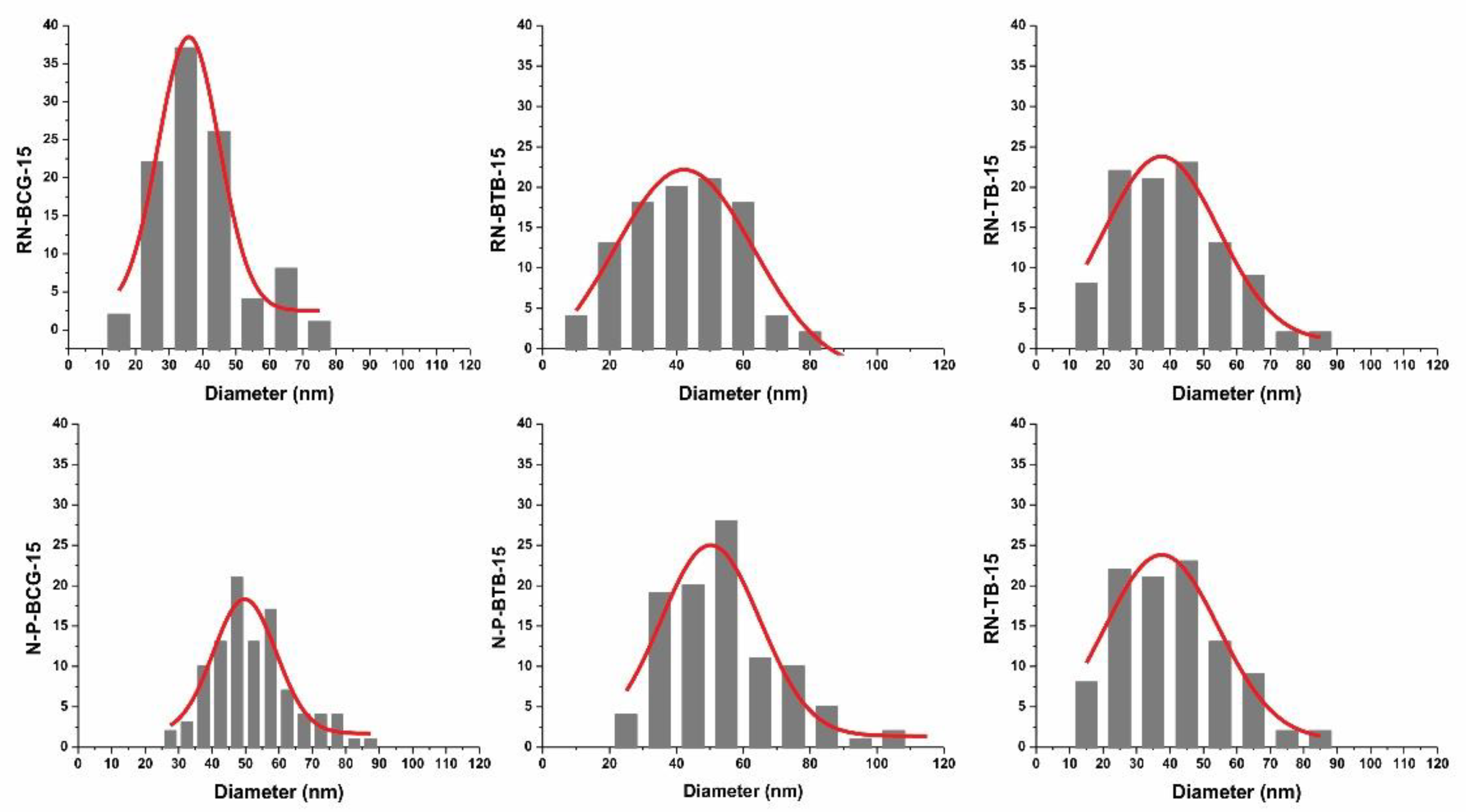
3.2. Electrospinning of Hybrid Solutions and Characterization of Nanofibers
3.3. Characterization of the Dyes
3.4. Responsivity Evaluations
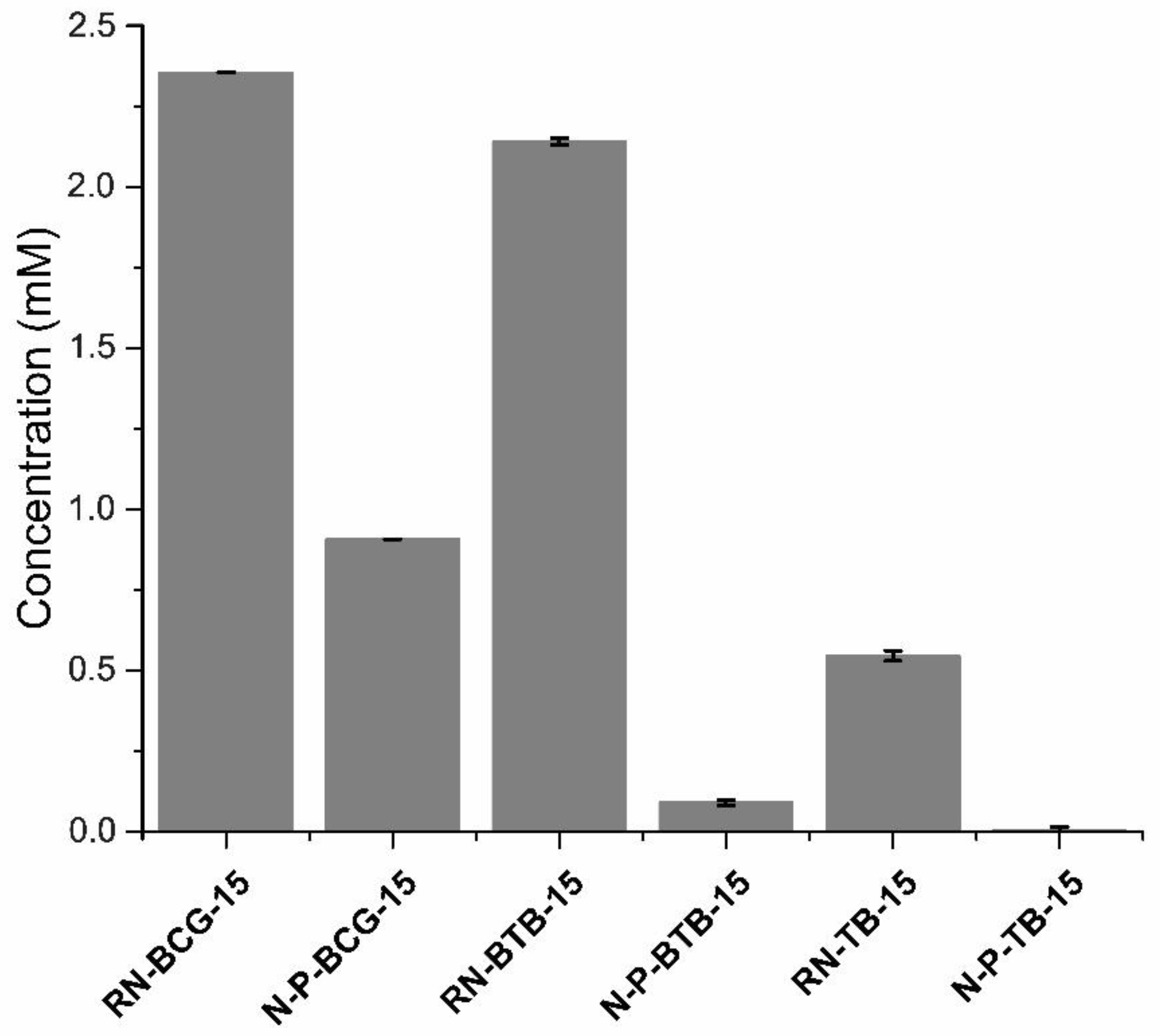
3.5. Leaching Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Bazbouz, M.B.; Tronci, G. Two-layer Electrospun System Enabling Wound Exudate Management and Visual Infection Response. Sensors 2019, 19, 991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Dong, T.; Li, Y.; Sun, M.; Qi, Y.; Liu, J.; Kuss, M.A.; Chen, S.; Duan, B. State-of-the-art review of advanced electrospun nanofiber yarn-based textiles for biomedical applications. Appl. Mater. Today 2022, 27, 101473. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Ghazalian, M.; Afshar, S.; Rostami, A.; Rashedi, S.; Bahrami, S.H. Fabrication and characterization of chitosan-polycaprolactone core-shell nanofibers containing tetracycline hydrochloride. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128163. [Google Scholar] [CrossRef]
- Lin, J. Needleless Electrospinning: A Practical Way to Mass Production of Nanofibers. Text. Sci. Eng. 2012, 2, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Sun, R.; Liu, C.; Xiong, J.; Qin, X. Mass production of nanofibers from needleless electrospinning by a novel annular spinneret. Mater. Des. 2019, 179, 107885. [Google Scholar] [CrossRef]
- Niu, H.; Lin, T. Fiber generators in needleless electrospinning. J. Nanomater. 2012, 2012, 725950. [Google Scholar] [CrossRef]
- Karim, M.; Fathi, M.; Soleimanian-Zad, S.; Spigno, G. Development of sausage packaging with zein nanofibers containing tetradecane produced via needle-less electrospinning method. Food Packag. Shelf Life 2022, 33, 100911. [Google Scholar] [CrossRef]
- Jirsak, O.; Sysel, P.; Sanetrnik, F.; Hruza, J.; Chaloupek, J. Polyamic acid nanofibers produced by needleless electrospinning. J. Nanomater. 2010, 2010, 842831. [Google Scholar] [CrossRef]
- Panda, P.K.; Gangwar, A.; Thite, A.G. Optimization of Nylon 6 electrospun nanofiber diameter in needle-less wire electrode using central composite design and response surface methodology. J. Ind. Text. 2022, 51, 7279S–7292S. [Google Scholar] [CrossRef]
- Callam, M.J.; Ruckley, C.V.; Harper, D.R.; Dale, J.J. Chronic ulceration of the leg: Extent of the problem and provision of care. Br. Med. J. (Clin. Res. Ed.) 1985, 290, 1855–1856. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Raheja, A.; Natarajan, T.S.; Chandra, T.S. Development of universal pH sensing electrospun nanofibers. Sens. Actuators B Chem. 2012, 161, 1097–1101. [Google Scholar] [CrossRef]
- Tassanawat, S.; Phandee, A.; Magaraphan, R.; Nithitanakul, M.; Manuspiya, H. pH-Sensitive PP/Clay Nanocomposites for Beverage Smart Packaging. In Proceedings of the 2nd IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Bangkok, Thailand, 16–19 January 2007; pp. 478–482. [Google Scholar]
- Van der Schueren, L.U.; Mollet, T.U.; Ceylan, Ö.U.; De Clerck, K.T.W. The development of polyamide 6.6 nanofibres with a pH-sensitive function by electrospinning. Eur. Polym. J. 2010, 46, 2229–2239. [Google Scholar] [CrossRef]
- Razaq, S.; Wilkins, R.J.; Urban, J.P. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur. Spine J. 2003, 12, 341–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldman, S.D.; Couto, D.C.; Omelon, S.J.; Kandel, R.A. Effect of sodium bicarbonate on extracellular pH, matrix accumulation, and morphology of cultured articular chondrocytes. Tissue Eng. 2004, 10, 1633–1640. [Google Scholar] [CrossRef]
- Van der Schueren, L.; De Clerck, K.; Brancatelli, G.; Rosace, G.; Van Damme, E.; De Vos, W. Novel cellulose and polyamide halochromic textile sensors based on the encapsulation of Methyl Red into a sol–gel matrix. Sens. Actuators B Chem. 2012, 162, 27–34. [Google Scholar] [CrossRef]
- Masuda, T.; Maruyama, H.; Arai, F.; Anada, T.; Fukuda, T.; Suzuki, O. Development of a pH indicator immobilized-gel-sheet for microenviroment analysis. In Proceedings of the 2009 International Symposium on Micro-NanoMechatronics and Human Science, Nagoya, Japan, 9–11 November 2009; pp. 362–367. [Google Scholar]
- Nakao, M.; Inoue, S.; Yoshinobu, T.; Iwasaki, H. High-resolution pH imaging sensor for microscopic observation of microorganisms. Sens. Actuators B Chem. 1996, 34, 234–239. [Google Scholar] [CrossRef]
- Van der Schueren, L.; Hemelsoet, K.; Van Speybroeck, V.; De Clerck, K. The influence of a polyamide matrix on the halochromic behaviour of the pH-sensitive azo dye Nitrazine Yellow. Dye. Pigment. 2012, 94, 443–451. [Google Scholar] [CrossRef]
- Van der Schueren, L.; De Meyer, T.; Steyaert, I.; Ceylan, Ö.; Hemelsoet, K.; Van Speybroeck, V.; De Clerck, K. Polycaprolactone and polycaprolactone/chitosan nanofibres functionalised with the pH-sensitive dye Nitrazine Yellow. Carbohydr. Polym. 2013, 91, 284–293. [Google Scholar] [CrossRef]
- Kurečič, M.; Maver, T.; Virant, N.; Ojstršek, A.; Gradišnik, L.; Hribernik, S.; Kolar, M.; Maver, U.; Kleinschek, K.S. A multifunctional electrospun and dual nano-carrier biobased system for simultaneous detection of pH in the wound bed and controlled release of benzocaine. Cellulose 2018, 25, 7277–7297. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Zaslavski, E.; Vasilyev, G.; Boas, M.; Zussman, E. Tunable pH-Responsive Chitosan-Poly(acrylic acid) Electrospun Fibers. Biomacromolecules 2018, 19, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Gao, J.; Wang, L.; Hu, X. Composite chitosan/poly(ethylene oxide) electrospun nanofibrous mats as novel wound dressing matrixes for the controlled release of drugs. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Rosace, G.; Guido, E.; Colleoni, C.; Brucale, M.; Piperopoulos, E.; Milone, C.; Plutino, M.R. Halochromic resorufin-GPTMS hybrid sol-gel: Chemical-physical properties and use as pH sensor fabric coating. Sens. Actuators B Chem. 2017, 241, 85–95. [Google Scholar] [CrossRef]
- De Meyer, T.; Steyaert, I.; Hemelsoet, K.; Hoogenboom, R.; Van Speybroeck, V.; De Clerck, K. Halochromic properties of sulfonphthaleine dyes in a textile environment: The influence of substituents. Dye. Pigment. 2016, 124, 249–257. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Batov, D.V.; Petrova, L.S.; Vladimirtzeva, E.L.; Odintzova, O.I. The study of interactions between textile auxiliary polyelectrolytes by isothermal titration calorimetry. J. Mol. Liq. 2022, 359, 119286. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Parameswaranpillai, J.; Siengchin, S. Removal of Methylene Blue Dye from Aqueous Solution using PDADMAC Modified ZSM-5 Zeolite as a Novel Adsorbent. J. Polym. Environ. 2021, 29, 3185–3198. [Google Scholar] [CrossRef]
- Bračič, M.; Hansson, P.; Pérez, L.; Zemljič, L.F.; Kogej, K. Interaction of Sodium Hyaluronate with a Biocompatible Cationic Surfactant from Lysine: A Binding Study. Langmuir 2015, 31, 12043–12053. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Virant, N.; Ojstršek, A. Complementary Assessment of Commercial Photoluminescent Pigments Printed on Cotton Fabric. Polymers 2019, 11, 1216. [Google Scholar] [CrossRef] [Green Version]
- Ojstršek, A.; Vouk, P.; Fakin, D. Adsorption of Pollutants from Colored Wastewaters after Natural Wool Dyeing. Materials 2022, 15, 1488. [Google Scholar] [CrossRef]
- Kurečič, M.; Smole, M.S. Electrospinning: Nanofibre Production Method. Tekstilec 2013, 56, 4–9. [Google Scholar] [CrossRef]
- Williams, L.; Hatton, F.L.; Willcock, H.; Mele, E. Electrospinning of stimuli-responsive polymers for controlled drug delivery: pH- and temperature-driven release. Biotechnol. Bioeng. 2022, 119, 1177–1188. [Google Scholar] [CrossRef]
- Maver, T.; Maver, U.; Pivec, T.; Kurecic, M.; Persin, Z.; Stana-Kleinschek, K. Advanced Wound Care. In Bioactive Polysaccharide Materials for Modern Wound Healing; Springer: Cham, Switzerland, 2018; pp. 1–8. [Google Scholar] [CrossRef]
- Lim, Y.-M.; Gwon, H.-J.; Jeun, J.; Nho, Y.-C. Preparation of Cellulose-based Nanofibers Using Electrospinning. In Nanofibers; InTech: Tezpur, India, 2010. [Google Scholar] [CrossRef] [Green Version]
- Mohan, T.; Kargl, R.; Tradt, K.E.; Kulterer, M.R.; Braćić, M.; Hribernik, S.; Stana-Kleinschek, K.; Ribitsch, V. Antifouling coating of cellulose acetate thin films with polysaccharide multilayers. Carbohydr. Polym. 2015, 116, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Römhild, K.; Niemz, F.-G.; Mohan, T.; Hribernik, S.; Kurecic, M.; Ganser, C.; Teichert, C.; Spirk, S. The Cellulose Source Matters—Hollow Semi Spheres or Fibers by Needleless Electrospinning. Macromol. Mater. Eng. 2015, 301, 42–47. [Google Scholar] [CrossRef]
- Tarus, B.; Fadel, N.; Al-Oufy, A.; El-Messiry, M. Effect of polymer concentration on the morphology and mechanical characteristics of electrospun cellulose acetate and poly (vinyl chloride) nanofiber mats. Alex. Eng. J. 2016, 55, 2975–2984. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.E. An investigation of the movement of Newtonian liquids initiated and sustained by the oscillation of mechanical cilia. Aspen Emphysema Conf. 1967, 10, 309–321. [Google Scholar] [CrossRef]
- Tazraei, P.; Riasi, A.; Takabi, B. The influence of the non-Newtonian properties of blood on blood-hammer through the posterior cerebral artery. Math. Biosci. 2015, 264, 119–127. [Google Scholar] [CrossRef]
- He, H.; Kara, Y.; Molnar, K. Effect of needle characteristic on fibrous PEO produced by electrospinning. Resolut. Discov. 2018, 4, 7–11. [Google Scholar] [CrossRef]
- Bernardino, K.; Ribeiro, M.C.C. Role of density and electrostatic interactions in the viscosity and non-newtonian behavior of ionic liquids—A molecular dynamics study. Phys. Chem. Chem. Phys. 2022, 24, 6866–6879. [Google Scholar] [CrossRef]
- Ozaki, H.; Kuratani, K.; Sano, H.; Kiyobayashi, T. A Monte-Carlo simulation of ionic conductivity and viscosity of highly concentrated electrolytes based on a pseudo-lattice model. J. Chem. Phys. 2017, 147, 034904. [Google Scholar] [CrossRef]
- Hayati, I.; Bailey, A.I.; Tadros, T.F. Investigations into the mechanisms of electrohydrodynamic spraying of liquids: I. Effect of electric field and the environment on pendant drops and factors affecting the formation of stable jets and atomization. J. Colloid Interface Sci. 1987, 117, 205–221. [Google Scholar] [CrossRef]
- Baumgarten, P.K. Electrostatic spinning of acrylic microfibers. J. Colloid Interface Sci. 1971, 36, 71–79. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded nanofibers formed during electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Huang, L.; Nagapudi, K.; Apkarian, R.P.; Chaikof, E.L. Engineered collagen–PEO nanofibers and fabrics. J. Biomater. Sci. Polym. Ed. 2001, 12, 979–993. [Google Scholar] [CrossRef] [Green Version]
- Zong, X.; Kim, K.; Fang, D.; Ran, S.; Hsiao, B.S.; Chu, B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 2002, 43, 4403–4412. [Google Scholar] [CrossRef]
- Jiang, H.; Fang, D.; Hsiao, B.S.; Chu, B.; Chen, W. Optimization and Characterization of Dextran Membranes Prepared by Electrospinning. Biomacromolecules 2004, 5, 326–333. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Phiriyawirut, M.; Phaechamud, T. Gallic Acid-loaded Cellulose Acetate Electrospun Nanofibers: Thermal Properties, Mechanical Properties, and Drug Release Behavior. Open J. Polym. Chem. 2012, 02, 21–29. [Google Scholar] [CrossRef]
- Bai, X.; Son, S.J.; Zhang, S.; Liu, W.; Jordan, E.K.; Frank, J.A.; Venkatesan, T.; Lee, S.B. Synthesis of superparamagnetic nanotubes as MRI contrast agents and for cell labeling. Nanomedicine 2008, 3, 163–174. [Google Scholar] [CrossRef]
- Wongsasulak, S.; Patapeejumruswong, M.; Weiss, J.; Supaphol, P.; Yoovidhya, T. Electrospinning of food-grade nanofibers from cellulose acetate and egg albumen blends. J. Food Eng. 2010, 98, 370–376. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Shimoda, Y.; Koda, S. Effects of hydrodynamic interaction on the equivalent conductivity minimum of electrolyte solutions in solvents of low dielectric constant. J. Chem. Phys. 2013, 138, 024503. [Google Scholar] [CrossRef] [PubMed]
- Erbasa, A.; de la Cruz, M.O. Morphology-enhanced conductivity in dry ionic liquids. Phys. Chem. Chem. Phys. 2016, 18, 6441–6450. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef] [Green Version]
- Perrin, B.D.D.D.; Serjeant, E.P. pKa Prediction for Organic Acids and Bases; Springer: Dordrecht, The Netherlands, 1981; p. 146. [Google Scholar] [CrossRef]
- Farrell, D.; Miranda, E.S.; Webb, H.; Georgi, N.; Crowley, P.B.; McIntosh, L.P.; Nielsen, J.E. Titration_DB: Storage and analysis of NMR-monitored protein pH titration curves. Proteins Struct. Funct. Bioinform. 2010, 78, 843–857. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, T.; Ensing, B.; Rogge, S.M.J.; De Clerck, K.; Meijer, E.J.; Van Speybroeck, V. Acidity Constant (pKa) Calculation of Large Solvated Dye Molecules: Evaluation of Two Advanced Molecular Dynamics Methods. Chemphyschem 2016, 17, 3447–3459. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.F. Properties of proton transfer in hydrogen-bonded systems and its experimental evidences and applications in biology. Prog. Biophys. Mol. Biol. 2013, 112, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Devarayan, K.; Kim, B.-S. Reversible and universal pH sensing cellulose nanofibers for health monitor. Sens. Actuators B Chem. 2015, 209, 281–286. [Google Scholar] [CrossRef]
- McDonagh, C.; Burke, C.S.; MacCraith, B.D. Optical chemical sensors. Chem. Rev. 2008, 108, 400–422. [Google Scholar] [CrossRef]
- Mohr, G.J.; Müller, H.; Bussemer, B.; Stark, A.; Carofiglio, T.; Trupp, S.; Heuermann, R.; Henkel, T.; Escudero, D.; González, L. Design of acidochromic dyes for facile preparation of pH sensor layers. Anal. Bioanal. Chem. 2008, 392, 1411–1418. [Google Scholar] [CrossRef]
- Ding, B.; Wang, M.; Yu, J.; Sun, G. Gas Sensors Based on Electrospun Nanofibers. Sensors 2009, 9, 1609–1624. [Google Scholar] [CrossRef]

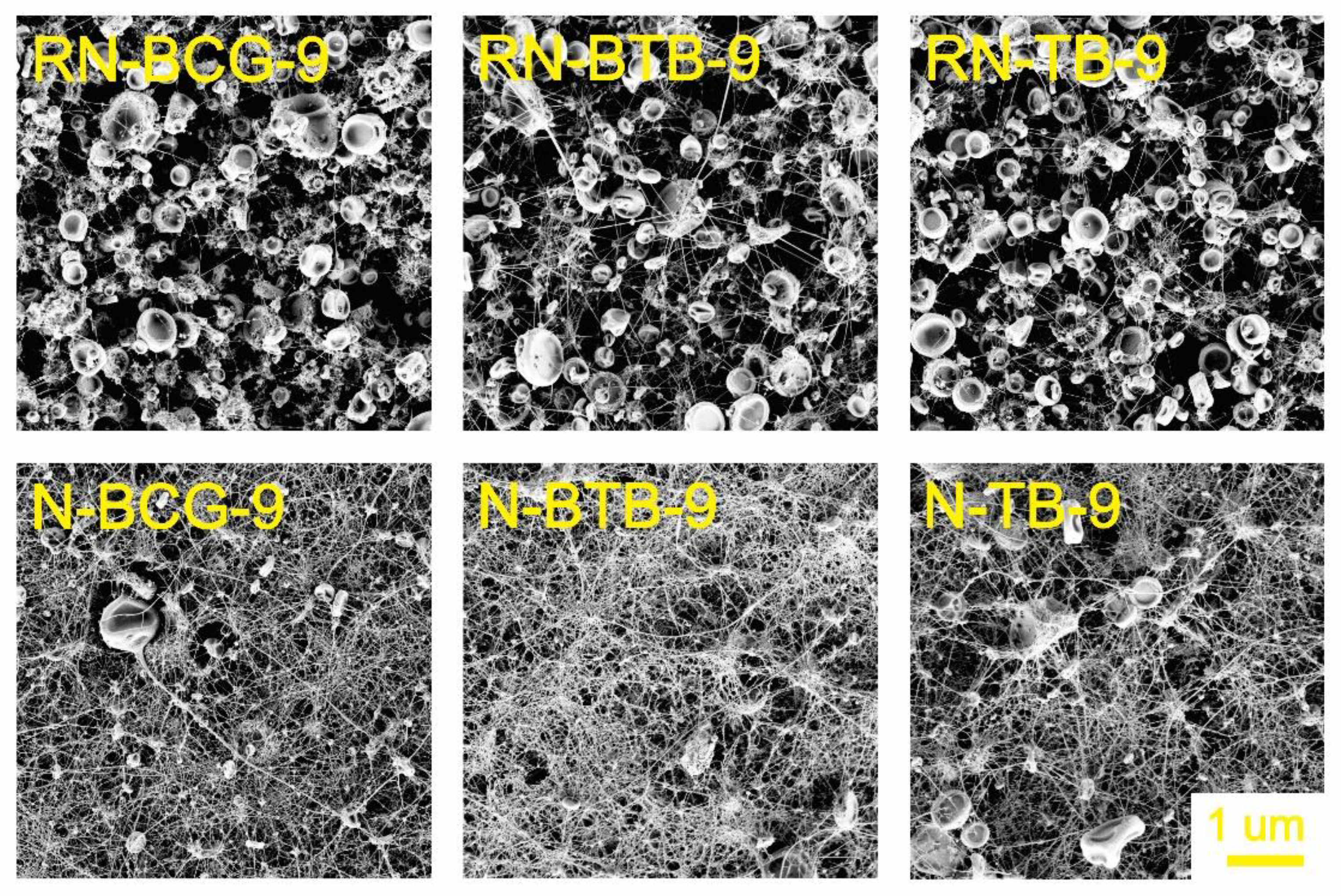
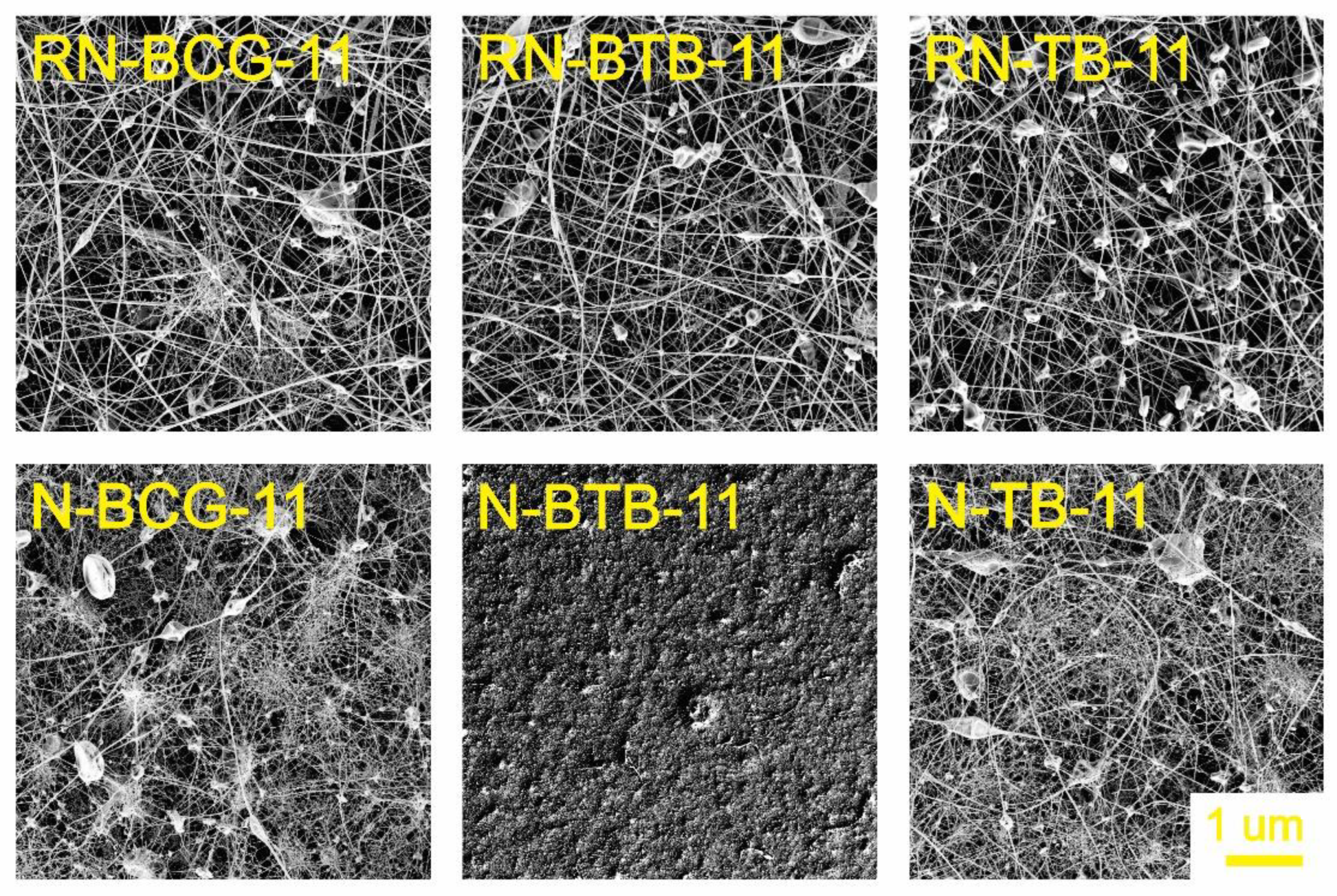

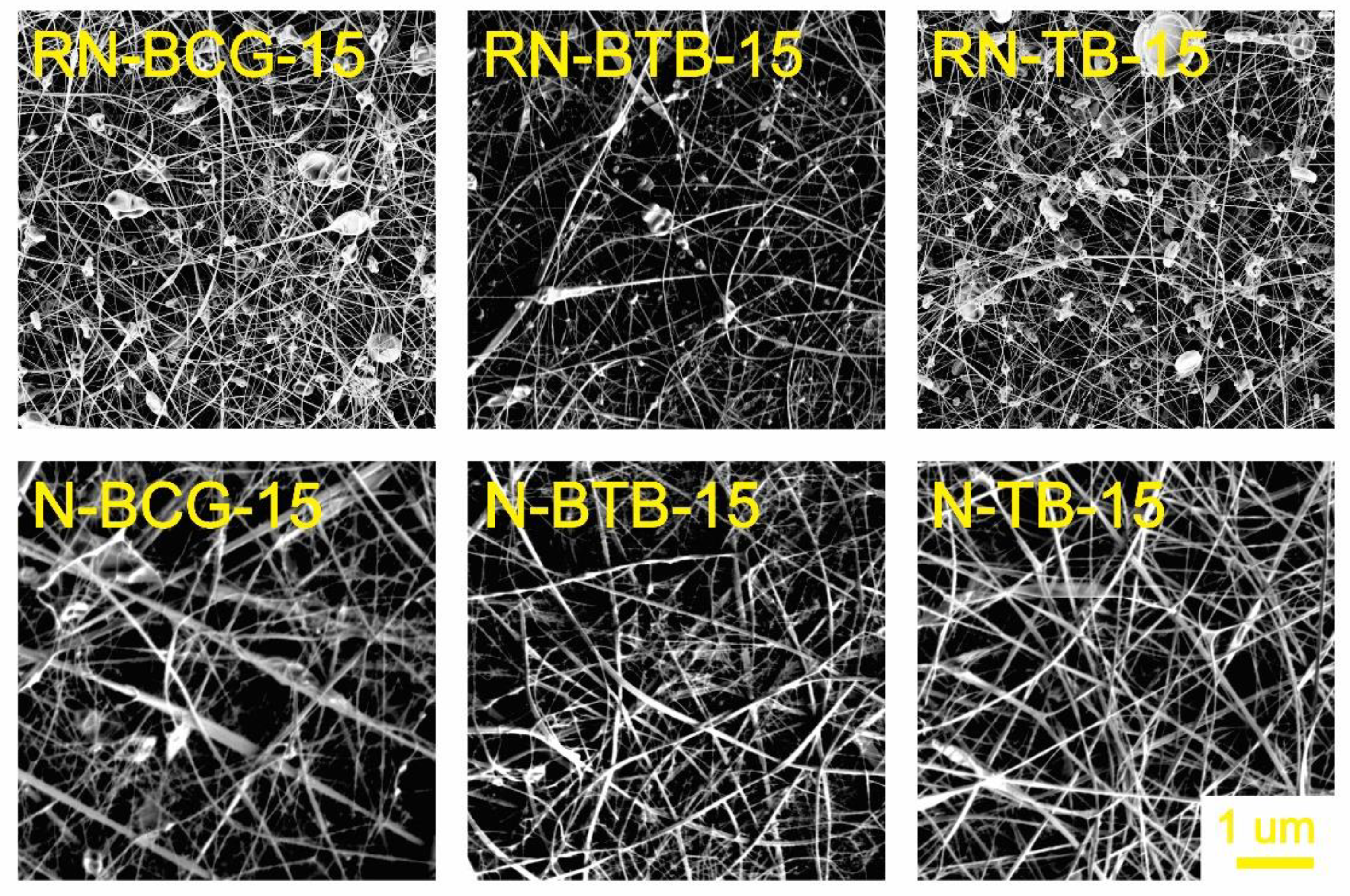

| Sample Code | CA Concentration (wt%) | PDADMAC (wt%) | BCG (wt%) | BTB (wt%) | TB (wt%) |
|---|---|---|---|---|---|
| R-BCG-9 | 9 | - | 0.3 | - | - |
| R-BTB-9 | 9 | - | - | 0.3 | - |
| R-TB-9 | 9 | - | - | - | 0.3 |
| R-BCG-11 | 11 | - | 0.3 | - | - |
| R-BTB-11 | 11 | - | - | 0.3 | - |
| R-TB-11 | 11 | - | - | - | 0.3 |
| R-BCG-13 | 13 | - | 0.3 | - | - |
| R-BTB-13 | 13 | - | - | 0.3 | - |
| R-TB-13 | 13 | - | - | - | 0.3 |
| R-BCG-15 | 15 | - | 0.3 | - | - |
| R-BTB-15 | 15 | - | - | 0.3 | - |
| R-TB-15 | 15 | - | - | - | 0.3 |
| H-P-BCG-9 | 9 | 4 | 0.3 | - | - |
| H-P-BTB-9 | 9 | 4 | - | 0.3 | - |
| H-P-TB-9 | 9 | 4 | - | - | 0.3 |
| H-P-BCG-11 | 11 | 4 | 0.3 | - | - |
| H-P-BTB-11 | 11 | 4 | - | 0.3 | - |
| H-P-TB-11 | 11 | 4 | - | - | 0.3 |
| H-P-BCG-13 | 13 | 4 | 0.3 | - | - |
| H-P-BTB-13 | 13 | 4 | - | 0.3 | - |
| H-P-TB.13 | 13 | 4 | - | - | 0.3 |
| H-P-BCG-15 | 15 | 4 | 0.3 | - | - |
| H-P-BTB-15 | 15 | 4 | - | 0.3 | - |
| H-P-TB-15 | 15 | 4 | - | - | 0.3 |
| Sample Code | CA Concentration (wt%) | PDADMAC (wt%) | BCG (wt%) | BTB (wt%) | TB (wt%) |
|---|---|---|---|---|---|
| RN-BCG-9 | 9 | - | 0.3 | - | - |
| RN-BTB-9 | 9 | - | - | 0.3 | - |
| RN-TB-9 | 9 | - | - | - | 0.3 |
| RN-BCG-11 | 11 | - | 0.3 | - | - |
| RN-BTB-11 | 11 | - | - | 0.3 | - |
| RN-TB-11 | 11 | - | - | - | 0.3 |
| RN-BCG-13 | 13 | - | 0.3 | - | - |
| RN-BTB-13 | 13 | - | - | 0.3 | - |
| RN-TB-13 | 13 | - | - | - | 0.3 |
| RN-BCG-15 | 15 | - | 0.3 | - | - |
| RN-BTB-15 | 15 | - | - | 0.3 | - |
| RN-TB-15 | 15 | - | - | - | 0.3 |
| N-P-BCG-9 | 9 | 4 | 0.3 | - | - |
| N-P-BTB-9 | 9 | 4 | - | 0.3 | - |
| N-P-TB-9 | 9 | 4 | - | - | 0.3 |
| N-P-BCG-11 | 11 | 4 | 0.3 | - | - |
| N-P-BTB-11 | 11 | 4 | - | 0.3 | - |
| N-P-TB-11 | 11 | 4 | - | - | 0.3 |
| N-P-BCG-13 | 13 | 4 | 0.3 | - | - |
| N-P-BTB-13 | 13 | 4 | - | 0.3 | - |
| N-P-BTB.13 | 13 | 4 | - | - | 0.3 |
| N-P-BCG-15 | 15 | 4 | 0.3 | - | - |
| N-P-BTB-15 | 15 | 4 | - | 0.3 | - |
| N-P-TB-15 | 15 | 4 | - | - | 0.3 |
| Reference Sample | Conductivity (µS.cm) | Sample | Conductivity (µS.cm) |
|---|---|---|---|
| R-BCG-15 | 73.16 ± 0.3 | H-P-BCG-15 | 400.40 ± 0.1 |
| R-BTB-15 | 84.38 ± 0.2 | H-P-BTB-15 | 347.40 ± 0.3 |
| R-TB-15 | 38.29 ± 0.5 | H-P-TB-15 | 304.70 ± 0.7 |
| R-BCG-13 | 114.70 ± 0.1 | H-P-BCG-13 | 448.70 ± 0.6 |
| R-BTB-13 | 442.00 ± 0.7 | H-P-BTB-13 | 711.00 ± 1.1 |
| R-TB-13 | 138.25 ± 0.2 | H-P-TB-13 | 449.60 ± 0.2 |
| R-BCG-11 | 268.60 ± 0.6 | H-P-BCG-11 | 598.80 ± 0.1 |
| R-BTB-11 | 426.90 ± 0.5 | H-P-BTB-11 | 626.90 ± 0.4 |
| R-TB-11 | 88.70 ± 0.1 | H-P-TB-11 | 352.10 ± 0.5 |
| R-BCG-9 | 210.90 ± 0.8 | H-P-BCG-9 | 343.30 ± 0.3 |
| R-BTB-9 | 357.90 ± 1.2 | H-P-BTB-9 | 481.80 ± 1.0 |
| R-TB-9 | 92.40 ± 0.8 | H-P-TB-9 | 319.10 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elveren, B.; Hribernik, S.; Kurečič, M. Fabrication of Polysaccharide-Based Halochromic Nanofibers via Needle-Less Electrospinning and Their Characterization: A Study of the Leaching Effect. Polymers 2022, 14, 4239. https://doi.org/10.3390/polym14194239
Elveren B, Hribernik S, Kurečič M. Fabrication of Polysaccharide-Based Halochromic Nanofibers via Needle-Less Electrospinning and Their Characterization: A Study of the Leaching Effect. Polymers. 2022; 14(19):4239. https://doi.org/10.3390/polym14194239
Chicago/Turabian StyleElveren, Beste, Silvo Hribernik, and Manja Kurečič. 2022. "Fabrication of Polysaccharide-Based Halochromic Nanofibers via Needle-Less Electrospinning and Their Characterization: A Study of the Leaching Effect" Polymers 14, no. 19: 4239. https://doi.org/10.3390/polym14194239





