Increased Mortality with Intermediate Ascitic Polymorphonuclear Cell Counts Amongst Patients with Cirrhosis: Time to Redefine the Care Approach
Abstract
1. Introduction
2. Methods
Categorization of Cohort and Statistical Analysis
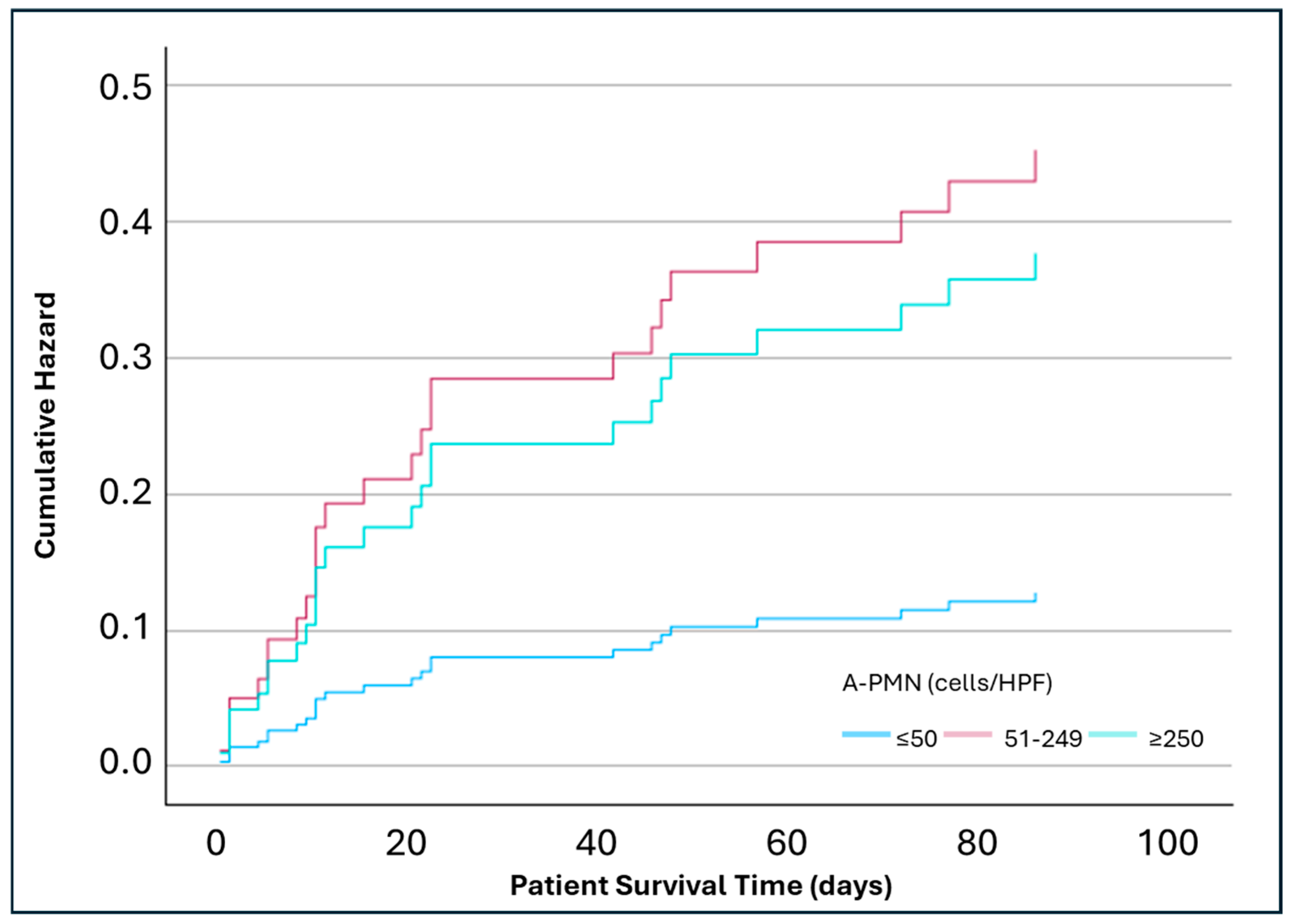
3. Results
3.1. Baseline Characteristics of the Cohort
3.2. Mortality Risk Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACLF | Acute-on-chronic liver failure |
| A-PMN | Ascitic polymorphonuclear cells |
| APRI | Aspartate Aminotransferase to Platelet Ratio Index |
| CI | Confidence interval |
| CLIF | Chronic Liver Failure Consortium |
| CT | Computed tomography |
| CTP | Child–Turcotte Pugh |
| DM | Diabetes mellitus |
| EASL | European Association for the Study of the Liver |
| Fib-4 | Fibrosis 4 |
| GI | Gastrointestinal |
| HCV | Hepatitis C virus |
| HPF | High-power field |
| HR | Hazard ratio |
| MELD | Model for End-Stage Liver Disease |
| ROC | Receiver operating characteristic |
| SBP | Spontaneous bacterial peritonitis |
| SD | Standard deviation |
| WBC | White blood cell |
References
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.C. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef]
- Gines, P.; Quintero, E.; Arroyo, V.; Teres, J.; Bruguera, M.; Rimola, A.; Caballería, J.; Rodés, J.; Rozman, C. Compensated cirrhosis: Natural history and prognostic factors. Hepatology 1987, 7, 122–128. [Google Scholar] [CrossRef]
- Groszmann, R.J.; Wongcharatrawee, S. The hepatic venous pressure gradient: Anything worth doing should be done right. Hepatology 2004, 39, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; De, A.; Mehtani, R.; Angeli, P.; Maiwall, R.; Satapathy, S.; Singal, A.K.; Saraya, A.; Sharma, B.C.; Eapen, C.E.; et al. Asia-Pacific association for study of liver guidelines on management of ascites in liver disease. Hepatol. Int. 2023, 17, 792–826. [Google Scholar] [CrossRef]
- Biggins, S.W.; Angeli, P.; Garcia-Tsao, G.; Gines, P.; Ling, S.C.; Nadim, M.K.; Wong, F.; Kim, W.R. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1014–1048. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J. Hepatol. 2010, 53, 397–417. [Google Scholar] [CrossRef]
- Shizuma, T. Spontaneous bacterial and fungal peritonitis in patients with liver cirrhosis: A literature review. World J. Hepatol. 2018, 10, 254–266. [Google Scholar] [CrossRef]
- Pinzello, G.; Simonetti, R.G.; Craxi, A.; Di Piazza, S.; Spano, C.; Pagliaro, L. Spontaneous bacterial peritonitis: A prospective investigation in predominantly nonalcoholic cirrhotic patients. Hepatology 1983, 3, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Cadranel, J.F.; Nousbaum, J.B.; Bessaguet, C.; Nahon, P.; Nguyen-Khac, E.; Moreau, R.; Thévenot, T.; Silvain, C.; Bureau, C.; Nouel, O.; et al. Low incidence of spontaneous bacterial peritonitis in asymptomatic cirrhotic outpatients. World J. Hepatol. 2013, 5, 104–108. [Google Scholar] [CrossRef]
- Chinnock, B.; Hendey, G.W.; Minnigan, H.; Butler, J.; Afarian, H. Clinical impression and ascites appearance do not rule out bacterial peritonitis. J. Emerg. Med. 2013, 44, 903–909. [Google Scholar] [CrossRef]
- Elzouki, A.N.; Hamad, A.; Almasri, H.; Ata, M.; Ashour, A.; Othman, M.; Badi, A.; Errayes, M.; Zahid, M.; Danjuma, M.; et al. Predictors of Short-Term Mortality Following First Episode of Spontaneous Bacterial Peritonitis in Hospitalized Cirrhotic Patients. Cureus 2021, 13, e18999. [Google Scholar] [CrossRef]
- Mahmud, N.; Reddy, K.R.; Taddei, T.H.; Kaplan, D.E. Type of Infection Is Associated with Prognosis in Acute-on-Chronic Liver Failure: A National Veterans Health Administration Study. Dig. Dis. Sci. 2023, 68, 1632–1640. [Google Scholar] [CrossRef]
- Karvellas, C.J.; Abraldes, J.G.; Arabi, Y.M.; Kumar, A.; Cooperative Antimicrobial Therapy of Septic Shock Database Research Group. Appropriate and timely antimicrobial therapy in cirrhotic patients with spontaneous bacterial peritonitis-associated septic shock: A retrospective cohort study. Aliment. Pharmacol. Ther. 2015, 41, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Rimola, A.; Garcia-Tsao, G.; Navasa, M.; Piddock, L.J.; Planas, R.; Bernard, B.; Inadomi, J.M. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: A consensus document. International Ascites Club. J. Hepatol. 2000, 32, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Navasa, M.; Gomez, J.; Colmenero, J.; Vila, J.; Arroyo, V.; Rodés, J. Bacterial infections in cirrhosis: Epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology 2002, 35, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Bernardi, M.; Balk, R.; Christman, B.; Moreau, R.; Garcia-Tsao, G.; Patch, D.; Soriano, G.; Hoefs, J.; Navasa, M. Sepsis in cirrhosis: Report on the 7th meeting of the International Ascites Club. Gut 2005, 54, 718–725. [Google Scholar] [CrossRef]
- Garcia-Tsao, G. Current Management of the Complications of Cirrhosis and Portal Hypertension: Variceal Hemorrhage, Ascites, and Spontaneous Bacterial Peritonitis. Dig. Dis. 2016, 34, 382–386. [Google Scholar] [CrossRef]
- Lutz, P.; Goeser, F.; Kaczmarek, D.J.; Schlabe, S.; Nischalke, H.D.; Nattermann, J.; Hoerauf, A.; Strassburg, C.P.; Spengler, U. Relative Ascites Polymorphonuclear Cell Count Indicates Bacterascites and Risk of Spontaneous Bacterial Peritonitis. Dig. Dis. Sci. 2017, 62, 2558–2568. [Google Scholar] [CrossRef]
- Schwabl, P.; Bucsics, T.; Soucek, K.; Mandorfer, M.; Bota, S.; Blacky, A.; Hirschl, A.M.; Ferlitsch, A.; Trauner, M.; Peck-Radosavljevic, M.; et al. Risk factors for development of spontaneous bacterial peritonitis and subsequent mortality in cirrhotic patients with ascites. Liver Int. 2015, 35, 2121–2128. [Google Scholar] [CrossRef]
- Simbrunner, B.; Rothenbacher, A.; Haslacher, H.; Bauer, D.; Chromy, D.; Bucsics, T.; Schwabl, P.; Paternostro, R.; Scheiner, B.; Trauner, M.; et al. Ascitic fluid polymorphic nuclear cell count impacts on outcome of cirrhotic patients with ascites. United Eur. Gastroenterol. J. 2019, 7, 651–661. [Google Scholar] [CrossRef]
- Dawit, L.; Lee, V.; Lehoang, D.; Furey, C.; Chowdhury, A.; Mai, T.A.; Angajala, V.; Park, J.H.; Khadarian, K.; She, R.; et al. Clinical Significance of Ascitic Fluid Polymorphonuclear Leukocyte Percentage in Patients with Cirrhosis without Spontaneous Bacterial Peritonitis. Clin. Transl. Gastroenterol. 2023, 14, e00614. [Google Scholar] [CrossRef]
- Saffo, S.; To, U.K.; Santoiemma, P.P.; Laurito, M.; Haque, L.; Rabiee, A.; Verna, E.C.; Angarone, M.P.; Garcia-Tsao, G. Changes in Ascitic Fluid Polymorphonuclear Cell Count After Antibiotics Are Associated with Mortality in Spontaneous Bacterial Peritonitis. Clin. Gastroenterol. Hepatol. 2022, 20, e1201–e1204. [Google Scholar] [CrossRef]
- Allah, A.-N.A.-A.G.; Hekal, R.E.A.; Dala, A.G.; Genanea, S.E.R.; AbdelAziz, H.A. Role of Ascitic Fluid Polymorphic Nuclear Cell Count and Prostaglandin E2 Prognostic Outcome of Cirrhotic Diseased Individual’s Mortality. Surg. Gastroenterol. Oncol. 2023, 28, 36–44. [Google Scholar] [CrossRef]
- Fernandes, S.R.; Santos, P.; Fatela, N.; Baldaia, C.; Tato Marinho, R.; Proenca, H.; Ramalho, F.; Velosa, J. Ascitic Calprotectin is a Novel and Accurate Marker for Spontaneous Bacterial Peritonitis. J. Clin. Lab. Anal. 2016, 30, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razik, A.; Mousa, N.; Elhammady, D.; Elhelaly, R.; Elzehery, R.; Elbaz, S.; Eissa, M.; El-Wakeel, N.; Eldars, W. Ascitic Fluid Calprotectin and Serum Procalcitonin as Accurate Diagnostic Markers for Spontaneous Bacterial Peritonitis. Gut Liver 2016, 10, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Aehling, N.F.; Hagenunger, A.; Krohn, S.; Zeller, K.; Jager, K.; Herber, A.; Engelmann, C.; Berg, T. Use of Bacterial DNA Concentration in Ascites as a Marker for Spontaneous Bacterial Peritonitis. J. Clin. Exp. Hepatol. 2024, 14, 101434. [Google Scholar] [CrossRef] [PubMed]
- Tawheed, A.; Yalniz, M.; Ozercan, M.; Bahcecioglu, I.H. Exploring the next frontier in diagnosing spontaneous bacterial peritonitis. World J. Hepatol. 2025, 17, 102044. [Google Scholar] [CrossRef]


| Characteristic | A-PMN ≤50 Cells/HPF (n = 86) | A-PMN 51–249 Cells/HPF (n = 18) | A-PMN ≥250 Cells/HPF (n = 13) | Total (n = 117) | p Value |
|---|---|---|---|---|---|
| Male gender—no. (%) | 66 (76.7) | 13 (72.2) | 12 (92.3) | 91 (77.8) | 0.375 A |
| Mean age (SD)—years | 54.3 (10.7) | 56.7 (7.2) | 56.7 (8.2) | 54.9 (10) | 0.514 B |
| Cirrhosis etiology—no./total no. (%) | 0.54 A | ||||
| Alcohol | 33/84 (39.3) | 5/17 (29.4) | 5/13 (38.5) | 43/114 (37.7) | |
| Hepatitis C virus | 28/84 (33.3) | 9/17 (52.9) | 6/13 (46.2) | 43/114 (37.7) | |
| Hepatitis B virus | 4/84 (4.8) | 0/17 (0) | 1/13 (7.7) | 5/114 (4.4) | |
| Metabolic-associated liver disease | 10/84 (11.9) | 0/17 (0) | 0/13 (0) | 10/114 (8.8) | |
| Autoimmune hepatitis | 1/84 (1.2) | 1/17 (5.9) | 0/13 (0) | 2/114 (1.8) | |
| Unknown/Cryptogenic | 8/84 (9.5) | 2/17 (11.8) | 1/13 (7.7) | 11/114 (9.6) | |
| Diabetes mellitus—no. (%) | 26 (30.2) | 1 (5.6) | 4 (30.8) | 31 (26.5) | 0.091 A |
| Chronic kidney disease—no. (%) | 12 (14) | 5 (27.8) | 3 (23.1) | 20 (17.1) | 0.305 A |
| Alcohol use—no./no. total (%) | 0.969 A | ||||
| No | 26/83 (31.3) | 7/18 (38.9) | 4/12 (33.3) | 37/113 (32.7) | |
| Yes, <6 months prior | 46/83 (55.4) | 9/18 (50) | 6/12 (50) | 61/113 (54) | |
| Yes, >6 months prior | 11/83 (13.3) | 2/18 (11.1) | 2/12 (16.7) | 15/113 (13.3) | |
| ACLF-EASL level 1 minimum criteria met C—no./no. total (%) | 29/86 (33.7) | 8/18 (44.4) | 7/13 (53.8) | 44/117 (37.6) | 0.305 A |
| APRI (n = 117)—mean (SD) | 2.4 (2.8) | 4.3 (10.2) | 1.4 (1.5) | 2.6 (4.7) | 0.195 B |
| CTP score (n = 117)—mean (SD) | 12.1 (1.3) | 12.1 (1.3) | 11.8 (0.6) | 12.1 (11.2) | 0.612 B |
| Fib-4 score (n = 117)—mean (SD) | 7.7 (6.7) | 12.5 (23.3) | 5.5 (5.3) | 8.2 (10.9) | 0.149 B |
| MELD score (n = 115)—mean (SD) | 19.3 (8.5) | 20.0 (8.7) | 22.3 (11.3) | 19.7 (8.8) | 0.510 B |
| Antibiotic agents—no./no. total (%) | 0.648 A | ||||
| 1 agent | 45/69 (65.2) | 7/15 (46.7) | 7/12 (58.3) | 59/96 (61.5) | |
| ≥2 agents | 23/69 (33.3) | 8/15 (53.3) | 5/12 (41.7) | 36/96 (37.5) | |
| Antibiotic initiation time from admission—no./no. total (%) | 0.850 A | ||||
| <24 h | 62/65 (95.4) | 14/15 (93.3) | 11/12 (91.7) | 87/92 (94.6) | |
| >24 h | 3/65 (4.6) | 1/15 (6.7) | 1/12 (8.3) | 5/92 (5.4) | |
| Reason for antibiotic use (for any infection—no./no. total (%) | 0.015 A | ||||
| Prophylaxis | 30/68 (44.1) | 5/15 (33.3) | 2/12 (16.7) | 37/95 (38.9) | |
| Preemptive | 17/68 (25) | 4/15 (26.7) | 0/12 (0) | 21/95 (22.1) | |
| Treatment | 21/68 (30.9) | 6/15 (40) | 10/12 (83.3) | 37/95 (38.9) | |
| Infection status (any infection)—no. (%) | 52 (60.5) | 11 (61.1) | 13 (100) | 76 (65) | 0.019 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habib, S.; Ball, M.; Thomas, C.; Murakami, T.; Patel, N.; Yarlagadda, S.; Patel, S.; Walker, C.; Takyar, V.; Patel, K.; et al. Increased Mortality with Intermediate Ascitic Polymorphonuclear Cell Counts Amongst Patients with Cirrhosis: Time to Redefine the Care Approach. Pathophysiology 2025, 32, 62. https://doi.org/10.3390/pathophysiology32040062
Habib S, Ball M, Thomas C, Murakami T, Patel N, Yarlagadda S, Patel S, Walker C, Takyar V, Patel K, et al. Increased Mortality with Intermediate Ascitic Polymorphonuclear Cell Counts Amongst Patients with Cirrhosis: Time to Redefine the Care Approach. Pathophysiology. 2025; 32(4):62. https://doi.org/10.3390/pathophysiology32040062
Chicago/Turabian StyleHabib, Shahid, Michael Ball, Chris Thomas, Traci Murakami, Nehali Patel, Sandeep Yarlagadda, Sarah Patel, Courtney Walker, Varun Takyar, Krunal Patel, and et al. 2025. "Increased Mortality with Intermediate Ascitic Polymorphonuclear Cell Counts Amongst Patients with Cirrhosis: Time to Redefine the Care Approach" Pathophysiology 32, no. 4: 62. https://doi.org/10.3390/pathophysiology32040062
APA StyleHabib, S., Ball, M., Thomas, C., Murakami, T., Patel, N., Yarlagadda, S., Patel, S., Walker, C., Takyar, V., Patel, K., Domingues, C., & Hsu, C.-H. (2025). Increased Mortality with Intermediate Ascitic Polymorphonuclear Cell Counts Amongst Patients with Cirrhosis: Time to Redefine the Care Approach. Pathophysiology, 32(4), 62. https://doi.org/10.3390/pathophysiology32040062






