Nutrition and Micronutrient Interactions in Autoimmune Thyroid Disorders: Implications for Cardiovascular Health
Abstract
1. Introduction
1.1. Research Hypotheses
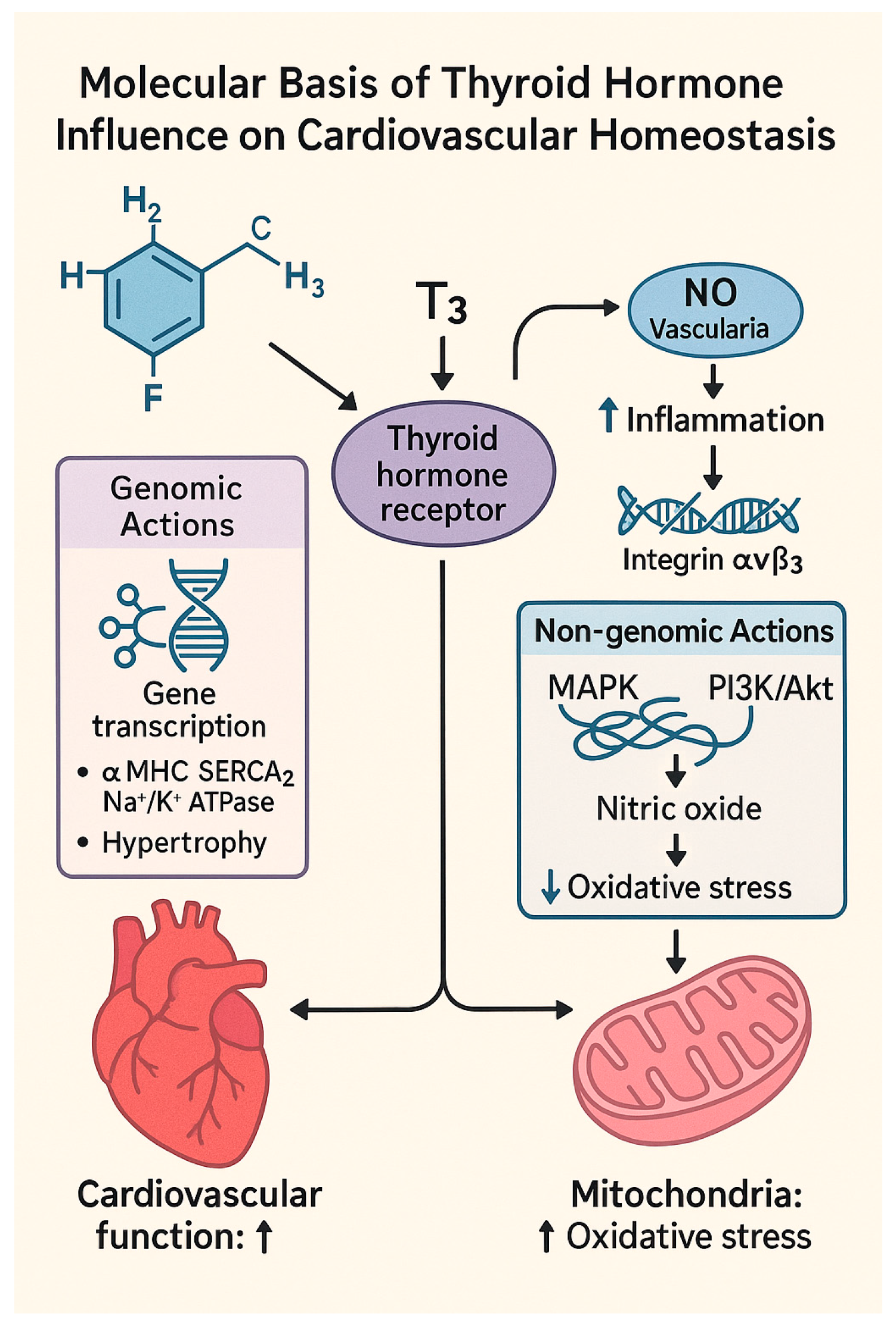
1.2. Molecular Basis of Thyroid Hormone Influence on Cardiovascular Homeostasis
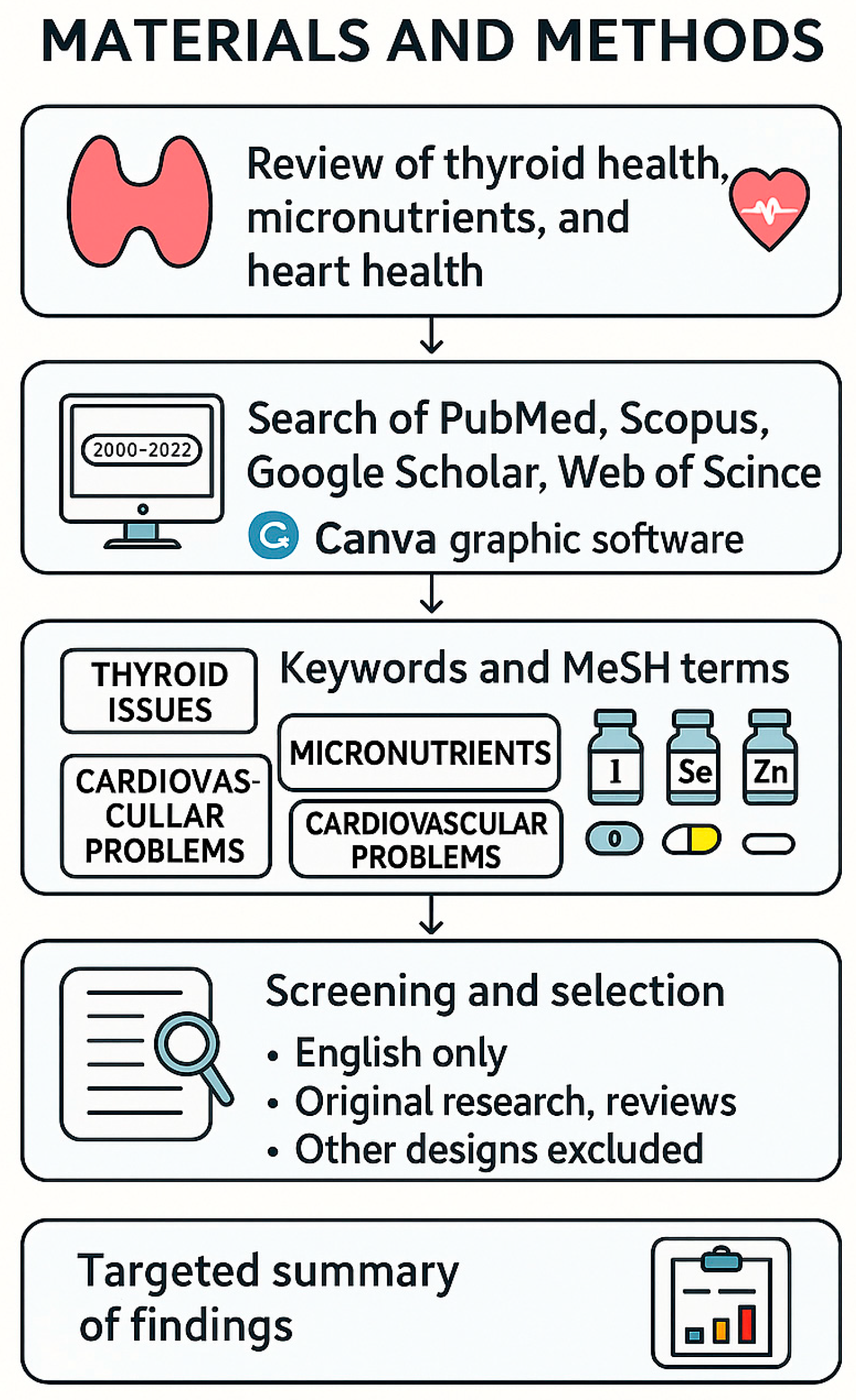
2. Materials and Methods
3. Sublinical Thyroid Dysfunction
4. Macronutrients in Thyroid and Cardiovascular Health
4.1. Protein
4.2. Carbohydrates
4.3. Fats
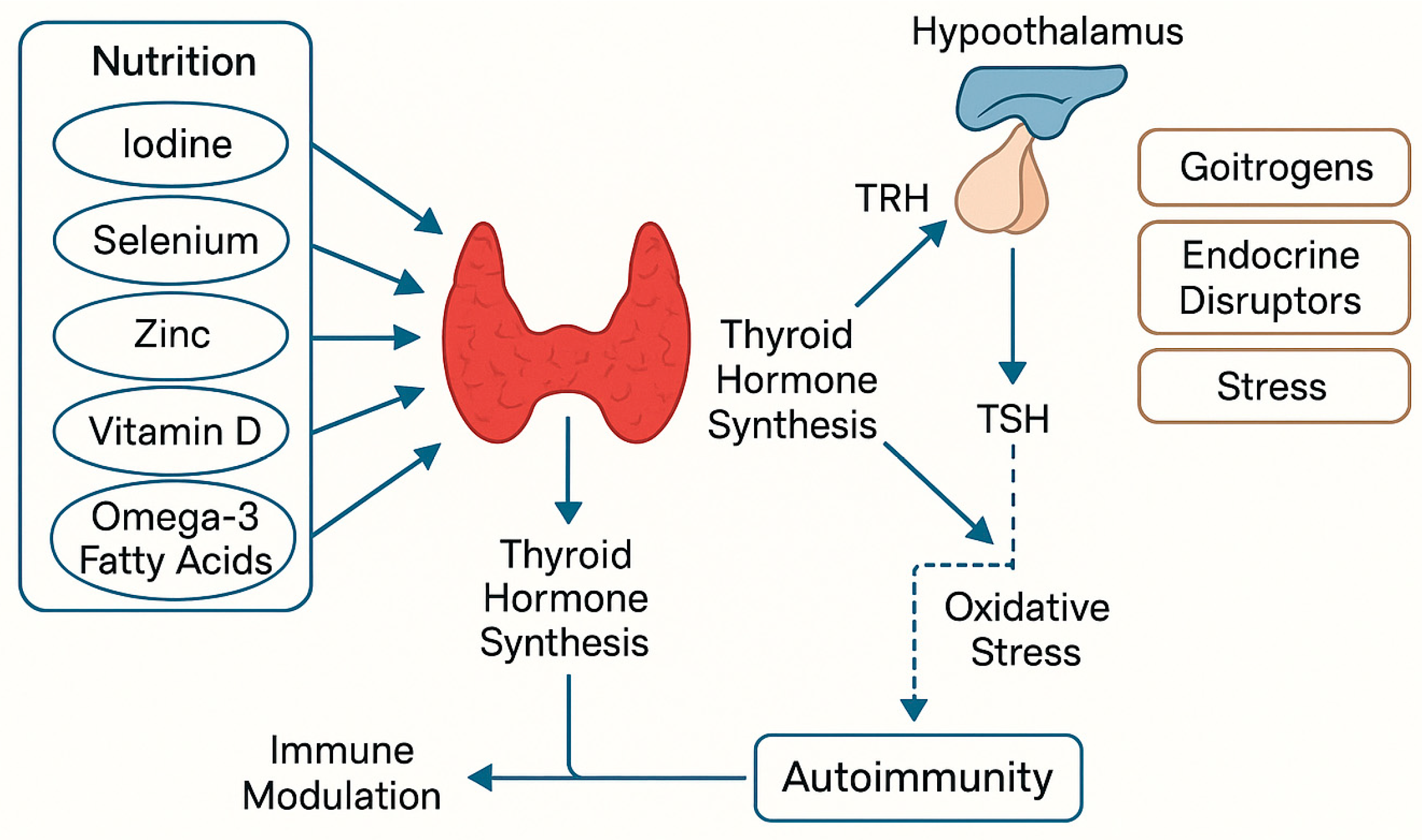
5. Relationship Between Nutrition and Hypothyroidism
5.1. Metabolic Changes in Hypothyroidism
5.2. Obesity and Body Composition
5.3. Environmental and Dietary Risk Factors
- Iodine deficiency;
- Exposure to endocrine-disrupting chemicals (e.g., plastics, pesticides);
- Radiation exposure;
- Chronic psychological stress affecting the hypothalamic–pituitary–thyroid axis;
- Consumption of goitrogenic foods (e.g., raw cabbage, broccoli, cauliflower);
- Viral infections and pollutants contributing to autoimmune activation [47].
5.4. Dietary Patterns Supporting Thyroid Health
6. Micronutrients in Thyroid and Cardiovascular Health
6.1. Iodine
6.2. Vitamin D
6.3. Iron
- Red meat and liver—highly bioavailable heme iron;
- Cocoa and dark chocolate—non-heme iron, enhanced by vitamin C;
- Spinach—iron-rich, though less bioavailable;
- Sardines and seafood—provide iron along with omega-3s;
- Pumpkin seeds—a plant-based source [40].
6.4. Selenium
6.5. Copper
6.6. Zinc
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OH | Overt Hypothyroidism |
| CVD | Cardiovascular Disease |
| TSH | Thyroid-Stimulating Hormone |
| T4 | Thyroxine |
| T3 | Triiodothyronine |
| BMI | Body Mass Index |
| HDL | High-Density Lipoprotein |
| LDL | Low-Density Lipoprotein |
| WHO | World Health Organization |
| TSAT | Transferrin Saturation |
| FCM | Ferritin Carboxymaltose |
| MetS | Metabolic Syndrome |
| TPO | Thyroid Peroxidase |
| TPOAbs | Thyroid Peroxidase Antibodies |
| RDI | Recommended Daily Intake |
| CH | Congenital Hypothyroidism |
| TgAbs | Thyroglobulin Antibodies |
| SHyper | Subclinical hyperthyroidism |
| FCM | Ferric Carboxymaltose |
| HPT Axis | Hypothalamic–Pituitary–Thyroid Axis |
| ROS | Reactive Oxygen Species |
| MAPK | Mitogen-Activated Protein Kinase |
| PI3K/Akt | Phosphoinositide 3-Kinase/Protein Kinase B Pathway |
| NO | Nitric Oxide |
References
- What Is the Association of Hypothyroidism with Risks of Cardiovascular Events and Mortality? A Meta-Analysis of 55 Cohort Studies Involving 1,898,314 Participants—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/28148249/ (accessed on 11 August 2024).
- Galetta, F.; Franzoni, F.; Fallahi, P.; Tocchini, L.; Braccini, L.; Santoro, G.; Antonelli, A. Changes in Heart Rate Variability and QT Dispersion in Patients with Overt Hypothyroidism. Eur. J. Endocrinol. 2008, 158, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Seol, S.-H.; Kim, D.-I.; Park, B.-M.; Kim, D.-K.; Song, P.-S.; Kim, K.-H.; Jin, H.-Y.; Seo, J.-S.; Jang, J.-S.; Yang, T.-H.; et al. Complete Atrioventricular Block Presenting with Syncope Caused by Severe Hypothyroidism. Cardiol. Res. 2012, 3, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Bassett, J.H.D.; Harvey, C.B.; Williams, G.R. Mechanisms of Thyroid Hormone Receptor-Specific Nuclear and Extra Nuclear Actions. Mol. Cell Endocrinol. 2003, 213, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Biondi, B. The Management of Thyroid Abnormalities in Chronic Heart Failure. Heart Fail. Clin. 2019, 15, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Verma, I.; Sood, R.; Juneja, S.; Kaur, S. Prevalence of Hypothyroidism in Infertile Women and Evaluation of Response of Treatment for Hypothyroidism on Infertility. Int. J. Appl. Basic. Med. Res. 2012, 2, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Bereda, G. Definition, Causes, Pathophysiology, and Management of Hypothyroidism. Mathews, J. Pharm. Sci. 2023, 7, 14. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H.; Wartofsky, L. LT4/LT3 Combination Therapy vs. Monotherapy with LT4 for Persistent Symptoms of Hypothyroidism: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 9218. [Google Scholar] [CrossRef] [PubMed]
- Naliwajko, S.K.; Markiewicz-Żukowska, R.; Sawicka, E.; Bartosiuk, E.; Omeljaniuk, W.J.; Borawska, M. Składniki mineralne w diecie pacjentek z chorobą Hashimoto. Bromatol. Chem. Toksykol. 2011, 44. Available online: https://yadda.icm.edu.pl/agro/element/bwmeta1.element.dl-catalog-eae0662a-5a19-480a-9fb7-41ae5de5686f (accessed on 16 July 2025).
- Ghasemi, A.; Zahediasl, S.; Hosseini-Esfahani, F.; Azizi, F. Gender Differences in the Relationship between Serum Zinc Concentration and Metabolic Syndrome. Ann. Hum. Biol. 2014, 41, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Chawla, R.; Filippini, T.; Dutt, C.; Cilloni, S.; Loomba, R.; Bargellini, A.; Orsini, N.; Dhillon, K.S.; Whelton, P. Blood Pressure Levels and Hypertension Prevalence in a High Selenium Environment: Results from a Cross-Sectional Study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, H.; Kato, T.S.; Noh, J.Y.; Yuasa, S.; Kawamura, A.; Fukuda, K.; Aizawa, Y. Thyroid Hormone Plays an Important Role in Cardiac Function: From Bench to Bedside. Front. Physiol. 2021, 12, 606931. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A. Thyroid Hormone Action by Genomic and Nongenomic Molecular Mechanisms. Methods Mol. Biol. 2025, 2876, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Giammanco, M.; Di Liegro, C.M.; Schiera, G.; Di Liegro, I. Genomic and Non-Genomic Mechanisms of Action of Thyroid Hormones and Their Catabolite 3,5-Diiodo-L-Thyronine in Mammals. Int. J. Mol. Sci. 2020, 21, 4140. [Google Scholar] [CrossRef] [PubMed]
- Rehman, G.; Kumari, N.; Bano, F.; Tyagi, R.K. Thyroid Hormone Receptor Beta: Relevance in Human Health and Diseases. Endocr. Metab. Sci. 2023, 13, 100144. [Google Scholar] [CrossRef]
- Wilkenfeld, S.R.; Lin, C.; Frigo, D.E. Communication between Genomic and Non-Genomic Signaling Events Coordinate Steroid Hormone Actions. Steroids 2018, 133, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Tóthová, Z.; Šemeláková, M.; Solárová, Z.; Tomc, J.; Debeljak, N.; Solár, P. The Role of PI3K/AKT and MAPK Signaling Pathways in Erythropoietin Signalization. Int. J. Mol. Sci. 2021, 22, 7682. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Dutta, A.; Chakraborty, A. The Interaction of Oxidative Stress with MAPK, PI3/AKT, NF-κB, and DNA Damage Kinases Influences the Fate of γ-Radiation-Induced Bystander Cells. Arch. Biochem. Biophys. 2022, 725, 109302. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.I.; Sarmento-Ribeiro, A.B.; Gonçalves, A.C. Zinc: From Biological Functions to Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 4822. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yu, P.; Chan, W.N.; Xie, F.; Zhang, Y.; Liang, L.; Leung, K.T.; Lo, K.W.; Yu, J.; Tse, G.M.K.; et al. Cellular Zinc Metabolism and Zinc Signaling: From Biological Functions to Diseases and Therapeutic Targets. Sig. Transduct. Target. Ther. 2024, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27521067/ (accessed on 11 August 2024).
- Sun, J.; Yao, L.; Fang, Y.; Yang, R.; Chen, Y.; Yang, K.; Tian, L. Relationship between Subclinical Thyroid Dysfunction and the Risk of Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Int. J. Endocrinol. 2017, 2017, 8130796. [Google Scholar] [CrossRef] [PubMed]
- Klubo-Gwiezdzinska, J.; Wartofsky, L. Hashimoto Thyroiditis: An Evidence-Based Guide to Etiology, Diagnosis and Treatment. Pol. Arch. Intern. Med. 2022, 132, 16222. [Google Scholar] [CrossRef] [PubMed]
- Wrońska, K.; Hałasa, M.; Szczuko, M. The Role of the Immune System in the Course of Hashimoto’s Thyroiditis: The Current State of Knowledge. Int. J. Mol. Sci. 2024, 25, 6883. [Google Scholar] [CrossRef] [PubMed]
- Macvanin, M.T.; Gluvic, Z.; Zafirovic, S.; Gao, X.; Essack, M.; Isenovic, E.R. The Protective Role of Nutritional Antioxidants against Oxidative Stress in Thyroid Disorders. Front. Endocrinol. 2023, 13, 1092837. [Google Scholar] [CrossRef] [PubMed]
- Bel Lassen, P.; Kyrilli, A.; Lytrivi, M.; Corvilain, B. Graves’ Disease, Multinodular Goiter and Subclinical Hyperthyroidism. Ann. D’endocrinologie 2019, 80, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Danailova, Y.; Velikova, T.; Nikolaev, G.; Mitova, Z.; Shinkov, A.; Gagov, H.; Konakchieva, R. Nutritional Management of Thyroiditis of Hashimoto. Int. J. Mol. Sci. 2022, 23, 5144. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Rayman, M.P. Multiple Nutritional Factors and the Risk of Hashimoto’s Thyroiditis. Thyroid 2017, 27, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Giovinazzo, S.; Barbalace, M.C.; Cristani, M.; Alibrandi, A.; Vicchio, T.M.; Giuffrida, G.; Aguennouz, M.H.; Malaguti, M.; Angeloni, C.; et al. Influence of Dietary Habits on Oxidative Stress Markers in Hashimoto’s Thyroiditis. Thyroid 2021, 31, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, E.; Zegan, M.; Michota-Katulska, E. Zalecenia dietetyczne w niedoczynności tarczycy przy współwystępowaniu choroby Hashimoto. Bromatol. Chem. Toksykol. 2015, 48, 2. [Google Scholar]
- Kawicka, A.; Regulska-Ilow, B.; Regulska-Ilow, B. Metabolic Disorders and Nutritional Status in Autoimmune Thyroid Diseases. Postep. Hig. Med. Dosw. 2015, 69, 80–90. [Google Scholar] [CrossRef] [PubMed]
- The Importance of Nutritional Factors and Dietary Management of Hashimoto’s Thyroiditis—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/32588591/ (accessed on 16 August 2024).
- Kolasa, K. Niedoczynność Tarczycy–Opis Zaburzenia i Dietoterapia. In Wybrane Zagadnienia z Zakresu Bromatologii. Tom 2; Babicz, M., Kropiwiec-Domańska, K., Szymonowska, U., Eds.; Wydawnictwo Uniwersytetu Przyrodniczego w Lublinie: Lublin, Poland, 2022; pp. 63–74. [Google Scholar] [CrossRef]
- Woody, S. Running Head: DIET FOR HASHIMOTO’S THYROIDITIS Woody! Available online: https://files.achs.edu/resource/theses-and-capstone-projects/woody.pdf (accessed on 12 March 2025).
- WHO. Updates Guidelines on Fats and Carbohydrates. Available online: https://www.who.int/news/item/17-07-2023-who-updates-guidelines-on-fats-and-carbohydrates (accessed on 15 December 2024).
- Tzotzas, T.; Krassas, G.E.; Konstantinidis, T.; Bougoulia, M. Changes in Lipoprotein(a) Levels in Overt and Subclinical Hypothyroidism before and during Treatment. Thyroid 2000, 10, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.d.F.d.S.; Reuters, V.S.; Ferreira, M.M.; Almeida, C.P.; Reis, F.A.A.; Buescu, A.; Costa, A.J.L.; Vaisman, M. Lipid Profile in Different Degrees of Hypothyroidism and Effects of Levothyroxine Replacement in Mild Thyroid Failure. Transl. Res. 2008, 151, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Lipid Profile in Thyroid Dysfunction Patients|Semantic Scholar. Available online: https://www.semanticscholar.org/paper/Lipid-Profile-in-Thyroid-Dysfunction-Patients-Sangeeta-Singh/82fed1644815b34168d921d279cddc085e091c57 (accessed on 11 August 2024).
- Shulhai, A.-M.; Rotondo, R.; Petraroli, M.; Patianna, V.; Predieri, B.; Iughetti, L.; Esposito, S.; Street, M.E. The Role of Nutrition on Thyroid Function. Nutrients 2024, 16, 2496. [Google Scholar] [CrossRef] [PubMed]
- Ihnatowicz, P.; Drywień, M.; Wątor, P.; Wojsiat, J. The Importance of Nutritional Factors and Dietary Management of Hashimoto’s Thyroiditis. Ann. Agric. Environ. Med. 2020, 27, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xu, M.; Wu, M.; Wang, X.; Li, F.; Zhang, J.; You, L.; Pan, X.; Feng, W.; Wu, J.; et al. Obesity Is Associated with Subclinical Hypothyroidism in the Presence of Thyroid Autoantibodies: A Cross-Sectional Study. BMC Endocr. Disord. 2022, 22, 94. [Google Scholar] [CrossRef] [PubMed]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Valea, A.; Carsote, M.; Moldovan, C.; Georgescu, C. Chronic Autoimmune Thyroiditis and Obesity. Arch. Balk. Med. Union. 2018, 53, 64–69. [Google Scholar]
- Szwajkosz, K.; Zwolak, A.; Dudzińska, M.; Świrska, J.; Oszywa-Chabros, A.; Wawryniuk, A.; Łuczyk, R.; Daniluk, J. Nadwaga i Otyłość a Niedoczynność Tarczycy= Overweight and Obesity in Hypothyroidism. J. Educ. Health Sport. 2016, 6, 419–428. [Google Scholar] [CrossRef]
- Babić Leko, M.; Gunjača, I.; Pleić, N.; Zemunik, T. Environmental Factors Affecting Thyroid-Stimulating Hormone and Thyroid Hormone Levels. Int. J. Mol. Sci. 2021, 22, 6521. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.L.; Chernock, R.D.; Mansour, M. Environmental Factors and Anatomic Pathology of the Thyroid Gland: Review of Literature. Diagn. Histopathol. 2020, 26, 207–215. [Google Scholar] [CrossRef]
- Karbownik-Lewińska, M.; Stępniak, J.; Iwan, P.; Lewiński, A. Iodine as a Potential Endocrine Disruptor—A Role of Oxidative Stress. Endocrine 2022, 78, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Whalen, K.A.; Judd, S.; McCullough, M.L.; Flanders, W.D.; Hartman, T.J.; Bostick, R.M. Paleolithic and Mediterranean Diet Pattern Scores Are Inversely Associated with All-Cause and Cause-Specific Mortality in Adults. J. Nutr. 2017, 147, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Bechthold, A.; Boeing, H.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Iqbal, K.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; Schlesinger, S.; et al. Food Groups and Risk of Coronary Heart Disease, Stroke and Heart Failure: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 1071–1090. [Google Scholar] [CrossRef] [PubMed]
- Ventriglio, A.; Sancassiani, F.; Contu, M.P.; Latorre, M.; Di Salvatore, M.; Fornaro, M.; Bhugra, D. Mediterranean Diet and Its Benefits on Health and Mental Health: A Literature Review. Clin. Pr. Epidemiol. Ment. Health 2020, 16, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Snetselaar, L.G.; de Jesus, J.M.; DeSilva, D.M.; Stoody, E.E. Dietary Guidelines for Americans, 2020–2025: Understanding the Scientific Process, Guidelines, and Key Recommendations. Nutr. Today 2021, 56, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Roser, M. Micronutrient Deficiency.-References-Scientific Research Publishing. 2017. Available online: https://www.scirp.org/reference/referencespapers?referenceid=2913858 (accessed on 16 July 2025).
- Babiker, A.; Alawi, A.; Al Atawi, M.; Al Alwan, I. The Role of Micronutrients in Thyroid Dysfunction. Sudan. J. Paediatr. 2020, 20, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.R.S.M.; Freire, F.L.d.A.; Dantas-Komatsu, R.C.S.; Silva, E.P.d.; Queiroz, S.I.M.L.; de Lira, N.R.D.; Diniz, R.V.Z.; Lima, S.C.V.C.; Pedrosa, L.F.C.; Lopes, M.M.G.D.; et al. Lack of Association between Inadequate Micronutrient Intake and Prognosis in Outpatients with Heart Failure. Nutrients 2022, 14, 788. [Google Scholar] [CrossRef] [PubMed]
- Larsen, D.; Singh, S.; Brito, M. Thyroid, Diet, and Alternative Approaches. J. Clin. Endocrinol. Metab. 2022, 107, 2973–2981. [Google Scholar] [CrossRef] [PubMed]
- Guideline: Fortification of Food-Grade Salt with Iodine for the Prevention and Control of Iodine Deficiency Disorders; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, The Switzerland, 2014; ISBN 978-92-4-150792-9.
- Iodine: Biochemistry, Deficiency and Application in Clinical Nutrition. Available online: https://www.researchgate.net/publication/311419610_Iodine_Biochemistry_Deficiency_and_Application_in_Clinical_Nutrition (accessed on 11 August 2024).
- Tykarski, A.; Filipiak, K.J.; Rajzer, M. Zalecenia ESH 2023 dotyczące postępowania w nadciśnieniu tętniczym Próba komentarza na temat zmian w stosunku do zaleceń ESH/ESC 2018 i różnic w porównaniu z zaleceniami PTNT 2019. Nadciśnienie Tętnicze W Prakt. 2023, 9, 45–84. [Google Scholar]
- Kypridemos, C.; Guzman-Castillo, M.; Hyseni, L.; Hickey, G.L.; Bandosz, P.; Buchan, I.; Capewell, S.; O’Flaherty, M. Estimated Reductions in Cardiovascular and Gastric Cancer Disease Burden through Salt Policies in England: An IMPACTNCD Microsimulation Study. BMJ Open 2017, 7, e013791. [Google Scholar] [CrossRef] [PubMed]
- Zalecenia Dietetyczne Dotyczące Spożywania Jodu—W Poszukiwaniu Konsensusu Między Kardiologami a Endokrynologami|Pyka|Folia Cardiologica. Available online: https://journals.viamedica.pl/folia_cardiologica/article/view/61505 (accessed on 11 August 2024).
- Park, S.-J.; Chen, L.; Wallace, T.C.; Lee, H.-J. The Association between Iodine Intake and Thyroid Disease in Iodine-Replete Regions: The Korean Genome and Epidemiology Study. Nutr. Res. Pract. 2024, 19, e24. [Google Scholar]
- Jod-Jego Rola, Źródła Pokarmowe, Nadmiar i Niedobór. Available online: http://www.mp.pl/social/article/74618 (accessed on 11 August 2024).
- KARMAŃSKA, A. DZIAŁANIE WITAMINY D. Available online: https://publicum.umed.lodz.pl/docstore/download/AML6b51cc6ba25c44ccaadf201f63e58c81/MONOGRAFIA_12_2021.pdf (accessed on 16 August 2024).
- Kunachowicz, H.; Iwanow, K.; Ratkovska, B.; Przygoda, B.; Nadolna, I. Nowelizacja tabel składu i wartości odżywczej żywności. Zmiany w ciągu ostatnich lat. Zyw. Człowieka Metab. Suplement. 2005, 32. Available online: http://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-496f8886-4687-45d9-a915-0bade066e419 (accessed on 19 August 2024).
- Liontiris, M.I.; Mazokopakis, E.E. A Concise Review of Hashimoto Thyroiditis (HT) and the Importance of Iodine, Selenium, Vitamin D and Gluten on the Autoimmunity and Dietary Management of HT Patients.Points That Need More Investigation. Hell. J. Nucl. Med. 2017, 20, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kunachowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Tabele Składu i Wartości Odżywczej Żywności, 2nd ed.; PZWL Wydawnictwo Lekarskie: Warszawa, Poland, 2017; Volume 2, ISBN 978-83-200-6258-8. [Google Scholar]
- Zimmermann, M.B.; Köhrle, J. The Impact of Iron and Selenium Deficiencies on Iodine and Thyroid Metabolism: Biochemistry and Relevance to Public Health. Thyroid 2002, 12, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Laurberg, P.; Andersen, S.; Pedersen, I.B.; Knudsen, N.; Carlé, A. Prevention of Autoimmune Hypothyroidism by Modifying Iodine Intake and the Use of Tobacco and Alcohol Is Manoeuvring between Scylla and Charybdis. Hormones 2013, 12, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Baczyk, M.; Ruchała, M.; Pisarek, M.; Pietz, L.; Wrotkowska, E.; Wojewoda-Korbelak, M.; Dziubandowska, A.; Gembicki, M.; Sowiński, J. Iodine prophylaxis in children population on the Wielkopolska Region area from year 1992 to 2005. Endokrynol. Pol. 2006, 57, 110–115. [Google Scholar] [PubMed]
- von Haehling, S.; Jankowska, E.A.; van Veldhuisen, D.J.; Ponikowski, P.; Anker, S.D. Iron Deficiency and Cardiovascular Disease. Nat. Rev. Cardiol. 2015, 12, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.F.; Zhang, J.; Pellicori, P.; Dicken, B.; Dierckx, R.; Shoaib, A.; Wong, K.; Rigby, A.; Goode, K.; Clark, A.L. Prevalence and Outcomes of Anemia and Hematinic Deficiencies in Patients With Chronic Heart Failure. JAMA Cardiol. 2016, 1, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Haddad, S.; Wang, Y.; Galy, B.; Korf-Klingebiel, M.; Hirsch, V.; Baru, A.M.; Rostami, F.; Reboll, M.R.; Heineke, J.; Flögel, U.; et al. Iron-Regulatory Proteins Secure Iron Availability in Cardiomyocytes to Prevent Heart Failure. Eur. Heart J. 2017, 38, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Melenovsky, V.; Petrak, J.; Mracek, T.; Benes, J.; Borlaug, B.A.; Nuskova, H.; Pluhacek, T.; Spatenka, J.; Kovalcikova, J.; Drahota, Z.; et al. Myocardial Iron Content and Mitochondrial Function in Human Heart Failure: A Direct Tissue Analysis. Eur. J. Heart Fail. 2017, 19, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F. Recent Developments in Trace Element Metabolism and Function: Newer Roles of Selenium in Nutrition. J. Nutr. 1989, 119, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Bastola, M.M.; Locatis, C.; Maisiak, R.; Fontelo, P. Selenium, Copper, Zinc and Hypertension: An Analysis of the National Health and Nutrition Examination Survey (2011–2016). BMC Cardiovasc. Disord. 2020, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Żywienia Dla Populacji Polski i Ich Zastosowanie; Narodowy Instytut Zdrowia Publicznego-Państwowy Zakład Higieny: Warsaw, Poland, 2020; Volume 83. [Google Scholar]
- Huwiler, V.V.; Maissen-Abgottspon, S.; Stanga, Z.; Mühlebach, S.; Trepp, R.; Bally, L.; Bano, A. Selenium Supplementation in Patients with Hashimoto Thyroiditis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Thyroid 2024, 34, 295–313. [Google Scholar] [CrossRef] [PubMed]
- National Agricultural Library. Available online: https://www.nal.usda.gov/ (accessed on 11 August 2024).
- Wang, J.; Lv, S.; Chen, G.; Gao, C.; He, J.; Zhong, H.; Xu, Y. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients 2015, 7, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Gać, P.; Czerwińska, K.; Macek, P.; Jaremków, A.; Mazur, G.; Pawlas, K.; Poręba, R. The Importance of Selenium and Zinc Deficiency in Cardiovascular Disorders. Env. Toxicol. Pharmacol. 2021, 82, 103553. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Alehagen, U.; Aaseth, J.O. Selenium—A Trace Element of Clinical Significance. Tidsskr. Den Nor. Legeforening 2020, 140. [Google Scholar] [CrossRef]
- Fávaro, D.I.; Hui, M.L.; Cozzolino, S.M.; Maihara, V.A.; Armelin, M.J.; Vasconcellos, M.B.; Yuyama, L.K.; Boaventura, G.T.; Tramonte, V.L. Determination of Various Nutrients and Toxic Elements in Different Brazilian Regional Diets by Neutron Activation Analysis. J. Trace Elem. Med. Biol. 1997, 11, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, R.A.; Sisson, S.B.; Baldwin, J.D.; Hord, N.; Eliot, K.; Anderson, L.; Gowin, M.J.; Scott, B.D.; Wortham, D. Comparison of Traditional and Intensive Cardiac Rehabilitation on Dietary Behavior and Clinical Risk Factor Outcomes: Secondary Analysis Research. J. Cardiopulm. Rehabil. Prev. 2025, 45, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Socha, K.; Dziemianowicz, M.; Omeljaniuk, W.J.; Soroczyńska, J.; Borawska, M.H. Nawyki Żywieniowe a Stężenie Selenu w Surowicy u Pacjentów z Chorobą Hashimoto. Probl. Hig. Epidemiol. 2012, 93, 824–827. [Google Scholar]
- Espinosa-Salas, S.; Gonzalez-Arias, M. Nutrition: Macronutrient Intake, Imbalances, and Interventions. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Zhou, Q.; Xue, S.; Zhang, L.; Chen, G. Trace Elements and the Thyroid. Front Endocrinol 2022, 13, 904889. [Google Scholar] [CrossRef] [PubMed]
- Blasig, S.; Kühnen, P.; Schuette, A.; Blankenstein, O.; Mittag, J.; Schomburg, L. Positive Correlation of Thyroid Hormones and Serum Copper in Children with Congenital Hypothyroidism. J. Trace Elem. Med. Biol. 2016, 37, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Maouche, N.; Meskine, D.; Alamir, B.; Koceir, E.-A. Trace Elements Profile Is Associated with Insulin Resistance Syndrome and Oxidative Damage in Thyroid Disorders: Manganese and Selenium Interest in Algerian Participants with Dysthyroidism. J. Trace Elem. Med. Biol. 2015, 32, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Stojsavljević, A.; Trifković, J.; Rasić-Milutinović, Z.; Jovanović, D.; Bogdanović, G.; Mutić, J.; Manojlović, D. Determination of Toxic and Essential Trace Elements in Serum of Healthy and Hypothyroid Respondents by ICP-MS: A Chemometric Approach for Discrimination of Hypothyroidism. J. Trace Elem. Med. Biol. 2018, 48, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Hess, S.Y.; Zimmermann, M.B. The Effect of Micronutrient Deficiencies on Iodine Nutrition and Thyroid Metabolism. Int. J. Vitam. Nutr. Res. 2004, 74, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Sobiecki, J.G. Vegetarianism and colorectal cancer risk in a low-selenium environment: Effect modification by selenium status? A possible factor contributing to the null results in British vegetarians. Eur. J. Nutr. 2017, 56, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Betsy, A.; Binitha, M.P.; Sarita, S. Zinc Deficiency Associated with Hypothyroidism: An Overlooked Cause of Severe Alopecia. Int. J. Trichology 2013, 5, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Stolińska, H.; Wolańska, D. Nutrients Important in Hypothyroidism. Żyw. Czł. Metabol. 2012, 39, 221–231. (In Polish) [Google Scholar]
- Błażewicz, A.; Wiśniewska, P.; Skórzyńska-Dziduszko, K. Selected Essential and Toxic Chemical Elements in Hypothyroidism—A Literature Review (2001–2021). Int. J. Mol. Sci. 2021, 22, 10147. [Google Scholar] [CrossRef] [PubMed]
- Beserra, J.B.; Morais, J.B.S.; Severo, J.S.; Cruz, K.J.C.; de Oliveira, A.R.S.; Henriques, G.S.; do Nascimento Marreiro, D. Relation Between Zinc and Thyroid Hormones in Humans: A Systematic Review. Biol. Trace Elem. Res. 2021, 199, 4092–4100. [Google Scholar] [CrossRef] [PubMed]
- Topuzoglu, G.; Erbay, A.R.; Karul, A.B.; Yensel, N. Concentrations of Copper, Zinc, and Magnesium in Sera from Patients with Idiopathic Dilated Cardiomyopathy. Biol. Trace Elem. Res. 2003, 95, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Eshak, E.S.; Iso, H.; Yamagishi, K.; Maruyama, K.; Umesawa, M.; Tamakoshi, A. Associations between Copper and Zinc Intakes from Diet and Mortality from Cardiovascular Disease in a Large Population-Based Prospective Cohort Study. J. Nutr. Biochem. 2018, 56, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Soinio, M.; Marniemi, J.; Laakso, M.; Pyorala, K.; Lehto, S.; Ronnemaa, T. Serum Zinc Level and Coronary Heart Disease Events in Patients with Type 2 Diabetes. Diabetes Care 2007, 30, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.S.; Koh, H.; Nam, K.H.; Lee, S.; Kim, J.; Lee, C.; Yun, H.-R.; Park, J.T.; Kang, E.W.; Chang, T.I.; et al. Alcohol Consumption and Progression of Chronic Kidney Disease: Results from the Korean Cohort Study for Outcome in Patients with Chronic Kidney Disease. Mayo Clin. Proc. 2020, 95, 293–305. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur, M.; Szymańska, M.; Malik, A.; Szlasa, W.; Popiołek-Kalisz, J. Nutrition and Micronutrient Interactions in Autoimmune Thyroid Disorders: Implications for Cardiovascular Health. Pathophysiology 2025, 32, 37. https://doi.org/10.3390/pathophysiology32030037
Mazur M, Szymańska M, Malik A, Szlasa W, Popiołek-Kalisz J. Nutrition and Micronutrient Interactions in Autoimmune Thyroid Disorders: Implications for Cardiovascular Health. Pathophysiology. 2025; 32(3):37. https://doi.org/10.3390/pathophysiology32030037
Chicago/Turabian StyleMazur, Michał, Magdalena Szymańska, Agnieszka Malik, Wojciech Szlasa, and Joanna Popiołek-Kalisz. 2025. "Nutrition and Micronutrient Interactions in Autoimmune Thyroid Disorders: Implications for Cardiovascular Health" Pathophysiology 32, no. 3: 37. https://doi.org/10.3390/pathophysiology32030037
APA StyleMazur, M., Szymańska, M., Malik, A., Szlasa, W., & Popiołek-Kalisz, J. (2025). Nutrition and Micronutrient Interactions in Autoimmune Thyroid Disorders: Implications for Cardiovascular Health. Pathophysiology, 32(3), 37. https://doi.org/10.3390/pathophysiology32030037






