Post-COVID-19 Femoral Head Osteonecrosis Exhibits Mast Cell Clusters, Fibrosis, and Vascular Thrombosis: Key Pathological Mechanisms in Long COVID-19 Bone Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Object
2.2. Morphological Analysis
2.3. Morphometric Analysis
2.4. Statistical Analysis
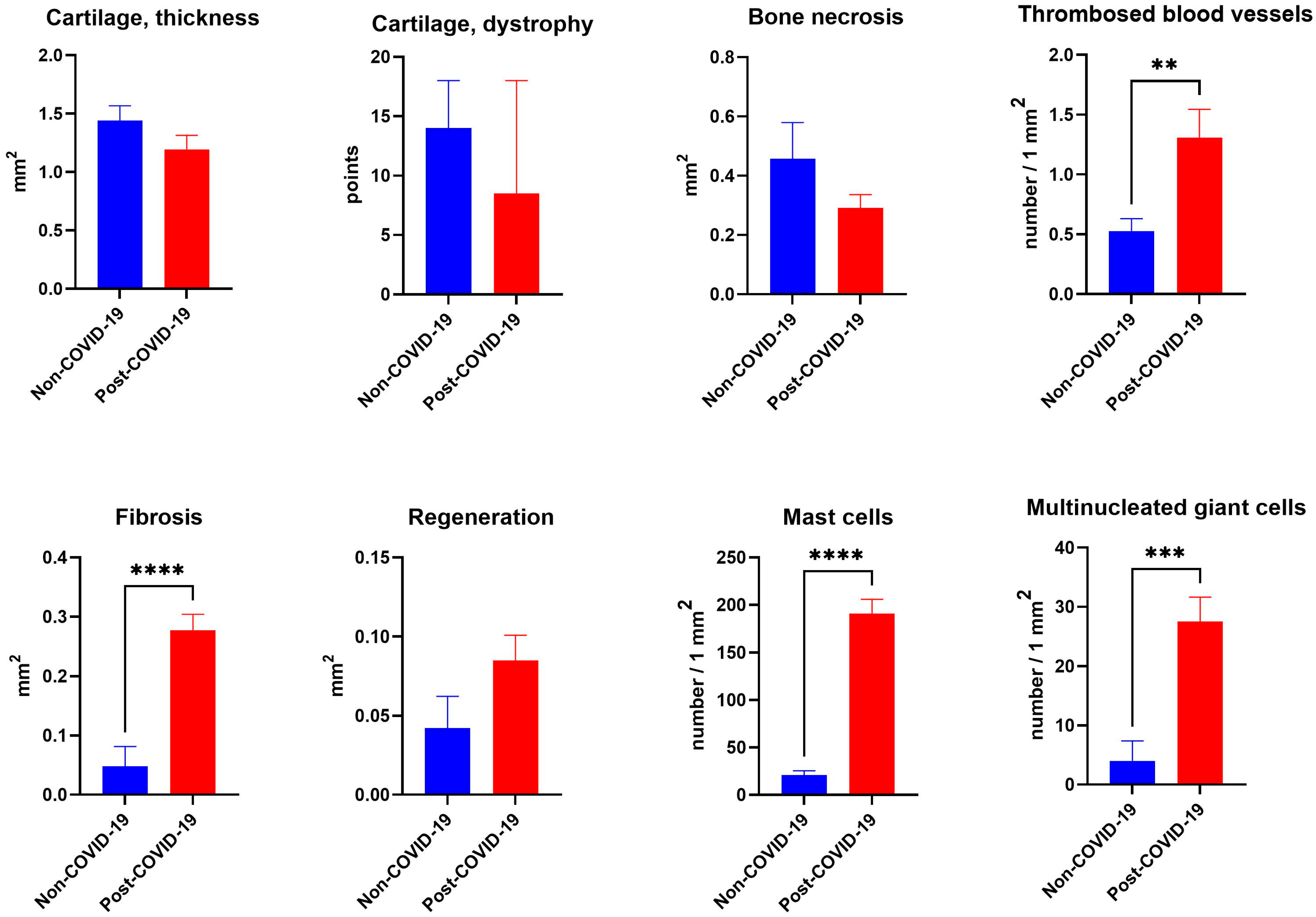
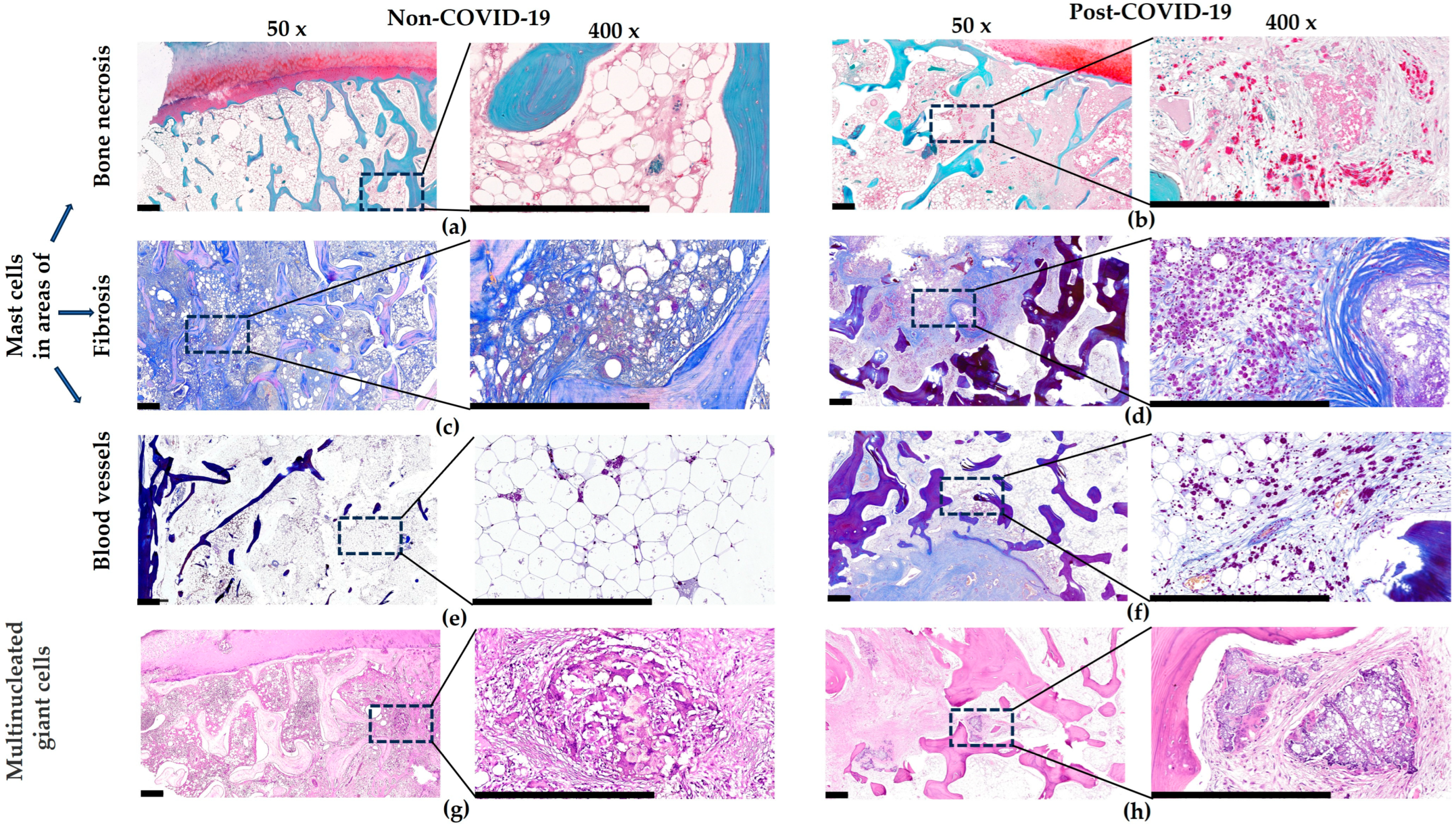
3. Results
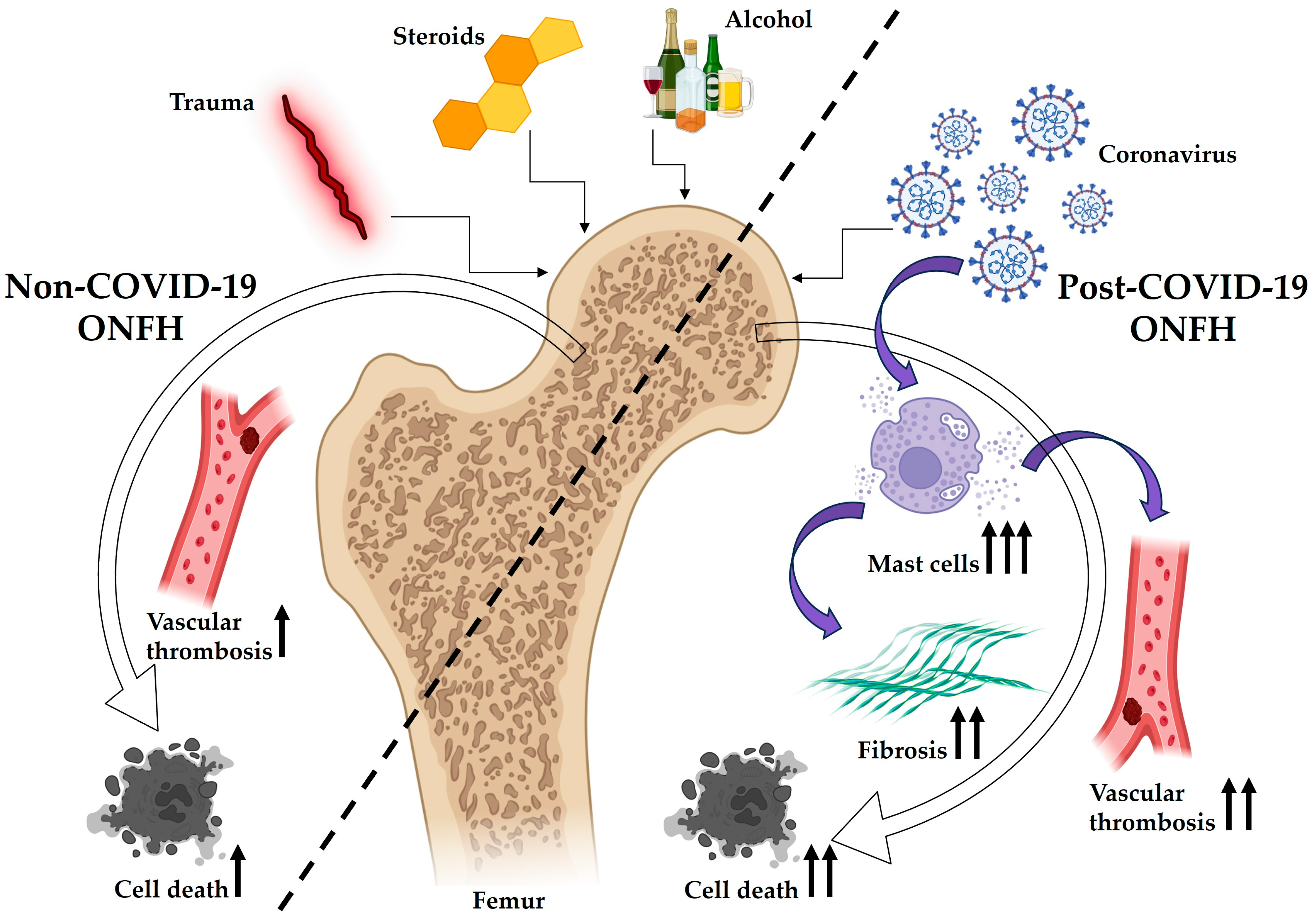
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ONFH | Osteonecrosis of the Femoral Head |
| CPA3 | Carboxypeptidase A3 |
| ACE2 | Angiotensin-Converting Enzyme 2 |
| HRH1 | Histamine Receptor H1 |
References
- George, G.; Lane, J.M. Osteonecrosis of the femoral head. JAAOS Glob. Res. Rev. 2022, 6, e21. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, C.; Meng, H.; Liu, G.; Li, H.; Gao, J.; Tian, H.; Peng, J. The role of structural deterioration and biomechanical changes of the necrotic lesion in collapse mechanism of osteonecrosis of the femoral head. Orthop. Surg. 2022, 14, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Konarski, W.; Poboży, T.; Konarska, K.; Śliwczyński, A.; Kotela, I.; Hordowicz, M.; Krakowiak, J. Osteonecrosis Related to Steroid and Alcohol Use—An Update on Pathogenesis. Healthcare 2023, 11, 1846. [Google Scholar] [CrossRef] [PubMed]
- Mustafin, R. Avascular Necrosis of Femoral Head in the Republic of Bashkortostan: A Clinical and Epidemiological Study. Creat. Surg. Oncol. 2020, 10, 100–107. [Google Scholar] [CrossRef]
- Li, L.; Zhao, S.; Leng, Z.; Chen, S.; Shi, Y.; Shi, L.; Li, J.; Mao, K.; Tang, H.; Meng, B. Pathological mechanisms and related markers of steroid-induced osteonecrosis of the femoral head. Ann. Med. 2024, 56, 2416070. [Google Scholar] [CrossRef] [PubMed]
- Odarchenko, D.I.; Dzyuba, G.G.; Erofeev, S.A.; Kuznetsov, N.K. Problems of diagnostics and treatment of aseptic necrosis of the femoral head in modern traumatology and orthopedics (literature review). Genius Orthop. 2021, 27, 270–276. [Google Scholar]
- Goncharov, E.N.; Koval, O.A.; Nikolaevich Bezuglov, E.; Aleksandrovich Vetoshkin, A.; Gavriilovich Goncharov, N.; Encarnación Ramirez, M.D.J.; Montemurro, N. Conservative treatment in avascular necrosis of the femoral head: A systematic review. Med. Sci. 2024, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Bialik, V.; Makarov, M.; Bialik, E.; Makarov, S.; Karateev, A.; Nesterenko, V.; Chernikova, A.; Kapitonov, D.; Gorelova, A. Avascular necrosis of bone tissue: Definition, epidemiology, types, risk factors, pathogenesis of the disease. Analytical review of the literature. Sci. Pract. Rheumatol. 2023, 61, 220–235. [Google Scholar] [CrossRef]
- Ko, Y.-S.; Ha, J.H.; Park, J.-W.; Lee, Y.-K.; Kim, T.-Y.; Koo, K.-H. Updating osteonecrosis of the femoral head. Hip Pelvis 2023, 35, 147. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Miao, Y.; Liu, K.; Zhu, B.; Xue, F.; Yin, J.; Zou, J.; Li, G.; Zhang, C.; Feng, Y. Less sclerotic microarchitecture pattern with increased bone resorption in glucocorticoid-associated osteonecrosis of femoral head as compared to alcohol-associated osteonecrosis of femoral head. Front. Endocrinol. 2023, 14, 1133674. [Google Scholar] [CrossRef] [PubMed]
- Hines, J.T.; Jo, W.-L.; Cui, Q.; Mont, M.A.; Koo, K.-H.; Cheng, E.Y.; Goodman, S.B.; Ha, Y.-C.; Hernigou, P.; Jones, L.C. Osteonecrosis of the femoral head: An updated review of ARCO on pathogenesis, staging and treatment. J. Korean Med. Sci. 2021, 36, e177. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Wang, Y.; Chen, Y.; Liu, Y.; Ma, M.; Ma, Z.; Wang, C.; Zeng, H.; Xue, L.; Yue, C. The dynamic feature of macrophage M1/M2 imbalance facilitates the progression of non-traumatic osteonecrosis of the femoral head. Front. Bioeng. Biotechnol. 2022, 10, 912133. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, B.; Sharma, K.; Kumar, N.; Mastana, S.; Singh, P. A molecular troika of angiogenesis, coagulopathy and endothelial dysfunction in the pathology of avascular necrosis of femoral head: A comprehensive review. Cells 2023, 12, 2278. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, S.; Zhang, B.; Dai, M. Relationship of idiopathic femoral head necrosis with blood lipid metabolism and coagulation function: A propensity score-based analysis. Front. Surg. 2023, 9, 938565. [Google Scholar] [CrossRef] [PubMed]
- Vicaş, R.M.; Bodog, F.D.; Fugaru, F.O.; Grosu, F.; Badea, O.; Lazăr, L.; Cevei, M.L.; Nistor-Cseppento, C.D.; Beiuşanu, G.C.; Holt, G. Histopathological and immunohistochemical aspects of bone tissue in aseptic necrosis of the femoral head. Rom. J. Morphol. Embryol. 2021, 61, 1249. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Zhang, A.; Qiu, B.; Chen, Y.; Kong, M. Femoral neck fracture after femoral head necrosis: A case report and review of the literature. BMC Musculoskelet. Disord. 2023, 24, 853. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, X.; Qian, H.; Liu, J.; Liu, G.; Wang, R.; Liu, R. Microarchitecture Alternations of Osteochondral Junction in Patients with Osteonecrosis of the Femoral Head. Calcif. Tissue Int. 2024, 114, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Polyakova, S.; Papichev, E.; Hakhverdyan, Y.; Sivordova, L.; Zavodovsky, B. New coronavirus infection—Direct and indirect impact on patients with diseases of the musculoskeletal system and connective tissue. Mod. Probl. Sci. Educ. 2021, 6, 176. [Google Scholar] [CrossRef]
- Harris, A.; Creecy, A.; Awosanya, O.D.; McCune, T.; Ozanne, M.V.; Toepp, A.J.; Kacena, M.A.; Qiao, X. SARS-CoV-2 and its multifaceted impact on bone health: Mechanisms and clinical evidence. Curr. Osteoporos. Rep. 2024, 22, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Shetty, G.M. Double Trouble—COVID-19 and the Widespread Use of Corticosteroids: Are We Staring at an Osteonecrosis Epidemic? Indian J. Orthop. 2021, 56, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Maffulli, N.; Shukla, T.; D’Ambrosi, R.; Singla, M.; Vaish, A.; Vaishya, R. The pandemic is gone but its consequences are here to stay: Avascular necrosis following corticosteroids administration for severe COVID-19. J. Orthop. Surg. Res. 2024, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- Torgashin, A.N.; Rodionova, S.S. Osteonecrosis in patients recovering from COVID-19: Mechanisms, diagnosis, and treatment at early-stage disease. Traumatol. Orthop. Russ. 2022, 28, 128–137. [Google Scholar] [CrossRef]
- Nejadhosseinian, M.; Haerian, H.; Tabatabaie Nejad, M.; Sadeghi, K.; Aghaghazvini, L.; Alikhani, M.; Loghman, M.; Faezi, S.T. Who is the convict; COVID-19 or corticosteroid? Late onset avascular necrosis of hips after COVID-19. A case report with literature review. Int. J. Rheum. Dis. 2023, 26, 2069–2072. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, S.; Ojha, M.M.; Gamanagatti, S.; Nag, H.L.; Kumar, V. Is COVID-19 an independent risk factor for the development of avascular necrosis of the hip? A retrospective study to evaluate the factors associated with avascular necrosis of the hip in patients who had COVID-19 infection. Int. Orthop. 2024, 48, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Panin, M.A.; Petrosyan, A.S.; Hadjicharalambous, K.K.; Boiko, A.V. Avascular necrosis of the femoral head after COVID-19: A case series. Traumatol. Orthop. Russ. 2022, 28, 110–117. [Google Scholar] [CrossRef]
- Klimenko, A.A.; Demidova, O.; Andriyashkina, Y.; Babadaeva, N.; Kondrashov, A.A.; Sahakyan, Y. Multifocal osteonecrosis as a consequence of the new coronavirus infection. Sci. Pract. Rheumatol. 2023, 61, 34–41. [Google Scholar] [CrossRef]
- Velchov, V.; Georgiev, P.; Tserovski, S.; Tsenkov, T.; Alexiev, V. Corticosteroid-associated avascular necrosis of the femoral head in patients with severe COVID-19: A single-center study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2023, 29, e940965. [Google Scholar] [CrossRef] [PubMed]
- Sakellariou, E.; Argyropoulou, E.; Karampinas, P.; Galanis, A.; Varsamos, I.; Giannatos, V.; Vasiliadis, E.; Kaspiris, A.; Vlamis, J.; Pneumaticos, S. A comprehensive review of COVID-19-infection-and steroid-treatment-associated bone avascular necrosis: A multi-study analysis. Diagnostics 2024, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Moody, H.R.; Heard, B.J.; Frank, C.B.; Shrive, N.G.; Oloyede, A.O. Investigating the potential value of individual parameters of histological grading systems in a sheep model of cartilage damage: The Modified Mankin method. J. Anat. 2012, 221, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Wöhrl, S.; Bielory, L. Mast cell biology at molecular level: A comprehensive review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.Y.; Tergaonkar, V.; Kumar, A.P.; Ahn, K.S. Mast cells: Therapeutic targets for COVID-19 and beyond. IUBMB Life 2021, 73, 1278–1292. [Google Scholar] [CrossRef] [PubMed]
- Gutowski, Ł.; Kanikowski, S.; Formanowicz, D. Mast cell involvement in the pathogenesis of selected musculoskeletal diseases. Life 2023, 13, 1690. [Google Scholar] [CrossRef] [PubMed]
- Ragipoglu, D.; Dudeck, A.; Haffner-Luntzer, M.; Voss, M.; Kroner, J.; Ignatius, A.; Fischer, V. The role of mast cells in bone metabolism and bone disorders. Front. Immunol. 2020, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Suurmond, J.; van der Velden, D.; Kuiper, J.; Bot, I.; Toes, R.E. Mast cells in rheumatic disease. Eur. J. Pharmacol. 2016, 778, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Kasprick, A.; Petersen, F. Revisiting the role of mast cells in autoimmunity. Autoimmun. Rev. 2015, 14, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, K.; Zuo, P.; Liu, Y.; Zhang, M.; Xie, S.; Zhang, H.; Chen, X.; Liu, C. Early decrease in blood platelet count is associated with poor prognosis in COVID-19 patients—Indications for predictive, preventive, and personalized medical approach. Epma J. 2020, 11, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Budnevsky, A.V.; Avdeev, S.N.; Kosanovic, D.; Ovsyannikov, E.S.; Savushkina, I.A.; Alekseeva, N.G.; Feigelman, S.N.; Shishkina, V.V.; Filin, A.A.; Esaulenko, D.I. Involvement of Mast Cells in the Pathology of COVID-19: Clinical and Laboratory Parallels. Cells 2024, 13, 711. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-B.; Zhu, S.-T.; Huang, X.-S.; Wang, X.-Y.; Wu, M.-L.; Li, X.; Liu, F.-L.; Chen, L.; Zheng, Y.-T.; Wang, J.-H. Mast cell degranulation-triggered by SARS-CoV-2 induces tracheal-bronchial epithelial inflammation and injury. Virol. Sin. 2024, 39, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Preza, Y.G.; Martínez-Martínez, R.; Meixueiro-Calderón, C.; Hernández, U.M.; Retana, E.A.; Ponce-Regalado, M.D.; Gamboa-Domínguez, A.; León-Contreras, J.C.; Muñoz-Cruz, S.; Hernández-Pando, R. Mast Cell Carboxypeptidase A3 Is Associated with Pulmonary Fibrosis Secondary to COVID-19. Int. J. Mol. Sci. 2024, 25, 12258. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cells and collagen fibrillogenesis. Histochem. Cell Biol. 2020, 154, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Wygrecka, M.; Dahal, B.; Kosanovic, D.; Petersen, F.; Taborski, B.; Preissner, K.; Schermuly, R.; Markart, P. Mast cells and fibroblasts work in concert to aggravate pulmonary fibrosis: Role of transmembrane stem cell factor (SCF) and PAR-2/PKCalpha/Raf-1/p44/42 signaling pathway. Pneumologie 2013, 67, V302. [Google Scholar] [CrossRef]
- Tchougounova, E.; Lundequist, A.; Fajardo, I.; Winberg, J.-O.; Åbrink, M.; Pejler, G. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J. Biol. Chem. 2005, 280, 9291–9296. [Google Scholar] [CrossRef] [PubMed]
- Overed-Sayer, C.; Rapley, L.; Mustelin, T.; Clarke, D.L. Are mast cells instrumental for fibrotic diseases? Front. Pharmacol. 2014, 4, 174. [Google Scholar] [CrossRef] [PubMed]
- Metsärinne, K.P.; Vehmaan-Kreula, P.; Kovanen, P.T.; Saijonmaa, O.; Baumann, M.; Wang, Y.; Nyman, T.; Fyhrquist, F.Y.; Eklund, K.K. Activated mast cells increase the level of endothelin-1 mRNA in cocultured endothelial cells and degrade the secreted Peptide. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Lundequist, A.; Tchougounova, E.; Åbrink, M.; Pejler, G. Cooperation between mast cell carboxypeptidase A and the chymase mouse mast cell protease 4 in the formation and degradation of angiotensin II. J. Biol. Chem. 2004, 279, 32339–32344. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.; D’Orléans-Juste, P.; Giaid, A. Potential role of endothelin-1 in pulmonary fibrosis: From the bench to the clinic. Am. J. Respir. Cell Mol. Biol. 2010, 42, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Uhal, B.D.; Li, X.; Piasecki, C.C.; Molina-Molina, M. Angiotensin signalling in pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2012, 44, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Papp, M.; Li, X.; Zhuang, J.; Wang, R.; Uhal, B.D. Angiotensin receptor subtype AT1 mediates alveolar epithelial cell apoptosis in response to ANG II. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2002, 282, L713–L718. [Google Scholar] [CrossRef] [PubMed]
- Krysko, O.; Bourne, J.H.; Kondakova, E.; Galova, E.A.; Whitworth, K.; Newby, M.L.; Bachert, C.; Hill, H.; Crispin, M.; Stamataki, Z. Severity of SARS-CoV-2 infection is associated with high numbers of alveolar mast cells and their degranulation. Front. Immunol. 2022, 13, 968981. [Google Scholar] [CrossRef] [PubMed]
- Motta Junior, J.d.S.; Miggiolaro, A.F.R.d.S.; Nagashima, S.; De Paula, C.B.V.; Baena, C.P.; Scharfstein, J.; De Noronha, L. Mast cells in alveolar septa of COVID-19 patients: A pathogenic pathway that may link interstitial edema to immunothrombosis. Front. Immunol. 2020, 11, 574862. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-L.; Xie, C.; Li, X.; Sun, J.; Zhao, J.; Wang, J.-H. Mast cell activation triggered by SARS-CoV-2 causes inflammation in brain microvascular endothelial cells and microglia. Front. Cell. Infect. Microbiol. 2024, 14, 1358873. [Google Scholar] [CrossRef] [PubMed]
- Silva-Aguiar, R.P.; Peruchetti, D.B.; Rocco, P.R.M.; Schmaier, A.H.; e Silva, P.M.R.; Martins, M.A.; Carvalho, V.F.; Pinheiro, A.A.S.; Caruso-Neves, C. Role of the renin-angiotensin system in the development of severe COVID-19 in hypertensive patients. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 319, L596–L602. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.E8. [Google Scholar] [CrossRef] [PubMed]
- Tsilioni, I.; Theoharides, T.C. Recombinant SARS-CoV-2 spike protein stimulates secretion of chymase, tryptase, and IL-1β from human mast cells, augmented by IL-33. Int. J. Mol. Sci. 2023, 24, 9487. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Suzuki, Y.; Takemasa, E.; Watanabe, R.; Mogi, M. Mast cells promote viral entry of SARS-CoV-2 via formation of chymase/spike protein complex. Eur. J. Pharmacol. 2022, 930, 175169. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liu, X.; Ou, H.; Li, X.; Liu, R.; Lv, X.; Xiao, S.; Hu, M.; Liang, T.; Chen, T. The histamine receptor H1 acts as an alternative receptor for SARS-CoV-2. MBio 2024, 15, e01088–01024. [Google Scholar] [CrossRef] [PubMed]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: The hunt for new therapeutic targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef] [PubMed]
- Praetzel, R.; Kepley, C. Human Lung Mast Cells as a Possible Reservoir for Coronavirus: A Novel Unrecognized Mechanism for SARS-CoV-2 Immune-Mediated Pathology. Int. J. Mol. Sci. 2024, 25, 6511. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Sattui, S.E.; van der Geest, K.S.; Brouwer, E.; Conway, R.; Putman, M.S.; Robinson, P.C.; Mackie, S.L. Giant cell arteritis and COVID-19: Similarities and discriminators. A systematic literature review. J. Rheumatol. 2021, 48, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Torres, V.; Soriano, V.; Calderón-Parra, J.; Martínez-Urbistondo, M.; Treviño, A.; de San Vicente, Z.; de Mendoza, C.; Ruiz-Irastorza, G. Increased incidence of giant cell arteritis and associated stroke during the COVID-19 pandemic in Spain: A nation-wide population study. Autoimmun. Rev. 2023, 22, 103341. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Houshmand, G.; Taghavi, S.; Kamali, M.; Faraji, M.; Naderi, N. Giant cell myocarditis following COVID-19 successfully treated by immunosuppressive therapy. Clin. Case Rep. 2022, 10, e6196. [Google Scholar] [CrossRef] [PubMed]
- Bollano, E.; Polte, C.L.; Mäyränpää, M.I.; Oldfors, A.; Bergh, N.; Lehtonen, J.; Kandolin, R. Cardiac sarcoidosis and giant cell myocarditis after COVID-19 infection. ESC Heart Fail. 2022, 9, 4298–4303. [Google Scholar] [CrossRef] [PubMed]
- Wenzhong, L.; Hualan, L. COVID-19: The CaMKII-like system of S protein drives membrane fusion and induces syncytial multinucleated giant cells. Immunol. Res. 2021, 69, 496–519. [Google Scholar] [CrossRef] [PubMed]
- Seitz, S.; Zustin, J.; Amling, M.; Rüther, W.; Niemeier, A. Massive accumulation of osteoclastic giant cells in rapid destructive hip disease. J. Orthop. Res. 2014, 32, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J.; Owen, D.; Millan, M. Granulomatous osteonecrosis in Crohn’s disease. Can. J. Gastroenterol. Hepatol. 2000, 14, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Boss, J.H.; Misselevich, I.; Norman, D.; Zinman, C. Giant-cell reparative granuloma-like lesion in the femoral heads of rats during the early repair phase of osteonecrosis. Eur. J. Orthop. Surg. Traumatol. 2004, 14, 7–9. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, Y.; Qi, Z.; Zho, J. The formation and function of the sclerosis rim in the femoral head: A biomechanical point of view. Med. Eng. Phys. 2015, 37, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Xie, L.; Chu, F. A sclerotic rim provides mechanical support for the femoral head in osteonecrosis. Orthopedics 2015, 38, e374–e379. [Google Scholar] [CrossRef] [PubMed]


| Non-COVID-19 (n = 47) M (SD) | Post-COVID-19 (n = 41) M (SD) | p-Value (One-Way ANOVA) | |
|---|---|---|---|
| Age, years | 53.7 (9.3) | 51.2 (10.6) | 0.907 |
| Gender, % | |||
| Male | 63.2 | 58.6 | 0.522 |
| Female | 36.8 | 41.4 | |
| BMI, kg/m2 | 29.7 (5.2) | 28.6 (2.7) | 0.986 |
| Etiologies of ONFH, % | |||
| tONFH | 45.6 | — | |
| sONFH | 29.2 | ||
| aONFH | 6.7 | ||
| iONFH | 18.5 | ||
| ONFH duration, months | 25.4 (7.2) | 14.1 (9.2) | 0.03 |
| Interval from COVID-19 and onset of ONFH, months | — | 11.3 (10.1) | |
| Steroid therapy for COVID-19, % | |||
| Yes | — | 16.8 | |
| No | 83.2 | ||
| Comorbidity, % | 12.8 | 7.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuliyeva, A.; Serejnikova, N.; Eshmotova, G.; Teslya, Y.; Ivina, A.; Zarov, A.; Panin, M.; Prizov, A.; Lyalina, V.; Shestakov, D.; et al. Post-COVID-19 Femoral Head Osteonecrosis Exhibits Mast Cell Clusters, Fibrosis, and Vascular Thrombosis: Key Pathological Mechanisms in Long COVID-19 Bone Degeneration. Pathophysiology 2025, 32, 36. https://doi.org/10.3390/pathophysiology32030036
Kuliyeva A, Serejnikova N, Eshmotova G, Teslya Y, Ivina A, Zarov A, Panin M, Prizov A, Lyalina V, Shestakov D, et al. Post-COVID-19 Femoral Head Osteonecrosis Exhibits Mast Cell Clusters, Fibrosis, and Vascular Thrombosis: Key Pathological Mechanisms in Long COVID-19 Bone Degeneration. Pathophysiology. 2025; 32(3):36. https://doi.org/10.3390/pathophysiology32030036
Chicago/Turabian StyleKuliyeva, Asya, Natalia Serejnikova, Gulnara Eshmotova, Yulya Teslya, Anastasia Ivina, Alexey Zarov, Michael Panin, Alexey Prizov, Vera Lyalina, Dmitry Shestakov, and et al. 2025. "Post-COVID-19 Femoral Head Osteonecrosis Exhibits Mast Cell Clusters, Fibrosis, and Vascular Thrombosis: Key Pathological Mechanisms in Long COVID-19 Bone Degeneration" Pathophysiology 32, no. 3: 36. https://doi.org/10.3390/pathophysiology32030036
APA StyleKuliyeva, A., Serejnikova, N., Eshmotova, G., Teslya, Y., Ivina, A., Zarov, A., Panin, M., Prizov, A., Lyalina, V., Shestakov, D., Fayzullin, A., Timashev, P., & Volkov, A. (2025). Post-COVID-19 Femoral Head Osteonecrosis Exhibits Mast Cell Clusters, Fibrosis, and Vascular Thrombosis: Key Pathological Mechanisms in Long COVID-19 Bone Degeneration. Pathophysiology, 32(3), 36. https://doi.org/10.3390/pathophysiology32030036







