Histochemical Assessment of Reticulin–Collagen Patterns in the Mid-Secretory Endometrium Predicts Recurrent Pregnancy Loss
Abstract
1. Introduction
2. Materials and Methods
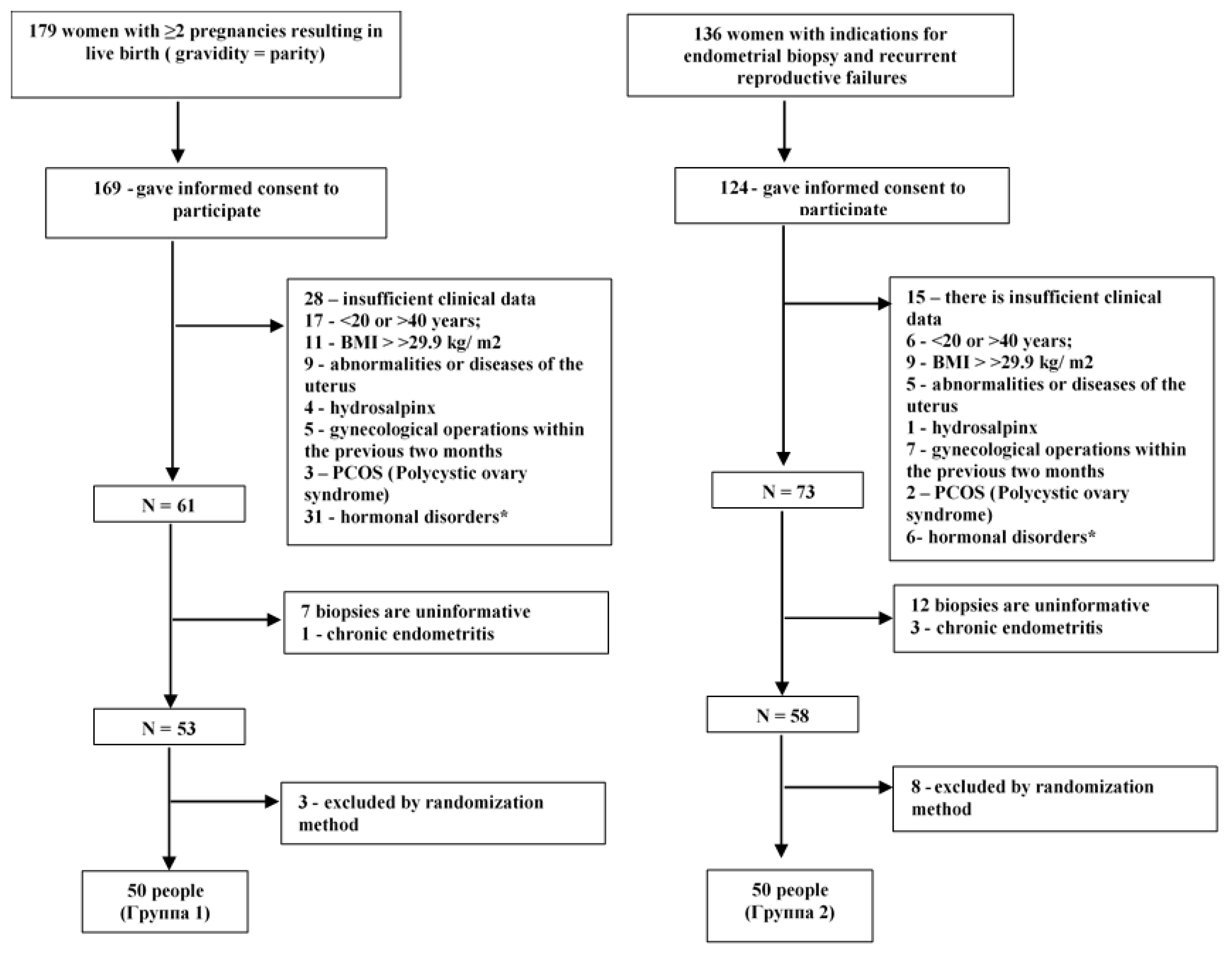
2.1. Study Design and Inclusion/Exclusion Criteria
2.2. Sample Size Calculation
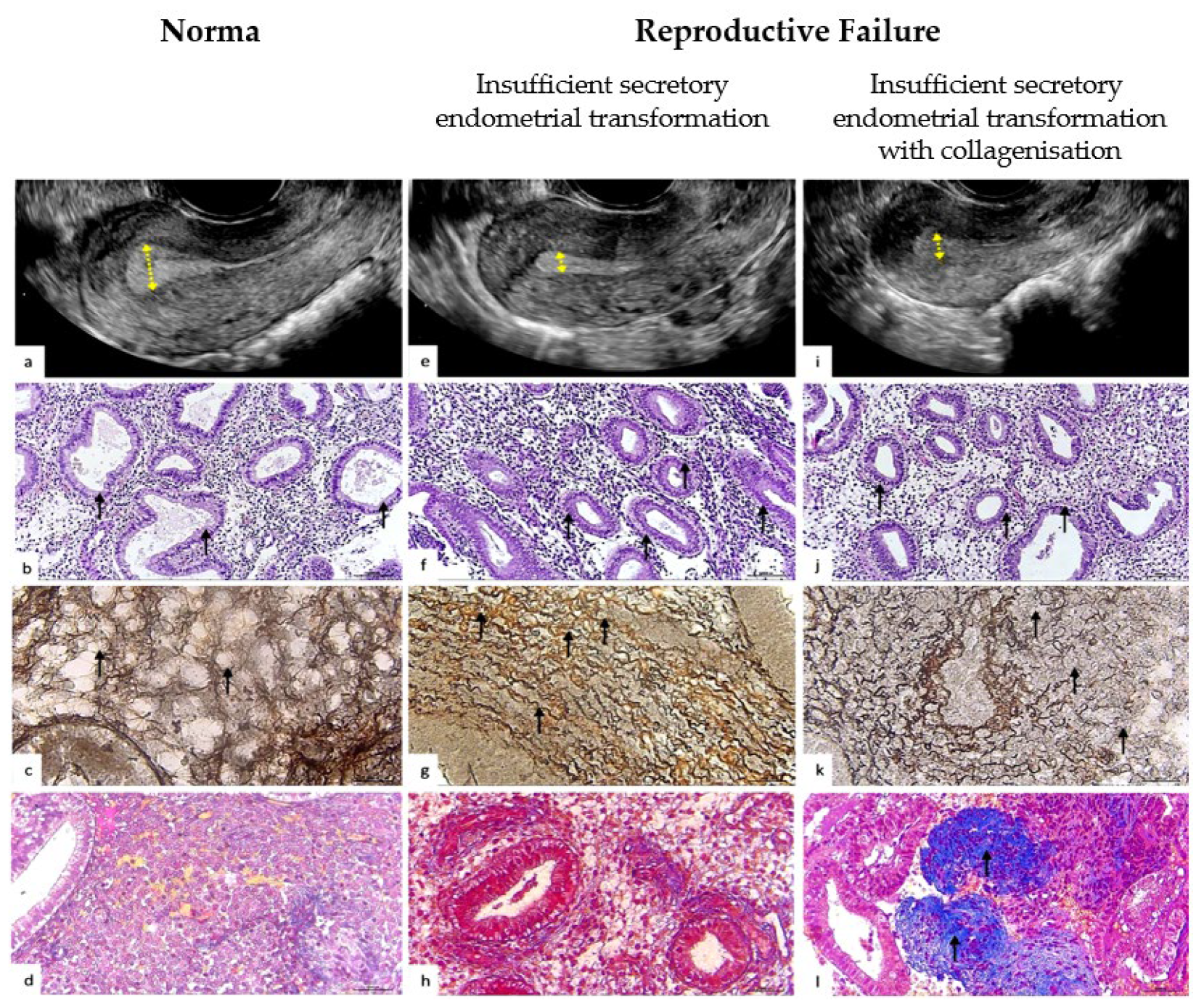
2.3. Methodology of Ultrasound Assessment of the Endometrium
2.4. Methods of Endometrial Biopsy Sampling and Their Histological Examination
2.4.1. Glandular Epithelium
2.4.2. Pinopodes
- ‑
- numerous, densely packed microvilli occupying most (>50%) of the apical membrane of epithelial cells.
- ‑
- few rare microvilli occupying less than half (<50%) of the apical membrane of epithelial cells
2.4.3. Reticulin Fibers of the Extracellular Matrix
2.4.4. Collagen Fibers of the Extracellular Matrix
2.4.5. Interpretation of the Histochemical Pattern of the Extracellular Matrix of the Endometrium by Reticulin–Collagen Phenotype
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of the Study Groups
3.2. Characterization of Histochemical Patterns of Reticulin and Collagen in the Endometrial Extracellular Matrix
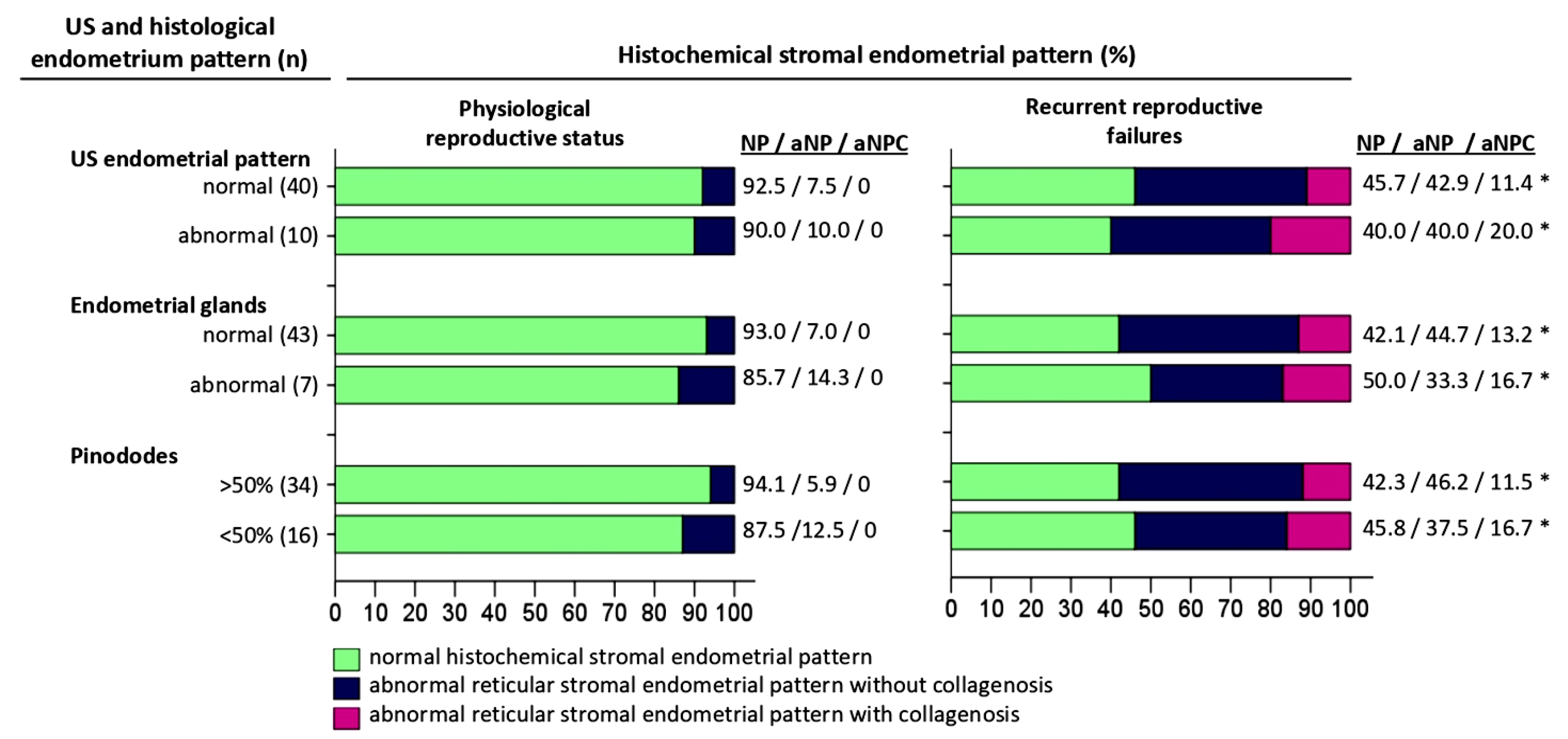
3.3. Comparative Analysis of Histochemical Patterns of the Endometrial Extracellular Matrix and Endometrial Patterns on Ultrasonography
3.4. Comparative Analysis of Extracellular Matrix Histochemical Patterns and Endometrial Glandular Epithelium Histological Features
3.5. Comparative Analysis of Histochemical Patterns of the Extracellular Matrix and the Number of Pinopodes in the Endometrial Epithelium in the Middle of the Secretory Phase
3.6. Comparative Analysis of Reproductive Outcomes in a Group of Women with Recurrent Reproductive Failure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muter, J.; Lynch, V.J.; McCoy, R.C.; Brosens, J.J. Human embryo implantation. Development 2023, 150, dev201507. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Spencer, T.E.; Johnson, G.A.; Burghardt, R.C.; Wu, G. Comparative aspects of implantation. Reproduction 2009, 138, 195–209. [Google Scholar] [CrossRef]
- Benagiano, G.; Mancuso, S.; Guo, S.W.; Di Renzo, G.C. Events Leading to the Establishment of Pregnancy and Placental Formation: The Need to Fine-Tune the Nomenclature on Pregnancy and Gestation. Int. J. Mol. Sci. 2023, 24, 15420. [Google Scholar] [CrossRef]
- O’Connor, B.B.; Pope, B.D.; Peters, M.M.; Ris-Stalpers, C.; Parker, K.K. The role of extracellular matrix in normal and pathological pregnancy: Future applications of microphysiological systems in reproductive medicine. Exp. Biol. Med. 2020, 245, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Pelham, R.J., Jr.; Wang, Y.l. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 1997, 94, 13661–13665. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, Y.-L.; Piao, Y.-S.; Bai, S.-X.; Xiao, Z.-J.; Jia, Y.-L.; Luo, S.-Y.; Zhuang, L.-Z. Effects of matrix proteins on the expression of matrix metalloproteinase-2, -9, and -14 and tissue inhibitors of metalloproteinases in human cytotrophoblast cells during the first trimester. Biol. Reprod. 2001, 65, 240–246. [Google Scholar] [CrossRef]
- Liu, R.; Dai, M.; Gong, G.; Chen, M.; Cao, C.; Wang, T.; Hou, Z.; Shi, Y.; Guo, J.; Zhang, Y.; et al. The role of extracellular matrix on unfavorable maternal–fetal interface: Focusing on the function of collagen in human fertility. J. Leather Sci. Eng. 2022, 4, 13. [Google Scholar] [CrossRef]
- Aplin, J.D.; Charlton, A.K.; Ayad, S. An immunohistochemical study of human endometrial extracellular matrix during the menstrual cycle and first trimester of pregnancy. Cell Tissue Res. 1988, 253, 231–240. [Google Scholar] [CrossRef]
- Kisling, A.; Lust, R.M.; Katwa, L.C. What is the role of peptide fragments of collagen I and IV in health and disease? Life Sci. 2019, 228, 30–34. [Google Scholar] [CrossRef]
- Kisalus, L.L.; Herr, J.C.; Little, C.D. Immunolocalization of extracellular matrix proteins and collagen synthesis in first-trimester human decidua. Anat. Rec. 1987, 218, 402–415. [Google Scholar] [CrossRef]
- Tang, V.W. Collagen, stiffness, and adhesion: The evolutionary basis of vertebrate mechanobiology. Mol. Biol. Cell. 2020, 31, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Schlunck, G.; Han, H.; Wecker, T.; Kampik, D.; Meyer-ter-Vehn, T.; Grehn, F. Substrate rigidity modulates cell matrix interactions and protein expression in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 2008, 49, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, A.K.; Buck, V.U.; Classen-Linke, I.; Leube, R.E. How Mechanical Forces Change the Human Endometrium during the Menstrual Cycle in Preparation for Embryo Implantation. Cells 2021, 10, 2008. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Y.; Carnicer-Lombarte, A.; Gardner, L.; Thomas, J.; Brosens, J.J.; Moffett, A.; Sharkey, A.M.; Franze, K.; Burton, G.J.; Oyen, M.L. Tissue stiffness at the human maternal-fetal interface. Hum. Reprod. 2019, 34, 1999–2008. [Google Scholar] [CrossRef]
- Okada, H.; Tsuzuki, T.; Murata, H. Decidualization of the human endometrium. Reprod. Med. Biol. 2018, 17, 220–227. [Google Scholar] [CrossRef]
- Ma, Z.; Sagrillo-Fagundes, L.; Mok, S.; Vaillancourt, C.; Moraes, C. Mechanobiological regulation of placental trophoblast fusion and function through extracellular matrix rigidity. Sci. Rep. 2020, 10, 5837. [Google Scholar] [CrossRef]
- Iwahashi, M.; Muragaki, Y.; Ooshima, A.; Nakano, R. Decreased type IV collagen expression by human decidual tissues in spontaneous abortion. J. Clin. Endocrinol. Metab. 1996, 81, 2925–2929. [Google Scholar] [CrossRef][Green Version]
- Fu, Q.; Sun, Y.; Tao, Y.; Piao, H.; Wang, X.; Luan, X.; Du, M.; Li, D. Involvement of the JAK-STAT pathway in collagen regulation of decidual NK cells. Am. J. Reprod. Immunol. 2017, 78, e12769. [Google Scholar] [CrossRef]
- Yoshii, N.; Hamatani, T.; Inagaki, N.; Hosaka, T.; Inoue, O.; Yamada, M.; Machiya, R.; Yoshimura, Y.; Odawara, Y. Successful implantation after reducing matrix metalloproteinase activity in the uterine cavity. Reprod. Biol. Endocrinol. 2013, 11, 37. [Google Scholar] [CrossRef]
- Fu, Q.; Tao, Y.; Piao, H.; Du, M.R.; Li, D.J. Trophoblasts and decidual stromal cells regulate decidual NK cell functions via interaction between collagen and LAIR-1. Am. J. Reprod. Immunol. 2014, 71, 368–378. [Google Scholar] [CrossRef]
- Lucas, E.S.; Dyer, N.P.; Murakami, K.; Lee, Y.H.; Chan, Y.-W.; Grimaldi, G.; Muter, J.; Brighton, P.J.; Moore, J.D.; Patel, G.; et al. Loss of Endometrial Plasticity in Recurrent Pregnancy Loss. Stem Cells 2016, 34, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Salker, M.; Teklenburg, G.; Molokhia, M.; Lavery, S.; Trew, G.; Aojanepong, T.; Mardon, H.J.; Lokugamage, A.U.; Rai, R.; Landles, C.; et al. Natural selection of human embryos: Impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS ONE 2010, 5, e10287. [Google Scholar] [CrossRef]
- Salker, M.S.; Nautiyal, J.; Steel, J.H.; Webster, Z.; Sućurović, S.; Nicou, M.; Singh, Y.; Lucas, E.S.; Murakami, K.; Chan, Y.-W.; et al. Disordered IL-33/ST2 activation in decidualizing stromal cells prolongs uterine receptivity in women with recurrent pregnancy loss. PLoS ONE 2012, 7, e52252. [Google Scholar] [CrossRef]
- Potiris, A.; Alyfanti, E.; Drakaki, E.; Mavrogianni, D.; Karampitsakos, T.; Machairoudias, P.; Topis, S.; Zikopoulos, A.; Skentou, C.; Panagopoulos, P.; et al. The Contribution of Proteomics in Understanding Endometrial Protein Expression in Women with Recurrent Implantation Failure. J. Clin. Med. 2024, 13, 2145. [Google Scholar] [CrossRef]
- Ticconi, C.; Pietropolli, A.; Di Simone, N.; Piccione, E.; Fazleabas, A. Endometrial Immune Dysfunction in Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2019, 20, 5332. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Zhu, Y.; Li, H.; He, F.; Liu, S.; Yang, X.; Wang, L.; Zeng, H.; Dai, J.; Hu, L. Recombinant humanized collagen remodels endometrial immune microenvironment of chronic endometritis through macrophage immunomodulation. Regen. Biomater. 2023, 10, rbad033. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M.; Huck, W.T. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 2013, 14, 467–473. [Google Scholar] [CrossRef]
- Wei, Y.; Deng, Z.; Yin, T. Are we closer to robust predictors of recurrent pregnancy loss by means of integrating different types of omics data? Expert. Rev. Mol. Diagn. 2024, 24, 561–563. [Google Scholar] [CrossRef]
- Messaoudi, S.; El Kasmi, I.; Bourdiec, A.; Crespo, K.; Bissonnette, L.; Le Saint, C.; Bissonnette, F.; Kadoch, I.-J. 15 years of transcriptomic analysis on endometrial receptivity: What have we learnt? Fertil. Res. Pract. 2019, 5, 9. [Google Scholar] [CrossRef]
- Fu, X.; Guo, X.; Xu, H.; Li, Y.; Jin, B.; Zhang, X.; Shu, C.; Fan, Y.; Yu, Y.; Tian, Y.; et al. Varied cellular abnormalities in thin vs. normal endometrium in recurrent implantation failure by single-cell transcriptomics. Reprod. Biol. Endocrinol. 2024, 22, 90. [Google Scholar] [CrossRef]
- Davalieva, K.; Kocarev, D.; Plaseska-Karanfilska, D. Decoding recurrent pregnancy loss: Insights from comparative proteomics studies. Biol Reprod. 2025, 112, 1–17. [Google Scholar] [CrossRef]
- Latifi, Z.; Fattahi, A.; Ranjbaran, A.; Nejabati, H.R.; Imakawa, K. Potential roles of metalloproteinases of endometrium-derived exosomes in embryo-maternal crosstalk during implantation. J. Cell Physiol. 2018, 233, 4530–4545. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Hum. Reprod. 2017, 32, 1786–1801. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, D.D.; Zhang, Y.; Song, J.Y.; Sun, Z.G. Comparison of Stimulated Cycles with Low Dose r-FSH versus Hormone Replacement Cycles for Endometrial Preparation Prior to Frozen-Thawed Embryo Transfer in Young Women with Polycystic Ovarian Syndrome: A Single-Center Retrospective Cohort Study from China. Drug Des. Devel Ther. 2021, 15, 2805–2813. [Google Scholar] [CrossRef]
- Chow, S.-C.; Wang, H.; Shao, J. Sample Size Calculations in Clinical Research; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Gonen, Y.; Casper, R.F.; Jacobson, W.; Blankier, J. Endometrial thickness and growth during ovarian stimulation: A possible predictor of implantation in in vitro fertilization. Fertil. Steril. 1989, 52, 446–450. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Q.; Li, Y. The effect of endometrial thickness and pattern measured by ultrasonography on pregnancy outcomes during IVF-ET cycles. Reprod. Biol. Endocrinol. 2012, 10, 100. [Google Scholar] [CrossRef]
- Ma, N.Z.; Chen, L.; Dai, W.; Bu, Z.Q.; Hu, L.L.; Sun, Y.P. Influence of endometrial thickness on treatment outcomes following in vitro fertilization/intracytoplasmic sperm injection. Reprod. Biol. Endocrinol. 2017, 15, 5. [Google Scholar] [CrossRef]
- McWilliams, G.D.; Frattarelli, J.L. Changes in measured endometrial thickness predict in vitro fertilization success. Fertil. Steril. 2007, 88, 74–81. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Q.; Wang, Y.G.; Li, Y.P. Endometrial pattern, thickness and growth in predicting pregnancy outcome following 3319 ivf cycle. Reprod. BioMed. Online 2014, 29, 291–298. [Google Scholar] [CrossRef]
- Zhang, C.H.; Chen, C.; Wang; Wang, Y.; Wen, S.-X.; Cao, Y.-P.; Qian, W.-P. An endometrial receptivity scoring system basing on the endometrial thickness, volume, echo, peristalsis, and blood flow evaluated by ultrasonography. Front Endocrinol 2022, 13, 907874. [Google Scholar] [CrossRef]
- Forrest, T.S.; Elyaderani, M.K.; Muilenburg, M.I.; Bewtra, C.; Kable, W.T.; Sullivan, P. Cyclic endometrial changes: US assessment with histologic correlation. Radiology 1988, 167, 233–237. [Google Scholar] [CrossRef]
- Li, T.C.; Nuttall, L.; Klentzeris, L.; Cooke, I.D. How well does ultrasonographic measurement of endometrial thickness predict the results of histological dating? Hum. Reprod. 1992, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Bahadur, A.; Mittal, S.; Malhotra, N.; Bhatt, A. Predictive value of endometrial thickness, pattern and sub-endometrial blood flows on the day of hCG by 2D doppler in in-vitro fertilization cycles: A prospective clinical study from a tertiary care unit. J. Hum. Reprod. Sci. 2011, 4, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Taizhanova, D.Z.; Zubkov, D.V.; Komlichenko, E.V.; Magalov, I.S.; Sorokina, M.A.; Bespalova, N.V.; Maidanova, Z.O. The possibilities of adverse pregnancy outcomes predicting based on laboratory markers of reproductive losses. Med. Ecol. 2024, 113, 77–84. [Google Scholar] [CrossRef]
- Johannisson, E.; Parker, R.A.; Landgren, B.M.; Diczfalusy, E. Morphometric analysis of the human endometrium in relation to peripheral hormone levels. Fertil. Steril. 1982, 38, 564–571. [Google Scholar] [CrossRef]
- Masson’s Trichrome Staining Protocol for Collagen Fibers. Available online: https://ihcworld.com/2024/01/26/massons-trichrome-staining-protocol-for-collagen-fibers/ (accessed on 25 March 2025).
- Reticulum Stain Kit (Modified Gomori’s) For the Histological Visualization of Reticular Fibers. Version 1 Last Updated 27 June 2018 ab236473. Available online: https://www.abcam.com/ps/products/236/ab236473/documents/ab236473%20-%20Reticulum%20Stain%20Kit%20(Modified%20Gomori's)%20v1a%20(website).pdf?srsltid=AfmBOordg8pj-ElMRK_eVCflx47RUjSyI7Cx2mc1eQAPUgjKyV6tzW6I (accessed on 25 March 2025).
- Noyes, R.W.; Hertig, A.T.; Rock, J. Dating the endometrial biopsy. Am. J. Obs. Obstet. Gynecol. 1975, 122, 262–263. [Google Scholar] [CrossRef]
- Hendrickson, M.R.; Kempson, R.L. Decision tree for endometrial dating. In Surgical Pathology of the Uterine Corpus; Benningtoon, J.L., Ed.; WB Saunders: Philadelphia, PA, USA, 1980; pp. 80–85. [Google Scholar]
- Murray, M.J.; Meyer, W.R.; Zaino, R.J.; Lessey, B.A.; Novotny, D.B.; Ireland, K.; Zeng, D.; Fritz, M.A. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil. Steril. 2004, 81, 1333–1343. [Google Scholar] [CrossRef]
- Dallenbach-Hellweg, G. The endometrium of infertility. A review. Pathol. Res. Pract. 1984, 178, 527–537. [Google Scholar] [CrossRef]
- Acosta, A.A.; Elberger, L.; Borghi, M.; Calamera, J.C.; Chemes, H.; Doncel, G.F.; Kliman, H.; Lema, B.; Lustig, L.; Papier, S. Endometrial dating and determination of the window of implantation in healthy fertile women. Fertil. Steril. 2000, 73, 788–798. [Google Scholar] [CrossRef]
- Jin, X.Y.; Zhao, L.J.; Luo, D.H.; Liu, L.; Dai, Y.D.; Hu, X.X.; Wang, Y.Y.; Lin, X.; Hong, F.; Li, T.C.; et al. Pinopode score around the time of implantation is predictive of successful implantation following frozen embryo transfer in hormone replacement cycles. Hum. Reprod. 2017, 32, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- Develioglu, O.H.; Nikas, G.; Hsiu, J.G.; Toner, J.P.; Jones, H.W., Jr. Detection of endometrial pinopodes by light microscopy. Fertil. Steril. 2000, 74, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Nikas, G.; Makrigiannakis, A.; Hovatta, O.; Jones, H.W., Jr. Surface morphology of the human endometrium. Basic and clinical aspects. Ann. N. Y Acad. Sci. 2000, 900, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Grishkina, A.A.; Chistyakova, G.N.; Remizova, I.I.; Melkozerova, O.A. Endometrial receptivity and expression of apoptosis and proliferation factors in the endometrium of women with hyperplasia and infertility. Bull. Gynecol. Obstet. Perinatol. 2019, 19, 37–39. [Google Scholar] [CrossRef]
- Boland, K.; Nguyen, G.C. Microscopic Colitis: A Review of Collagenous and Lymphocytic Colitis. Gastroenterol. Hepatol. 2017, 13, 671–677. [Google Scholar]
- Lazenby, A.J.; Yardley, J.H.; Giardiello, F.M.; Jessurun, J.; Bayless, T.M. Lymphocytic (“microscopic”) colitis: A comparative histopathologic study with particular reference to collagenous colitis. Hum. Pathol. 1989, 20, 18–28. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, T.T.; Zhang, L. Microscopic colitis: Lymphocytic colitis, collagenous colitis, and beyond. Hum. Pathol. 2023, 132, 89–101. [Google Scholar] [CrossRef]
- Iwahashi, M.; Nakano, R. Decreased type V collagen expression in human decidual tissues of spontaneous abortion during early pregnancy. J. Clin. Pathol. 1998, 51, 44–46. [Google Scholar] [CrossRef][Green Version]
- Foy, M.; Anézo, O.; Saule, S.; Planque, N. PRL-3/PTP4A3 phosphatase regulates integrin β1 in adhesion structures during migration of human ocular melanoma cells. Exp. Cell Res. 2017, 353, 88–99. [Google Scholar] [CrossRef]
- Shi, J.W.; Lai, Z.Z.; Yang, H.L.; Yang, S.-L.; Wang, C.-J.; Ao, D.; Ruan, L.-Y.; Shen, H.-H.; Zhou, W.-J.; Mei, J.; et al. Collagen at the maternal-fetal interface in human pregnancy. Int. J. Biol. Sci. 2020, 16, 2220–2234. [Google Scholar] [CrossRef]
- Rygiel, T.P.; Stolte, E.H.; de Ruiter, T.; van de Weijer, M.L.; Meyaard, L. Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR-1. Mol. Immunol. 2011, 49, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Lu, C.; Li, M.; Wu, M.; Wang, Q.; Wang, X.; Xu, L.; Zhang, J. The dynamic changes in myocardial collagen metabolism in experimental autoimmune myocarditis rats. Hell. J. Cardiol. 2018, 59, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yao, L.; Xin, M.; Gao, M. Dysregulation of collagen expression in peri-implantation endometrium of women with high ovarian response. J. Obs. Obstet. Gynaecol. Res. 2019, 45, 1035–1044. [Google Scholar] [CrossRef]
- Kaufmann, P.; Stark, J.; Stegner, H.E. The villous stroma of the human placenta. I. The ultrastructure of fixed connective tissue cells. Cell Tissue Res. 1977, 177, 105–121. [Google Scholar] [CrossRef]
- Okada, Y.; Asahina, T.; Kobayashi, T.; Goto, J.; Terao, T. Studies on the mechanism of edematous changes at the endometrial stroma for implantation. Semin. Thromb. Hemost. 2001, 27, 67–77. [Google Scholar] [CrossRef]
- Saadat, P.; Boostanfar, R.; Slater, C.C.; Tourgeman, D.E.; Stanczyk, F.Z.; Paulson, R.J. Accelerated endometrial maturation in the luteal phase of cycles utilizing controlled ovarian hyperstimulation: Impact of gonadotropin-releasing hormone agonists versus antagonists. Fertil. Steril. 2004, 82, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Corbacioglu, A.; Baysal, B. Effects of steroid hormone levels on the ultrasound appearance of the preovulatory endometrium in controlled ovarian hyperstimulation cycles. Int. J. Fertil. Steril. 2012, 5, 203–206. [Google Scholar]
- Nissinen, L.; Kähäri, V.M. Matrix metalloproteinases in inflammation. Biochim. Biophys. Acta. 2014, 1840, 2571–2580. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, W.J. The Role of Matrix Metalloproteinase in Inflammation with a Focus on Infectious Diseases. Int. J. Mol. Sci. 2022, 23, 10546. [Google Scholar] [CrossRef]
- Chen, K.; Xu, M.; Lu, F.; He, Y. Development of Matrix Metalloproteinases-Mediated Extracellular Matrix Remodeling in Regenerative Medicine: A Mini Review. Tissue Eng. Regen. Med. 2023, 20, 661–670. [Google Scholar] [CrossRef]
- Pezaro, S.; Pearce, G.; Reinhold, E. Hypermobile Ehlers-Danlos syndrome during pregnancy, birth and beyond. Br. J. Midwifery 2018, 26, 217–223. [Google Scholar] [CrossRef]
- Ganer Herman, H.; Volodarsky-Perel, A.; Ton Nu, T.N.; Machado-Gedeon, A.; Cui, Y.; Shaul, J.; Dahan, M.H. Pregnancy complications and placental histology following embryo transfer with a thinner endometrium. Hum. Reprod. 2022, 37, 1739–1745. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | Groups (N-100) | p-Value | |
|---|---|---|---|
| Physiological Reproductive Pattern (n-50) | Recurrent Reproductive Failure (n-50) | ||
| Age, mean ± SD | 31.14 ± 4.66 | 32.08 ± 4.37 | 0.236 |
| Body mass index (BMI), (kg/m2) | 24.4 (95% CI:23.5–25.3) | 24.9 (95% CI:24.0–25.7) | 0.389 |
| Pregnancies: number and outcomes | |||
| Gravida, median (IQR) | 2 (1) | 3 (1) | 0.002 |
| Clinical pregnancy loss, median (IQR) | 0 (0) | 2 (1) | - |
| Biochemical pregnancy loss, median (IQR) | 0 (0) | 1 (2) | - |
| Number of live births, median (IQR) | 2 (1) | 0 (0) | - |
| Preterm birth, median (IQR) | 1 (2) | 0 (0) | - |
| Term delivery, median (IQR) | 2 (1) | 0 (0) | - |
| Hormones | |||
| TSH (uU/mL) | 2.41 ± 0.86 | 2.62 ± 0.92 | 0.211 |
| Basal FSH (IU/L) | 7.81 (95% CI:7.2–8.4) | 7.49 (95% CI:6.6–8.3) | 0.934 |
| Basal LH (IU/L) | 4.92 ± 2.24 | 4.86 ± 1.42 | 0.667 |
| Basal serum estradiol (E2) (pg/mL) | 39.03 ± 17.48 | 42.40 ± 21.82 | 0.627 |
| Correspondence to Ovarian Cycle | Physiological Reproductive Status | Recurrent Reproductive Failure | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Normal reticulin–collagen pattern (NP) | 46 (92%) | 4 (8%) | 22 (44%) | 28 (56%) * |
| Natural cycles, n (%) | 46 (100%) | 4 (100%) | 7 (28.6%) | 9 (32.1%) |
| Stimulated cycles, n (%) | - | - | 15 (71.4%) | 19 (67.9%) |
| Pathological stromal reticulin pattern without collagenization (aNP) | 4 (8%) | 46 (92%) | 21 (42%) * | 29 (58%) |
| Natural cycles, n (%) | 4 (100%) | 46 (100%) | 6 (28.6%) | 10 (34.5%) |
| Stimulated cycles, n (%) | - | - | 15 (71.4%) | 19 (65.5%) |
| Pathological stromal reticulin pattern with collagenization (aNPC) | 0 (0%) | 50 (100%) | 7 (14%) * | 43 (86%) |
| Natural cycles, n (%) | - | 50 (100%) | 3 (42.9%) | 13 (30.2%) |
| Stimulated cycles, n (%) | - | - | 4 (57.1%) | 30 (69.8%) |
| US Pattern Correspondence to Cycle | Physiological Reproductive Status | Recurrent Reproductive Failure | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Total number, n (%) | 40 (80%) | 10 (20%) | 35 (70%) | 15 (30%) |
| Natural cycles, n (%) | 40 (100%) | 10 (100%) | 10 (28.6%) | 6 (40.0%) |
| Stimulated cycles, n (%) | - | - | 25 (71.4%) * | 9 (60.0%) |
| Correspondence to Cycle | Physiological Reproductive Status | Recurrent Reproductive Failure | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Total number, n (%) | 43 (86%) | 7 (14%) | 38 (76%) | 12 (24%) |
| Natural cycles, n (%) | 43 (100%) | 7 (100%) | 11 (28.9%) | 5 (41.7%) |
| Stimulated cycles, n (%) | - | - | 27 (71.1%) * | 7 (58.3%) |
| Relative Number of Pinopodes | Physiological Reproductive Status | Recurrent Reproductive Failure | ||
|---|---|---|---|---|
| >50% | <50% | >50% | <50% | |
| Total number, n (%) | 34 (68%) | 16(32%) | 26(52%) | 24(48%) |
| Natural cycles, n (%) | 34 (100%) | 16(100%) | 9(34.6%) | 7(29.2%) |
| Stimulated cycles, n (%) | - | - | 17(65.4%) | 17(70.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshakhtiyeva, N.; Klyuyev, D.; Amirbekova, Z.; Gatin, R.; Turmukhambetova, A.; Makhambetova, K.; Kadyrova, I.; Kamyshanskiy, Y. Histochemical Assessment of Reticulin–Collagen Patterns in the Mid-Secretory Endometrium Predicts Recurrent Pregnancy Loss. Pathophysiology 2025, 32, 24. https://doi.org/10.3390/pathophysiology32020024
Oshakhtiyeva N, Klyuyev D, Amirbekova Z, Gatin R, Turmukhambetova A, Makhambetova K, Kadyrova I, Kamyshanskiy Y. Histochemical Assessment of Reticulin–Collagen Patterns in the Mid-Secretory Endometrium Predicts Recurrent Pregnancy Loss. Pathophysiology. 2025; 32(2):24. https://doi.org/10.3390/pathophysiology32020024
Chicago/Turabian StyleOshakhtiyeva, Nazerke, Dmitriy Klyuyev, Zhanna Amirbekova, Rinat Gatin, Anar Turmukhambetova, Kamilya Makhambetova, Irina Kadyrova, and Yevgeniy Kamyshanskiy. 2025. "Histochemical Assessment of Reticulin–Collagen Patterns in the Mid-Secretory Endometrium Predicts Recurrent Pregnancy Loss" Pathophysiology 32, no. 2: 24. https://doi.org/10.3390/pathophysiology32020024
APA StyleOshakhtiyeva, N., Klyuyev, D., Amirbekova, Z., Gatin, R., Turmukhambetova, A., Makhambetova, K., Kadyrova, I., & Kamyshanskiy, Y. (2025). Histochemical Assessment of Reticulin–Collagen Patterns in the Mid-Secretory Endometrium Predicts Recurrent Pregnancy Loss. Pathophysiology, 32(2), 24. https://doi.org/10.3390/pathophysiology32020024







