Influence of Housing Temperature and Genetic Diversity on Allogeneic T Cell-Induced Tissue Damage in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Induction of aGVHD Using Inbred and Outbred Mice
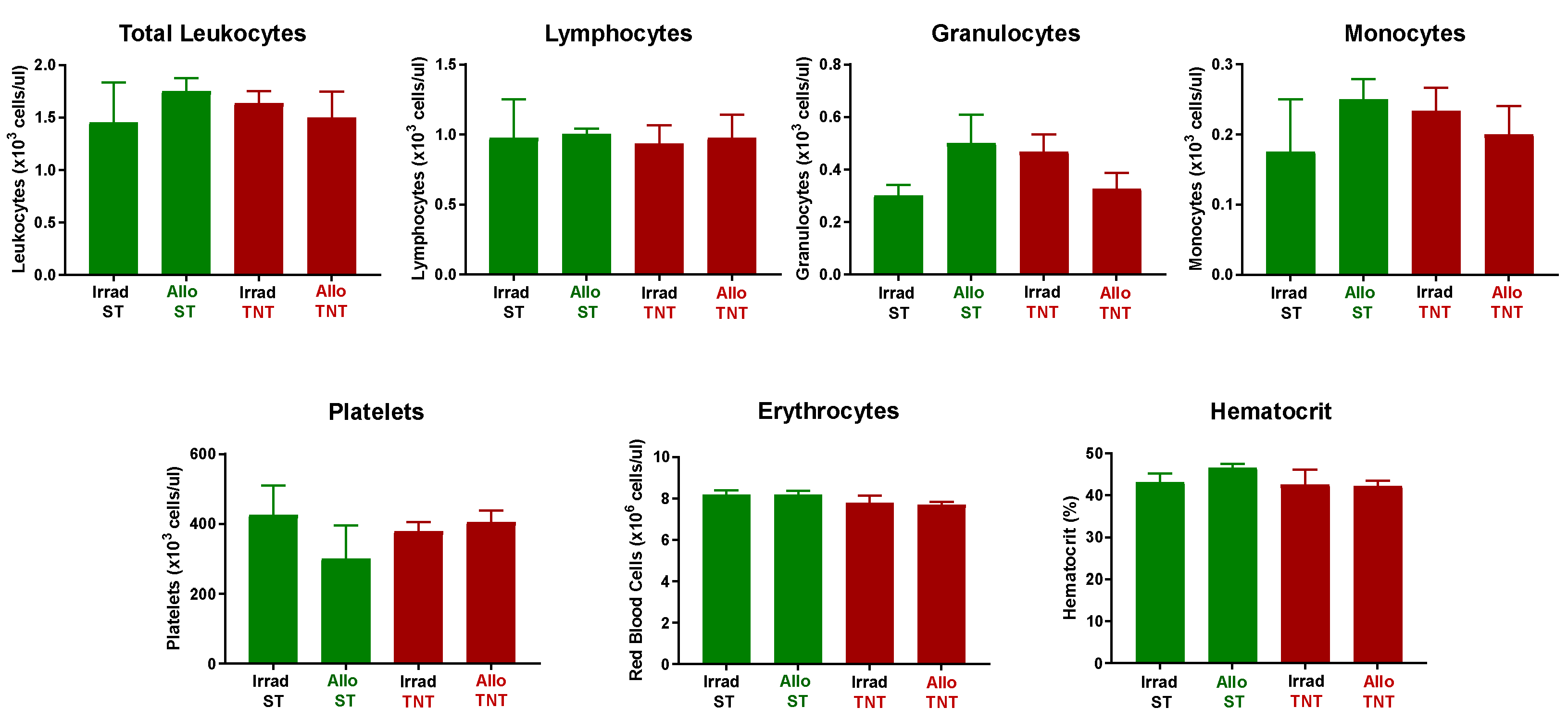
2.3. Complete Blood Cell Analyses and Plasma Cytokine Quantification
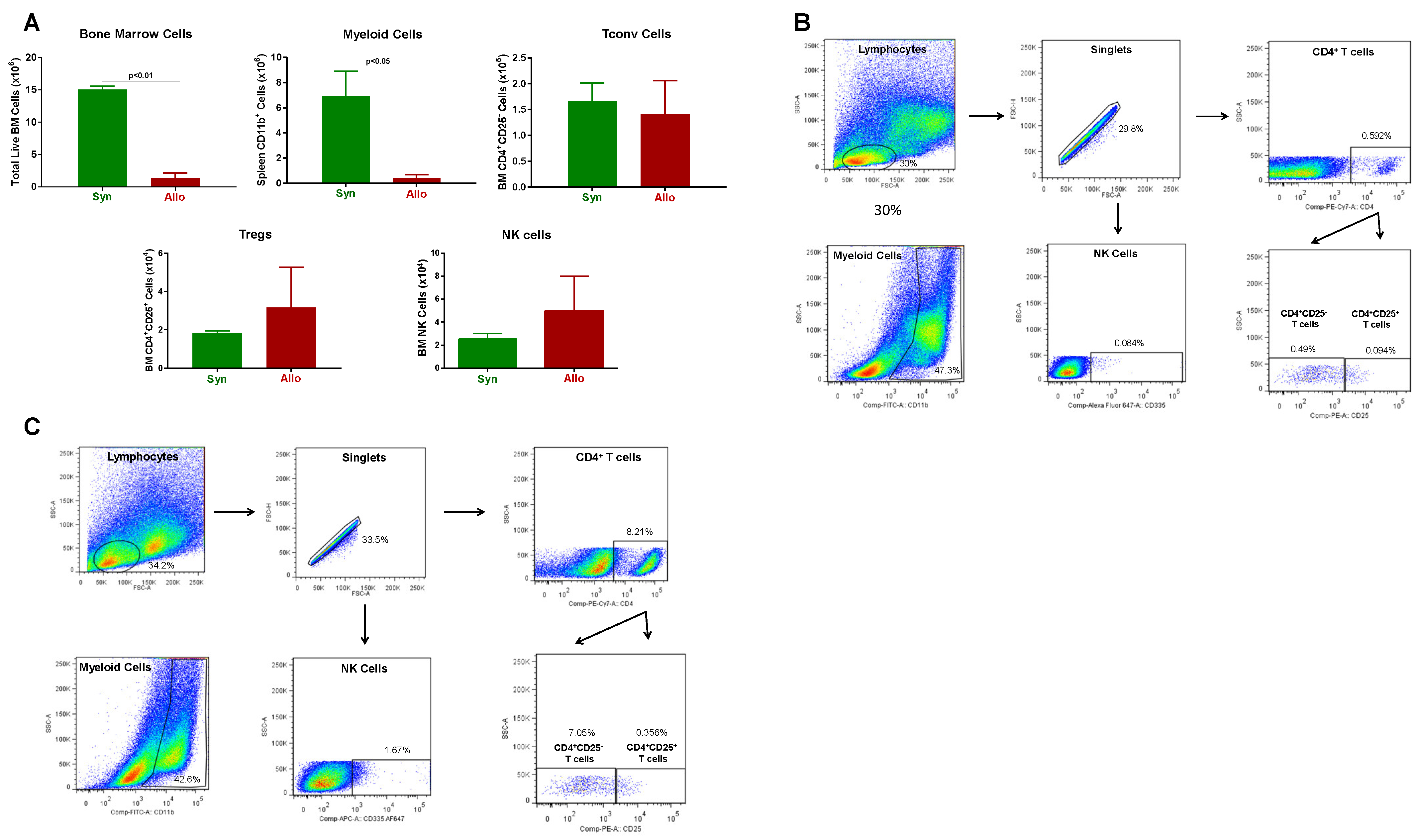
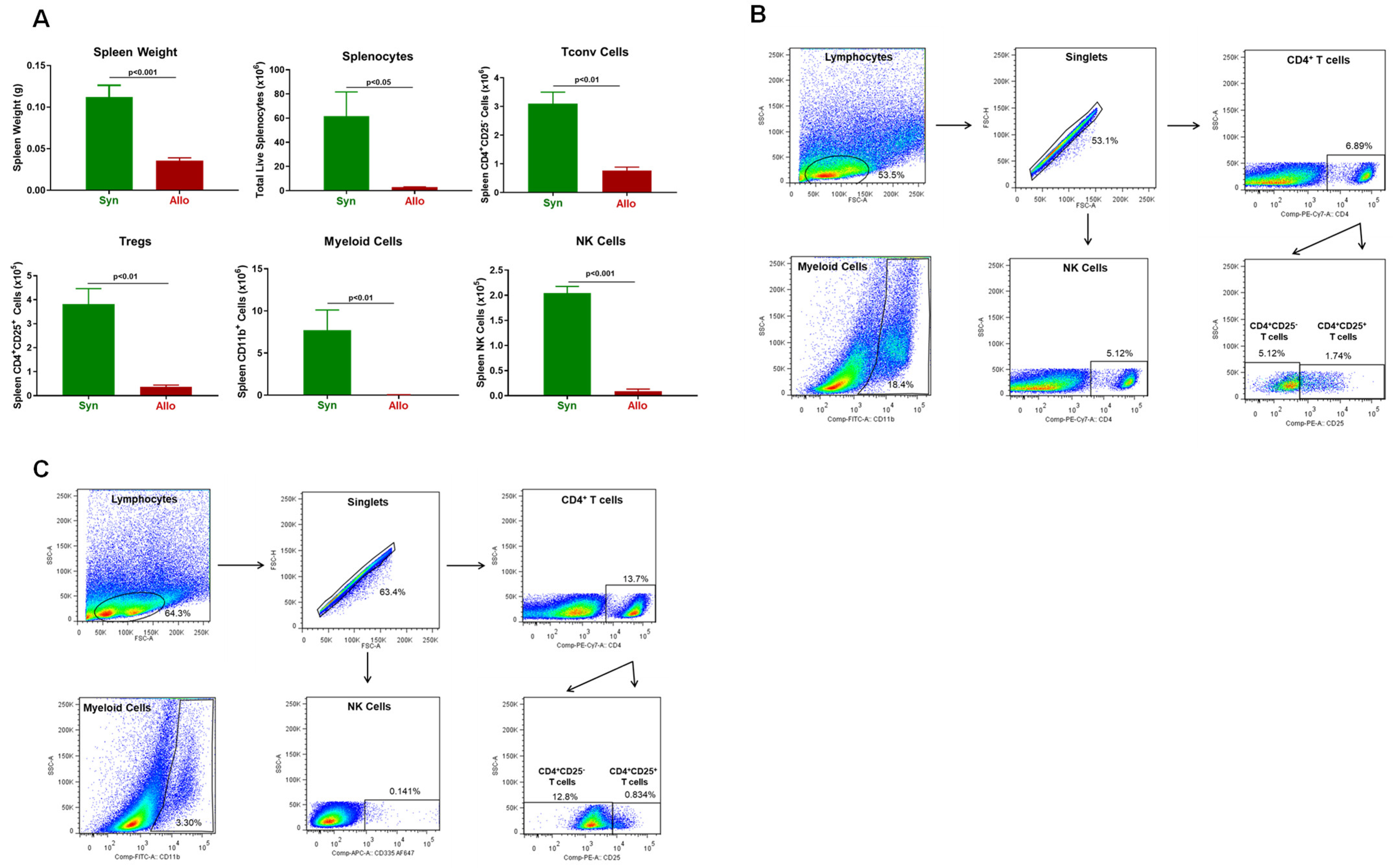
2.4. Immune Cell Analysis
2.5. Tissue Preparation for Blinded Histological Evaluation
2.6. Statistical Analyses
3. Results
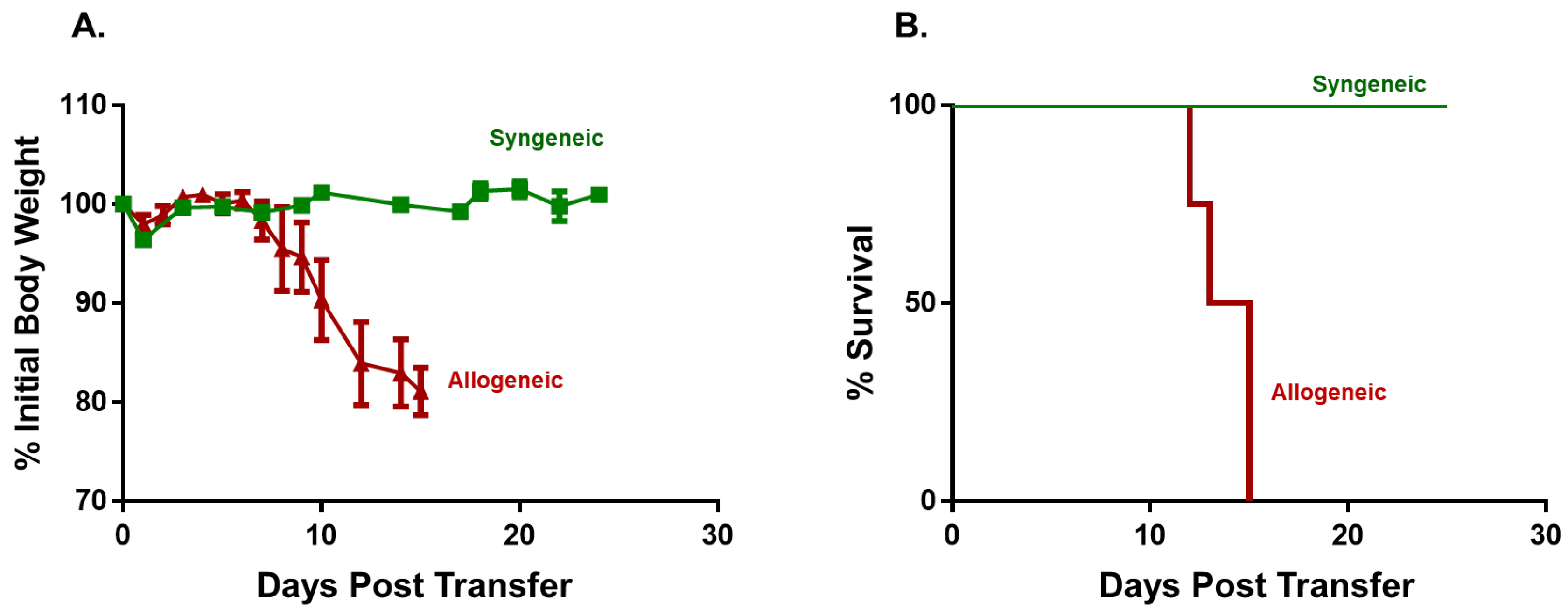
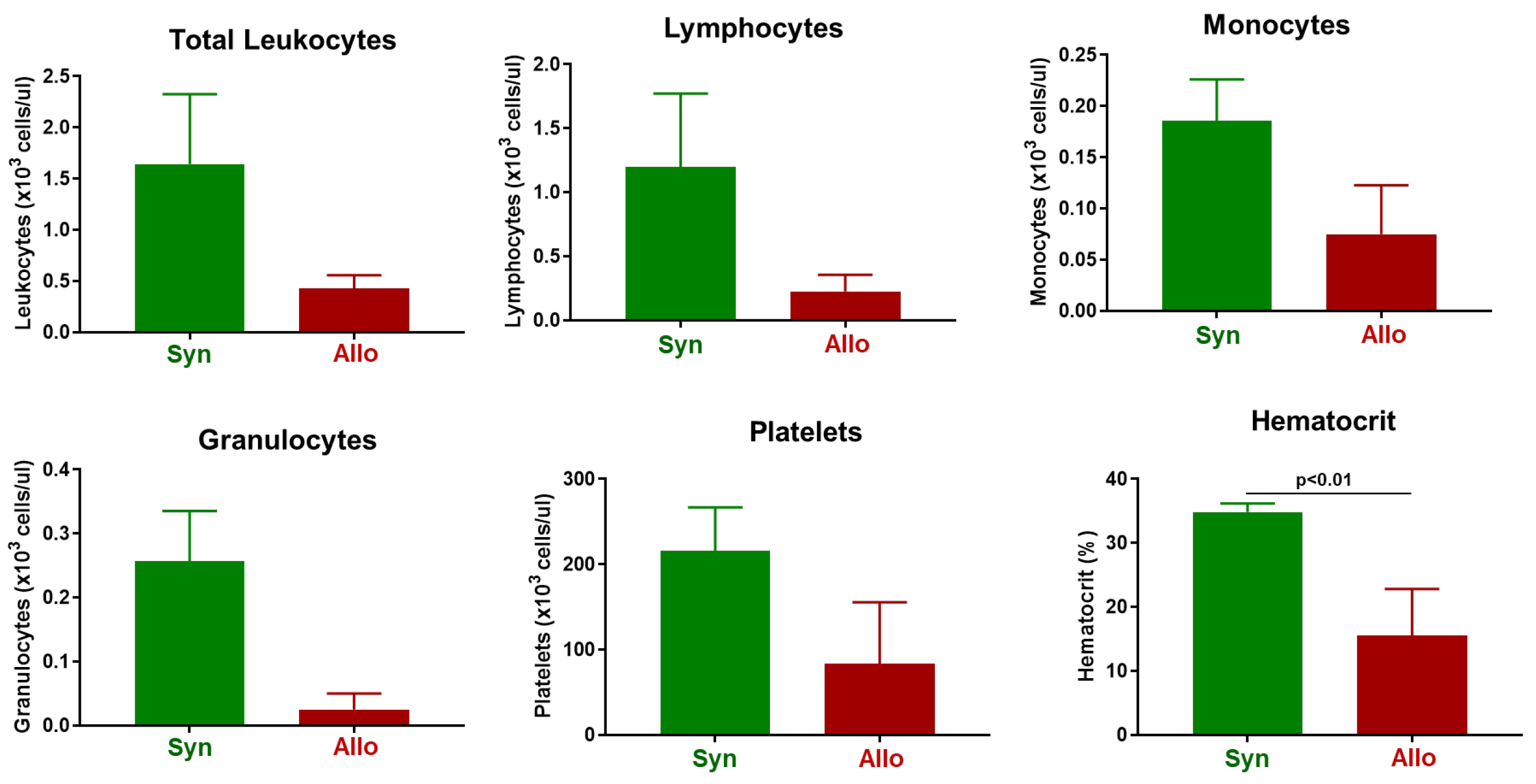
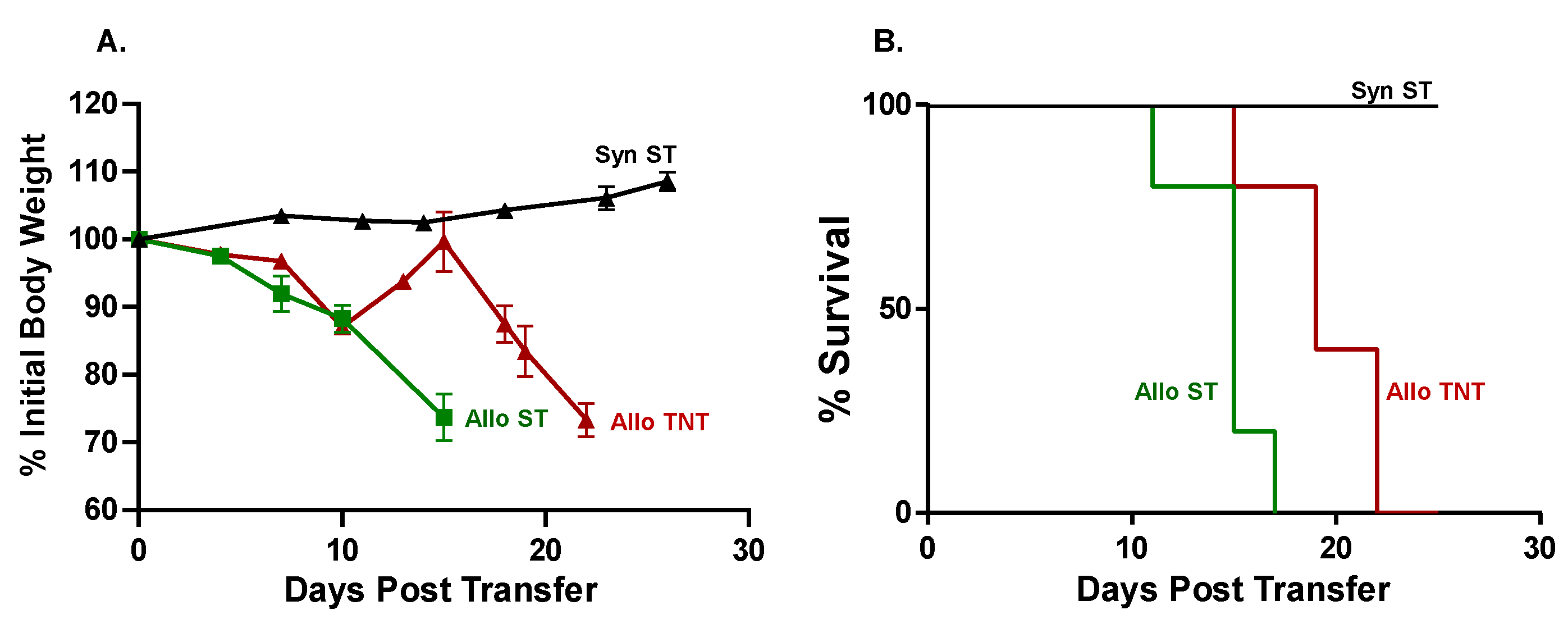
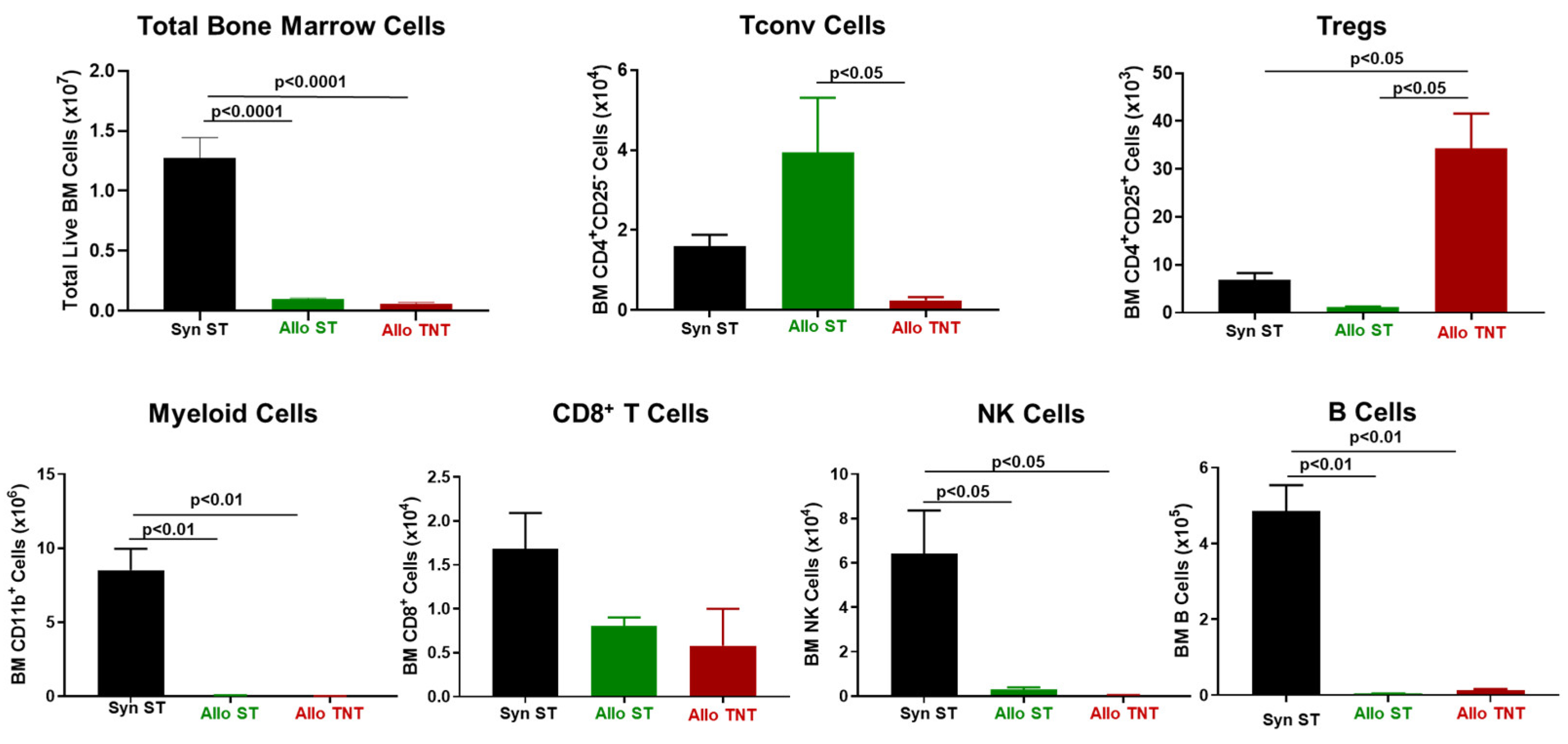
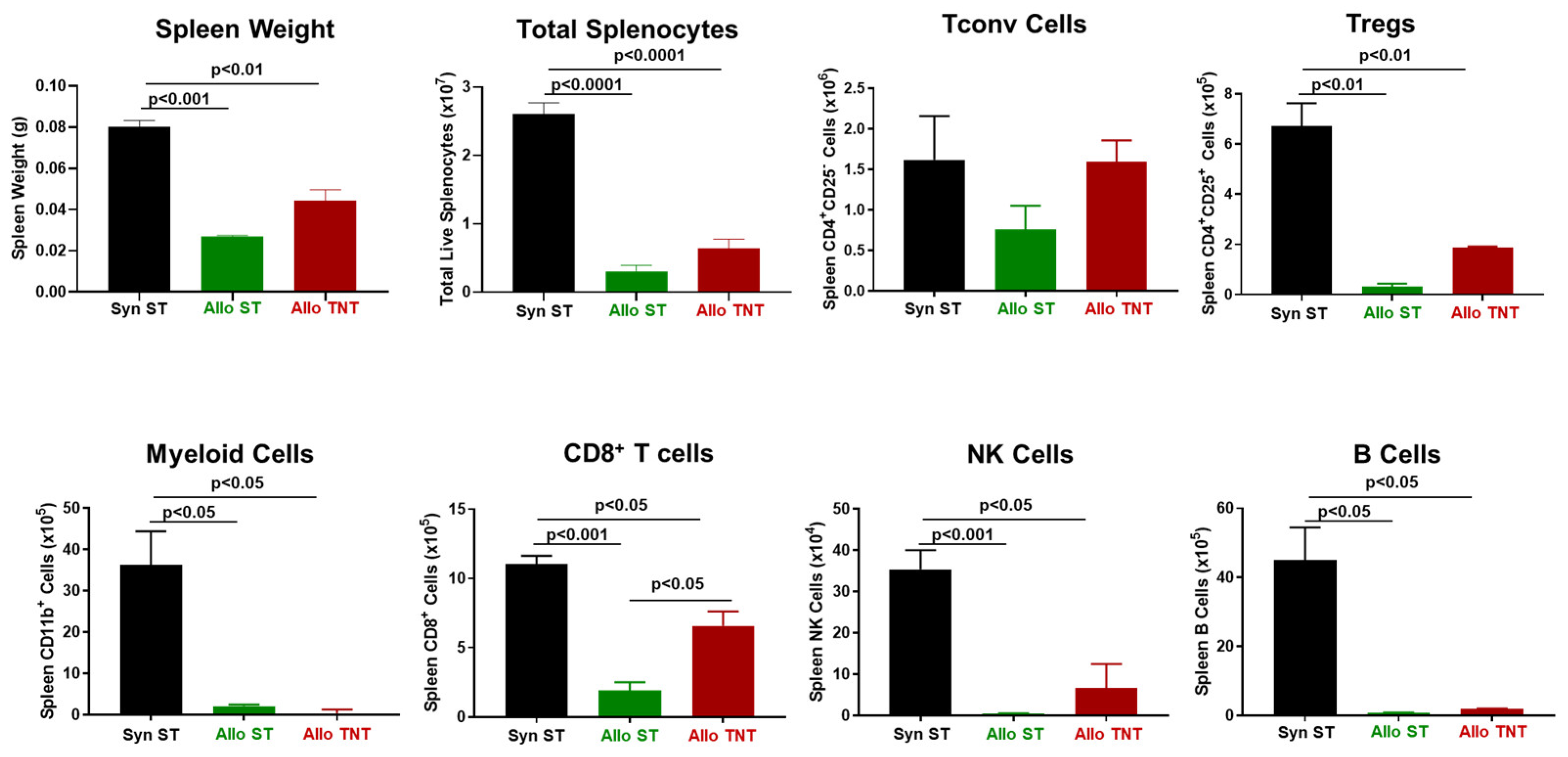
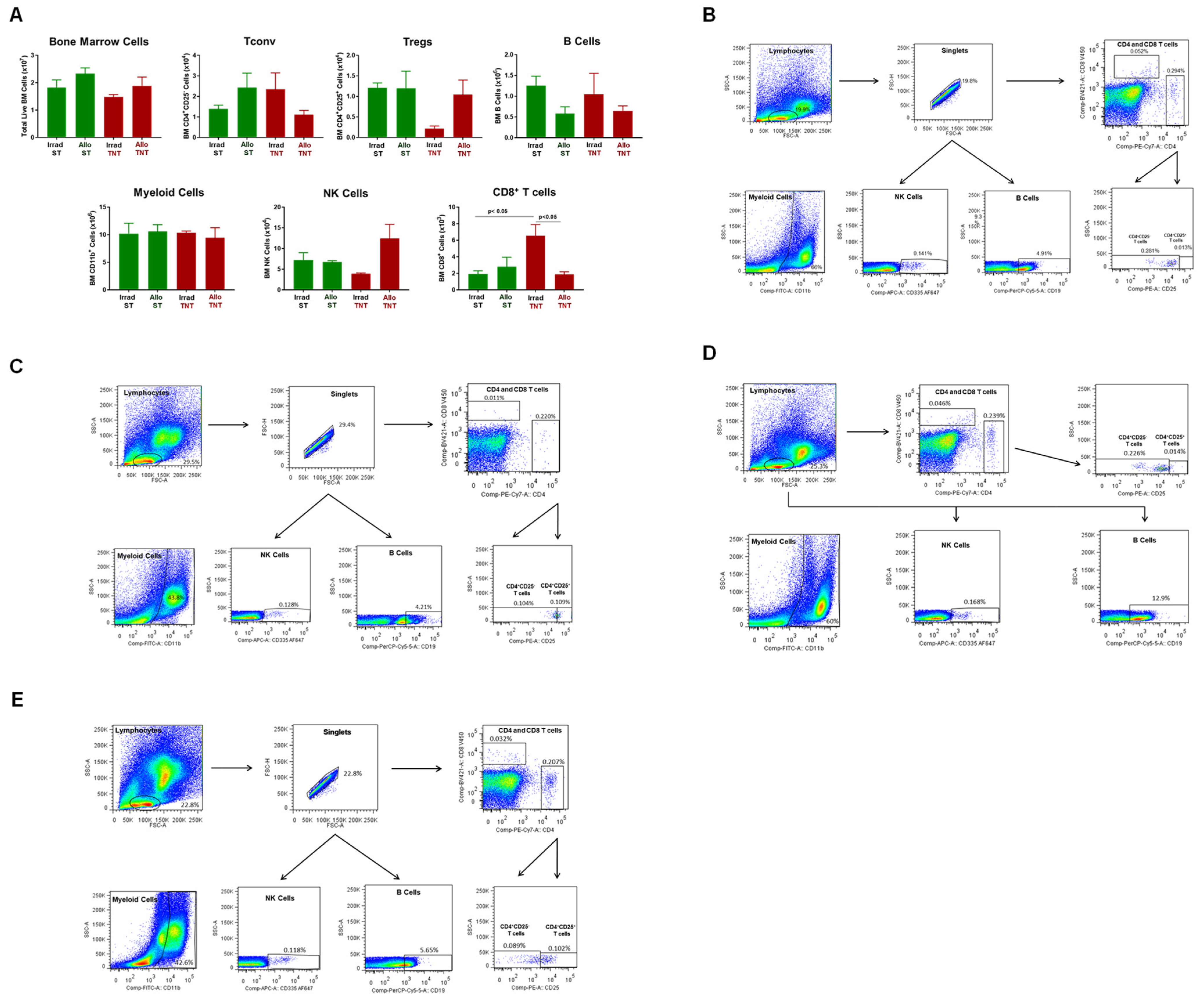
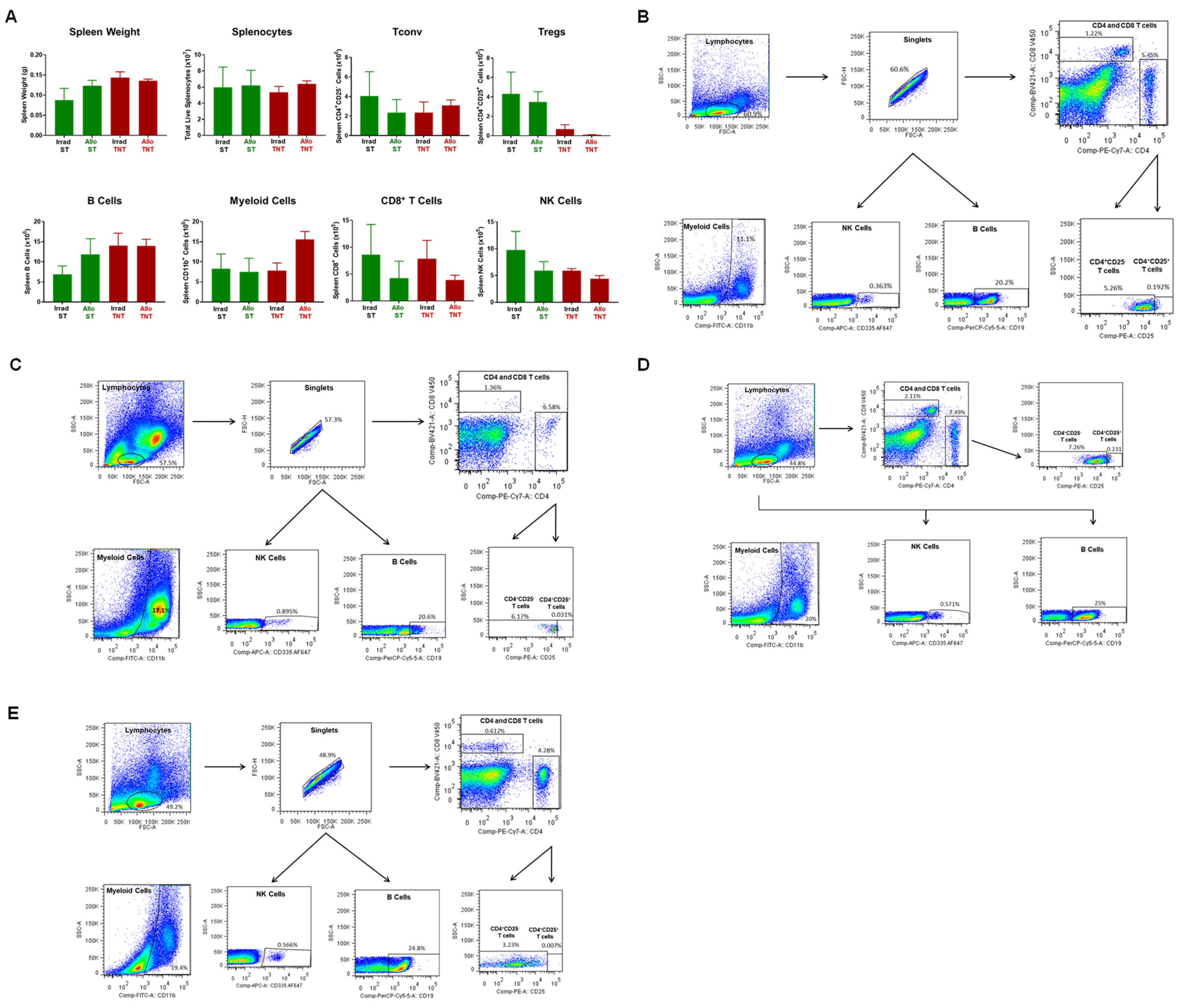
3.1. Induction and Characterization of Allogeneic T Cell-Mediated Tissue Damage in Inbred Mice
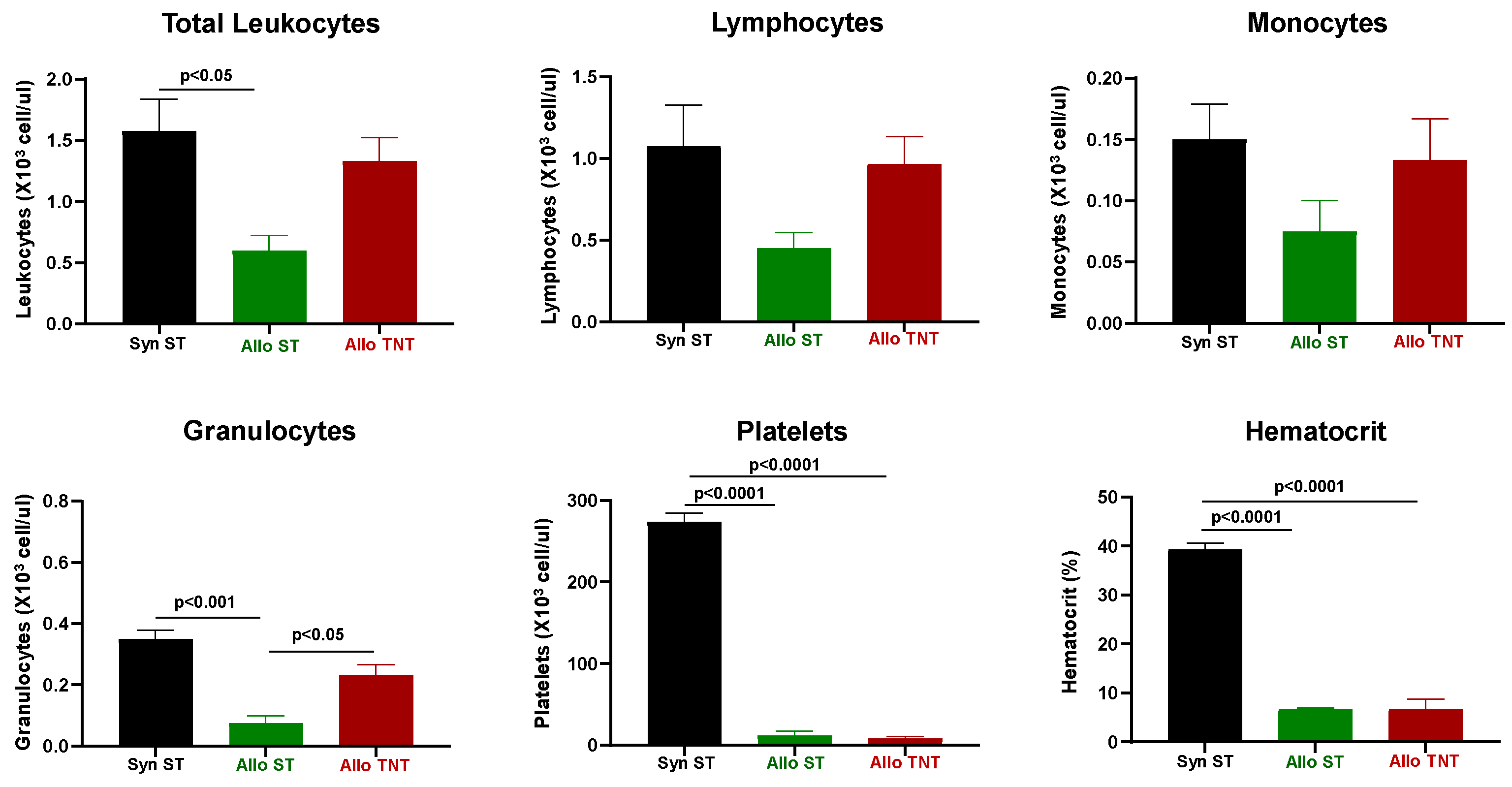
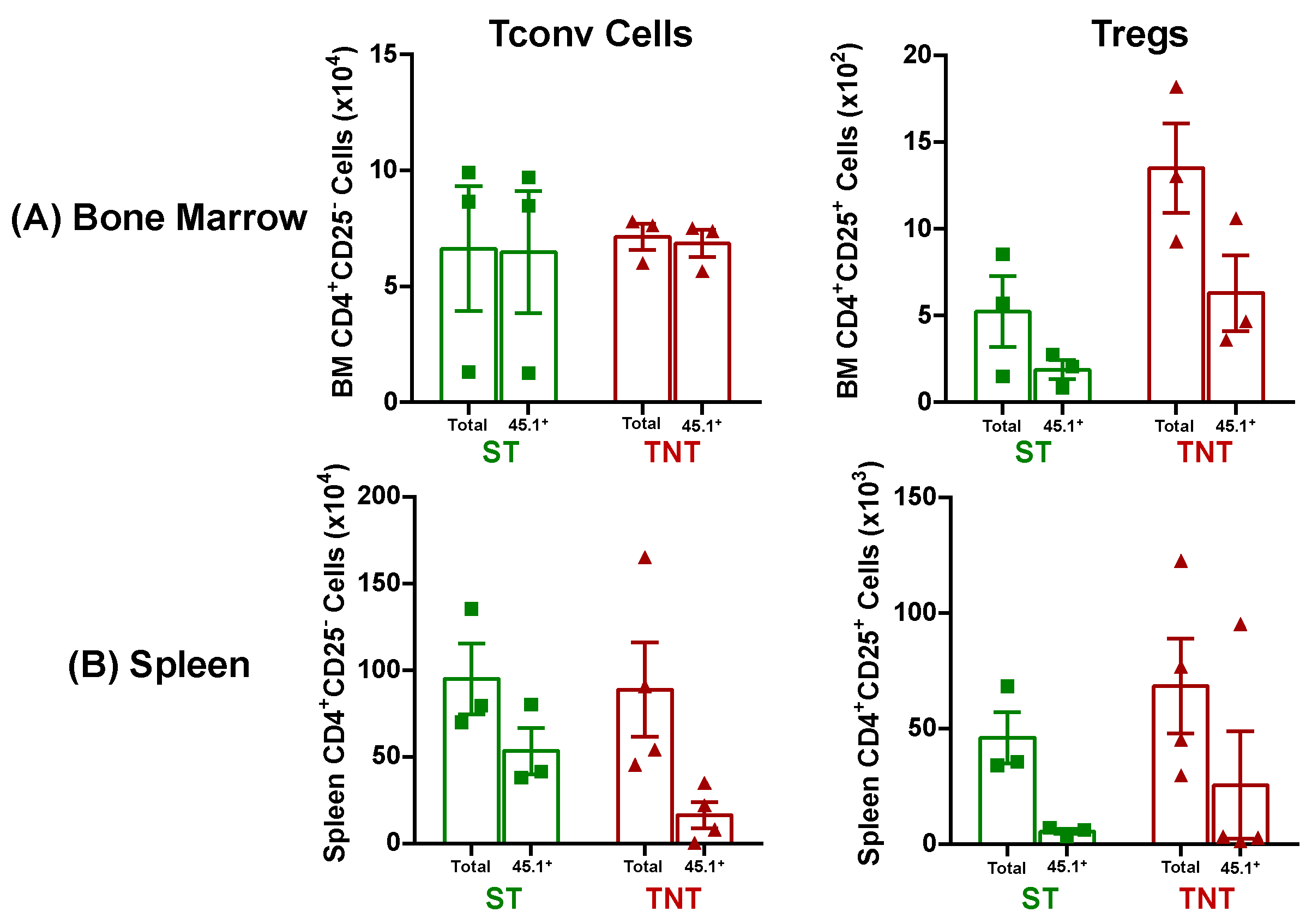
3.2. Onset and Severity of BM and Spleen Damage in Inbred Mice Housed at ST or TNT
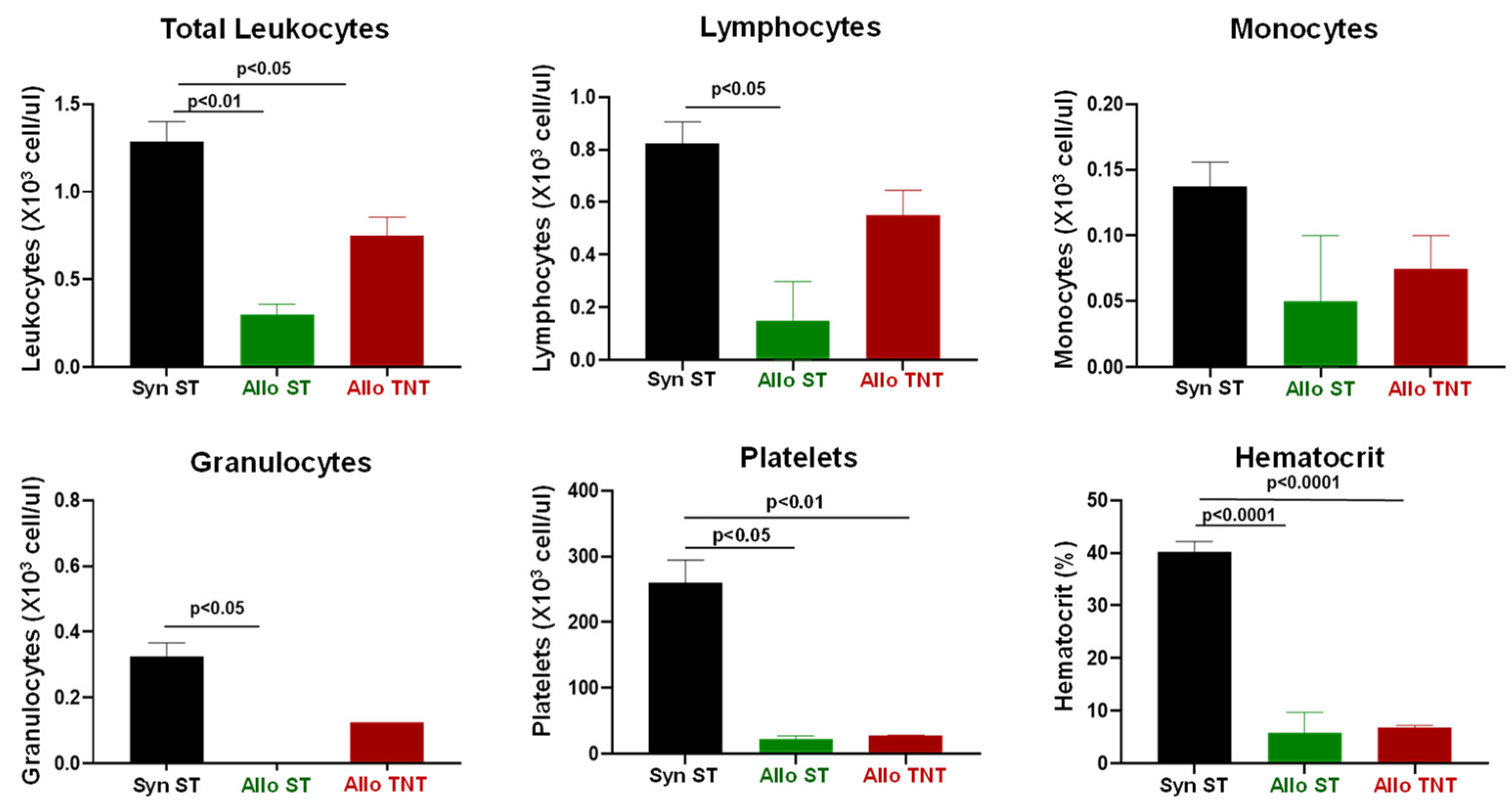
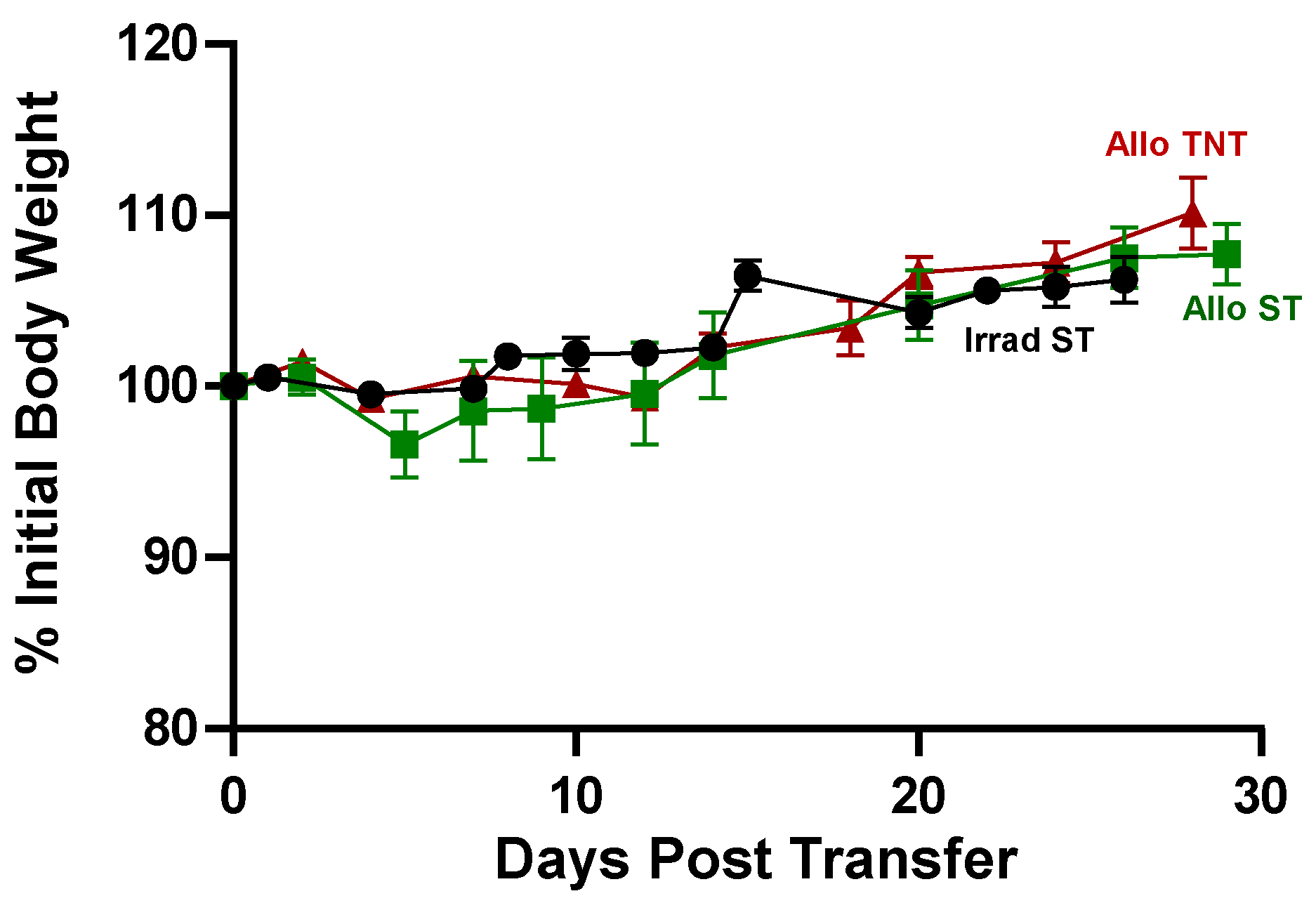
3.3. Adoptive Transfer of Allogeneic T Cells Fails to Induce Tissue Damage in Sub-Lethally Irradiated Outbred Mice
4. Discussion
4.1. T Cell Trafficking and Tissue Damage
4.2. Effect of Housing Temperature on Survival and Tissue Damage
4.3. Outbred Mice Are Resistant to T Cell-Mediated Tissue Damage
4.4. Potential Mechanism Involved in Suppressing Disease in Outbred Mice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niederwieser, D.; Baldomero, H.; Szer, J.; Gratwohl, M.; Aljurf, M.; Atsuta, Y.; Bouzas, L.F.; Confer, D.; Greinix, H.; Horowitz, M.; et al. Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. Bone Marrow Transplant. 2016, 51, 778–785. [Google Scholar] [CrossRef]
- Shimoni, A.; Labopin, M.; Savani, B.; Volin, L.; Ehninger, G.; Kuball, J.; Bunjes, D.; Schaap, N.; Vigouroux, S.; Bacigalupo, A.; et al. Long-term survival and late events after allogeneic stem cell transplantation from HLA-matched siblings for acute myeloid leukemia with myeloablative compared to reduced-intensity conditioning: A report on behalf of the acute leukemia working party of European group for blood and marrow transplantation. J. Hematol. Oncol. 2016, 9, 118. [Google Scholar] [PubMed]
- Zeiser, R.; Blazar, B.R. Acute Graft-versus-Host Disease—Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Alexander, T.; Greco, R. Hematopoietic stem cell transplantation and cellular therapies for autoimmune diseases: Overview and future considerations from the Autoimmune Diseases Working Party (ADWP) of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2022, 57, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, S.H.C.; Rambaldi, B.; Shapiro, R.M.; Romee, R. Key Aspects of the Immunobiology of Haploidentical Hematopoietic Cell Transplantation. Front. Immunol. 2020, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.R.; Betts, B.C.; Tkachev, V.; Kean, L.S.; Blazar, B.R. Current Concepts and Advances in Graft-Versus-Host Disease Immunology. Annu. Rev. Immunol. 2021, 39, 19–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Desierto, M.J.; Feng, X.; Biancotto, A.; Young, N.S. Immune-mediated bone marrow failure in C57BL/6 mice. Exp. Hematol. 2015, 43, 256–267. [Google Scholar] [CrossRef]
- Chewning, J.H.; Zhang, W.; Randolph, D.A.; Swindle, C.S.; Schoeb, T.R.; Weaver, C.T. Allogeneic Th1 cells home to host bone marrow and spleen and mediate IFNgamma-dependent aplasia. Biol. Blood Marrow Transplant. 2013, 19, 876–887. [Google Scholar] [CrossRef]
- McDaniel Mims, B.; Jones-Hall, Y.; Dos Santos, A.P.; Furr, K.; Enriquez, J.; Grisham, M.B. Induction of acute graft vs. host disease in lymphopenic mice. Pathophysiology 2019, 26, 233–244. [Google Scholar] [CrossRef]
- Shono, Y.; Ueha, S.; Wang, Y.; Abe, J.; Kurachi, M.; Matsuno, Y.; Sugiyama, T.; Nagasawa, T.; Imamura, M.; Matsushima, K. Bone marrow graft-versus-host disease: Early destruction of hematopoietic niche after MHC-mismatched hematopoietic stem cell transplantation. Blood 2010, 115, 5401–5411. [Google Scholar] [CrossRef]
- Cuthbert, R.J.; Iqbal, A.; Gates, A.; Toghill, P.J.; Russell, N.H. Functional hyposplenism following allogeneic bone marrow transplantation. J. Clin. Pathol. 1995, 48, 257–259. [Google Scholar] [CrossRef]
- Muskens, K.F.; Lindemans, C.A.; Belderbos, M.E. Hematopoietic Dysfunction during Graft-Versus-Host Disease: A Self-Destructive Process? Cells 2021, 10, 2051. [Google Scholar] [CrossRef]
- Shono, Y.; Shiratori, S.; Kosugi-Kanaya, M.; Ueha, S.; Sugita, J.; Shigematsu, A.; Kondo, T.; Hashimoto, D.; Fujimoto, K.; Endo, T.; et al. Bone marrow graft-versus-host disease: Evaluation of its clinical impact on disrupted hematopoiesis after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2014, 20, 495–500. [Google Scholar] [CrossRef][Green Version]
- Sprent, J.; Surh, C.D.; Agus, D.; Hurd, M.; Sutton, S.; Heath, W.R. Profound atrophy of the bone marrow reflecting major histocompatibility complex class II-restricted destruction of stem cells by CD4+ cells. J. Exp. Med. 1994, 180, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.R.; Koyama, M. Cytokines and costimulation in acute graft-versus-host disease. Blood 2020, 136, 418–428. [Google Scholar] [CrossRef]
- Schroeder, M.A.; DiPersio, J.F. Mouse models of graft-versus-host disease: Advances and limitations. Dis. Model. Mech. 2011, 4, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, J.L.; Pai, C.C.; Murphy, W.J. Preclinical modeling of hematopoietic stem cell transplantation—Advantages and limitations. FEBS J. 2016, 283, 1595–1606. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Preclinical models of acute and chronic graft-versus-host disease: How predictive are they for a successful clinical translation? Blood 2016, 127, 3117–3126. [Google Scholar] [CrossRef] [PubMed]
- Rayasam, A.; Drobyski, W.R. Translational Clinical Strategies for the Prevention of Gastrointestinal Tract Graft Versus Host Disease. Front. Immunol. 2021, 12, 779076. [Google Scholar] [CrossRef]
- Enriquez, J.; Mims, B.M.D.; Trasti, S.; Furr, K.L.; Grisham, M.B. Genomic, microbial and environmental standardization in animal experimentation limiting immunological discovery. BMC Immunol. 2020, 21, 50. [Google Scholar] [CrossRef]
- Khuat, L.T.; Vick, L.V.; Dunai, C.; Collins, C.P.; More, S.K.; Le, C.T.; Pai, C.S.; Stoffel, K.M.; Maverakis, E.; Canter, R.J.; et al. Increased efficacy of dual proinflammatory cytokine blockade on acute GVHD while maintaining GVT effects. Blood 2021, 138, 2583–2588. [Google Scholar] [CrossRef]
- Blazar, B.R.; Hill, G.R.; Murphy, W.J. Dissecting the biology of allogeneic HSCT to enhance the GvT effect whilst minimizing GvHD. Nat. Rev. Clin. Oncol. 2020, 17, 475–492. [Google Scholar] [CrossRef]
- Zeiser, R.; Socie, G.; Blazar, B.R. Pathogenesis of acute graft-versus-host disease: From intestinal microbiota alterations to donor T cell activation. Br. J. Haematol. 2016, 175, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Castor, M.G.; Pinho, V.; Teixeira, M.M. The role of chemokines in mediating graft versus host disease: Opportunities for novel therapeutics. Front. Pharmacol. 2012, 3, 23. [Google Scholar] [CrossRef]
- Mapara, M.Y.; Leng, C.; Kim, Y.M.; Bronson, R.; Lokshin, A.; Luster, A.; Sykes, M. Expression of chemokines in GVHD target organs is influenced by conditioning and genetic factors and amplified by GVHR. Biol. Blood Marrow Transplant. 2006, 12, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Dudakov, J.A.; Mertelsmann, A.M.; O’Connor, M.H.; Jenq, R.R.; Velardi, E.; Young, L.F.; Smith, O.M.; Boyd, R.L.; van den Brink, M.R.M.; Hanash, A.M. Loss of thymic innate lymphoid cells leads to impaired thymopoiesis in experimental graft-versus-host disease. Blood 2017, 130, 933–942. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Inohara, N.; Nunez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Gyurkocza, B.; Sandmaier, B.M. Conditioning regimens for hematopoietic cell transplantation: One size does not fit all. Blood 2014, 124, 344–353. [Google Scholar] [CrossRef]
- Bucsek, M.J.; Giridharan, T.; MacDonald, C.R.; Hylander, B.L.; Repasky, E.A. An overview of the role of sympathetic regulation of immune responses in infectious disease and autoimmunity. Int. J. Hyperthermia 2018, 34, 135–143. [Google Scholar] [CrossRef]
- Hylander, B.L.; Gordon, C.J.; Repasky, E.A. Manipulation of Ambient Housing Temperature To Study the Impact of Chronic Stress on Immunity and Cancer in Mice. J. Immunol. 2019, 202, 631–636. [Google Scholar] [CrossRef]
- Hylander, B.L.; Repasky, E.A. Thermoneutrality, Mice, and Cancer: A Heated Opinion. Trends Cancer 2016, 2, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Karp, C.L. Unstressing intemperate models: How cold stress undermines mouse modeling. J. Exp. Med. 2012, 209, 1069–1074. [Google Scholar] [CrossRef]
- Graham, J.B.; Swarts, J.L.; Mooney, M.; Choonoo, G.; Jeng, S.; Miller, D.R.; Ferris, M.T.; McWeeney, S.; Lund, J.M. Extensive Homeostatic T Cell Phenotypic Variation within the Collaborative Cross. Cell Rep. 2017, 21, 2313–2325. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.B.; Thomas, S.; Swarts, J.; McMillan, A.A.; Ferris, M.T.; Suthar, M.S.; Treuting, P.M.; Ireton, R.; Gale, M., Jr.; Lund, J.M. Genetic diversity in the collaborative cross model recapitulates human West Nile virus disease outcomes. MBio 2015, 6, e00493-15. [Google Scholar] [CrossRef]
- Martin, M.D.; Danahy, D.B.; Hartwig, S.M.; Harty, J.T.; Badovinac, V.P. Revealing the Complexity in CD8 T Cell Responses to Infection in Inbred C57B/6 versus Outbred Swiss Mice. Front. Immunol. 2017, 8, 1527. [Google Scholar] [CrossRef]
- Nikodemova, M.; Watters, J.J. Outbred ICR/CD1 mice display more severe neuroinflammation mediated by microglial TLR4/CD14 activation than inbred C57Bl/6 mice. Neuroscience 2011, 190, 67–74. [Google Scholar] [CrossRef]
- Reichenbach, D.K.; Li, Q.; Hoffman, R.A.; Williams, A.L.; Shlomchik, W.D.; Rothstein, D.M.; Demetris, A.J.; Lakkis, F.G. Allograft outcomes in outbred mice. Am. J. Transplant. 2013, 13, 580–588. [Google Scholar] [CrossRef]
- Von Herrath, M.G.; Nepom, G.T. Lost in translation: Barriers to implementing clinical immunotherapeutics for autoimmunity. J. Exp. Med. 2005, 202, 1159–1162. [Google Scholar] [CrossRef]
- Chen, J.; Lipovsky, K.; Ellison, F.M.; Calado, R.T.; Young, N.S. Bystander destruction of hematopoietic progenitor and stem cells in a mouse model of infusion-induced bone marrow failure. Blood 2004, 104, 1671–1678. [Google Scholar] [CrossRef]
- McKean, D.J.; Melvold, R.W.; David, C. Tryptic peptide comparison of Ia antigen alpha and beta polypeptides from the I-A mutant B6.C-H-2bm12 and its congenic parental strain B6. Immunogenetics 1981, 14, 41–51. [Google Scholar] [CrossRef]
- Reinoso Webb, C.; den Bakker, H.; Koboziev, I.; Jones-Hall, Y.; Rao Kottapalli, K.; Ostanin, D.; Furr, K.L.; Mu, Q.; Luo, X.M.; Grisham, M.B. Differential Susceptibility to T Cell-Induced Colitis in Mice: Role of the Intestinal Microbiota. Inflamm. Bowel Dis. 2018, 24, 361–379. [Google Scholar] [CrossRef]
- Kurmaeva, E.; Lord, J.D.; Zhang, S.; Bao, J.R.; Kevil, C.G.; Grisham, M.B.; Ostanin, D.V. T cell-associated alpha4beta7 but not alpha4beta1 integrin is required for the induction and perpetuation of chronic colitis. Mucosal Immunol. 2014, 7, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- McDaniel Mims, B.; Enriquez, J.; Pires Dos Santos, A.; Jones-Hall, Y.; Dowd, S.; Furr, K.L.; Grisham, M.B. Antibiotic administration exacerbates acute graft vs. host disease-induced bone marrow and spleen damage in lymphopenic mice. PLoS ONE 2021, 16, e0254845. [Google Scholar] [CrossRef]
- Baker, M.B.; Riley, R.L.; Podack, E.R.; Levy, R.B. Graft-versus-host-disease-associated lymphoid hypoplasia and B cell dysfunction is dependent upon donor T cell-mediated Fas-ligand function, but not perforin function. Proc. Natl. Acad. Sci. USA 1997, 94, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Ebens, C.L.; Perkey, E.; Radojcic, V.; Koch, U.; Scarpellino, L.; Tong, A.; Allen, F.; Wood, S.; Feng, J.; et al. Fibroblastic niches prime T cell alloimmunity through Delta-like Notch ligands. J. Clin. Investig. 2017, 127, 1574–1588. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Bhan, A.K. A case for regulatory B cells. J. Immunol. 2006, 176, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Agbogan, V.A.; Gastineau, P.; Tejerina, E.; Karray, S.; Zavala, F. CpG-Activated Regulatory B-Cell Progenitors Alleviate Murine Graft-Versus-Host-Disease. Front. Immunol. 2022, 13, 790564. [Google Scholar] [CrossRef]
- Candando, K.M.; Lykken, J.M.; Tedder, T.F. B10 cell regulation of health and disease. Immunol. Rev. 2014, 259, 259–272. [Google Scholar] [CrossRef]
- DiLillo, D.J.; Matsushita, T.; Tedder, T.F. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann. N. Y Acad. Sci. 2010, 1183, 38–57. [Google Scholar] [CrossRef]
- Hu, Y.; He, G.L.; Zhao, X.Y.; Zhao, X.S.; Wang, Y.; Xu, L.P.; Zhang, X.H.; Yu, X.Z.; Liu, K.Y.; Chang, Y.J.; et al. Regulatory B cells promote graft-versus-host disease prevention and maintain graft-versus-leukemia activity following allogeneic bone marrow transplantation. Oncoimmunology 2017, 6, e1284721. [Google Scholar] [CrossRef]
- Lee, K.M.; Fu, Q.; Huai, G.; Deng, K.; Lei, J.; Kojima, L.; Agarwal, D.; van Galen, P.; Kimura, S.; Tanimine, N.; et al. Suppression of allograft rejection by regulatory B cells induced via TLR signaling. JCI Insight 2022, 7, e152213. [Google Scholar] [CrossRef] [PubMed]
- Kalampokis, I.; Yoshizaki, A.; Tedder, T.F. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res. Ther. 2013, 15 (Suppl. S1), S1. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Leader, A.M.; Merad, M. MDSC: Markers, development, states, and unaddressed complexity. Immunity 2021, 54, 875–884. [Google Scholar] [CrossRef]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Ghobadinezhad, F.; Ebrahimi, N.; Mozaffari, F.; Moradi, N.; Beiranvand, S.; Pournazari, M.; Rezaei-Tazangi, F.; Khorram, R.; Afshinpour, M.; Robino, R.A.; et al. The emerging role of regulatory cell-based therapy in autoimmune disease. Front. Immunol. 2022, 13, 1075813. [Google Scholar] [CrossRef]
- Umansky, V.; Blattner, C.; Gebhardt, C.; Utikal, J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines 2016, 4, 36. [Google Scholar] [CrossRef]
- Rosser, E.C.; Mauri, C. Regulatory B cells: Origin, phenotype, and function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Rosser, E.C.; Mauri, C. The emerging field of regulatory B cell immunometabolism. Cell Metab. 2021, 33, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Roch, T.; Lampropoulou, V.; O’Connor, R.A.; Stervbo, U.; Hilgenberg, E.; Ries, S.; Dang, V.D.; Jaimes, Y.; Daridon, C.; et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 2014, 507, 366–370. [Google Scholar] [CrossRef]
- Leigh, N.D.; Kokolus, K.M.; O’Neill, R.E.; Du, W.; Eng, J.W.; Qiu, J.; Chen, G.L.; McCarthy, P.L.; Farrar, J.D.; Cao, X.; et al. Housing Temperature-Induced Stress Is Suppressing Murine Graft-versus-Host Disease through beta2-Adrenergic Receptor Signaling. J. Immunol. 2015, 195, 5045–5054. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Sarow, J.L.; MacDonald, C.R.; Chen, G.L.; Qiu, J.; Sharma, U.C.; Cao, X.; Herr, M.M.; Hahn, T.E.; Blazar, B.R.; et al. beta2-Adrenergic receptor activation on donor cells ameliorates acute GvHD. JCI Insight 2020, 5, 137788. [Google Scholar] [CrossRef] [PubMed]
- Arieta Kuksin, C.; Gonzalez-Perez, G.; Minter, L.M. CXCR4 expression on pathogenic T cells facilitates their bone marrow infiltration in a mouse model of aplastic anemia. Blood 2015, 125, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Feng, X.; Desierto, M.J.; Keyvanfar, K.; Young, N.S. IFN-gamma-mediated hematopoietic cell destruction in murine models of immune-mediated bone marrow failure. Blood 2015, 126, 2621–2631. [Google Scholar] [CrossRef] [PubMed]
- Young, N.S. Aplastic Anemia. N. Engl. J. Med. 2018, 379, 1643–1656. [Google Scholar] [CrossRef] [PubMed]
- Marin, N.; Mecha, M.; Espejo, C.; Mestre, L.; Eixarch, H.; Montalban, X.; Alvarez-Cermeno, J.C.; Guaza, C.; Villar, L.M. Regulatory lymphocytes are key factors in MHC-independent resistance to EAE. J. Immunol. Res. 2014, 2014, 156380. [Google Scholar] [CrossRef]
- Cai, Y.; Ma, S.; Liu, Y.; Gong, H.; Cheng, Q.; Hu, B.; Wu, Y.; Yu, X.; Dong, C.; Sun, K.; et al. Adoptively transferred donor IL-17-producing CD4(+) T cells augment, but IL-17 alleviates, acute graft-versus-host disease. Cell Mol. Immunol. 2018, 15, 233–245. [Google Scholar] [CrossRef]
- Iclozan, C.; Yu, Y.; Liu, C.; Liang, Y.; Yi, T.; Anasetti, C.; Yu, X.Z. T helper17 cells are sufficient but not necessary to induce acute graft-versus-host disease. Biol. Blood Marrow Transplant. 2010, 16, 170–178. [Google Scholar] [CrossRef]
- Wysocki, C.A.; Panoskaltsis-Mortari, A.; Blazar, B.R.; Serody, J.S. Leukocyte migration and graft-versus-host disease. Blood 2005, 105, 4191–4199. [Google Scholar] [CrossRef]
- Collison, L.W.; Vignali, D.A. In vitro Treg suppression assays. Methods Mol. Biol. 2011, 707, 21–37. [Google Scholar]
- Carpenter, K.C.; Zhou, Y.; Hakenjos, J.M.; Fry, C.D.; Nemzek, J.A. Thermoneutral Housing Temperature Improves Survival in a Murine Model of Polymicrobial Peritonitis. Shock 2020, 54, 688–696. [Google Scholar] [CrossRef]
- Li, J.; Dubois, W.; Thovarai, V.; Wu, Z.; Feng, X.; Peat, T.; Zhang, S.; Sen, S.K.; Trinchieri, G.; Chen, J.; et al. Attenuation of immune-mediated bone marrow damage in conventionally housed mice. Mol. Carcinog. 2020, 59, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Maron, R.; Hancock, W.W.; Slavin, A.; Hattori, M.; Kuchroo, V.; Weiner, H.L. Genetic susceptibility or resistance to autoimmune encephalomyelitis in MHC congenic mice is associated with differential production of pro- and anti-inflammatory cytokines. Int. Immunol. 1999, 11, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Lykken, J.M.; Candando, K.M.; Tedder, T.F. Regulatory B10 cell development and function. Int. Immunol. 2015, 27, 471–477. [Google Scholar] [CrossRef]
- Tedder, T.F. B10 cells: A functionally defined regulatory B cell subset. J. Immunol. 2015, 194, 1395–1401. [Google Scholar] [CrossRef]
- Ishio, T.; Sugita, J.; Tateno, T.; Hidaka, D.; Hayase, E.; Shiratori, S.; Okada, K.; Goto, H.; Onozawa, M.; Nakagawa, M.; et al. Hematogones Predict Better Outcome in Allogeneic Hematopoietic Stem Cell Transplantation Irrespective of Graft Sources. Biol. Blood Marrow Transplant. 2018, 24, 1990–1996. [Google Scholar] [CrossRef]
- Michonneau, D.; Peffault de Latour, R.; Porcher, R.; Robin, M.; Benbunan, M.; Rocha, V.; Ribaud, P.; Ferry, C.; Devergie, A.; Vanneaux, V.; et al. Influence of bone marrow graft B lymphocyte subsets on outcome after HLA-identical sibling transplants. Br. J. Haematol. 2009, 145, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Doki, N.; Haraguchi, K.; Hagino, T.; Igarashi, A.; Najima, Y.; Kobayashi, T.; Kakihana, K.; Okuyama, Y.; Sakamaki, H.; Ohashi, K. Clinical impact of hematogones on outcomes of allogeneic hematopoietic stem cell transplantation. Ann. Hematol. 2015, 94, 2055–2060. [Google Scholar] [CrossRef]
- Shima, T.; Miyamoto, T.; Kikushige, Y.; Mori, Y.; Kamezaki, K.; Takase, K.; Henzan, H.; Numata, A.; Ito, Y.; Takenaka, K.; et al. Quantitation of hematogones at the time of engraftment is a useful prognostic indicator in allogeneic hematopoietic stem cell transplantation. Blood 2013, 121, 840–848. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gu, L.; Fu, B.; Sui, X.; Xu, H. Abnormal expression of b10 cell frequencies: Possible relation to pathogenesis and disease severity of aplastic anemia. Rev. Assoc. Med. Bras. 2019, 65, 637–646. [Google Scholar] [CrossRef]
- Zaimoku, Y.; Patel, B.A.; Kajigaya, S.; Feng, X.; Alemu, L.; Quinones Raffo, D.; Groarke, E.M.; Young, N.S. Deficit of circulating CD19(+) CD24(hi) CD38(hi) regulatory B cells in severe aplastic anaemia. Br. J. Haematol. 2020, 190, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Kim, J.; Gonzalez-Matias, G.; Aggarwal, N.; Manley, A.L.; Wu, Z.; Solorzano, S.; Batchu, S.; Gao, S.; Chen, J.; et al. Granulocytic myeloid-derived suppressor cells to prevent and treat murine immune-mediated bone marrow failure. Blood Adv. 2023, 7, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Chen, L.; Wu, H.; Huo, J.; Jiang, Z.; Shao, Y.; Ren, X.; Huang, J.; Li, X.; Wang, M.; et al. Impaired immunosuppressive role of myeloid-derived suppressor cells in acquired aplastic anemia. Haematologica 2022, 107, 2834–2845. [Google Scholar] [CrossRef] [PubMed]




| Cytokine | Syngeneic | Allogeneic | p Value |
|---|---|---|---|
| GM-CSF | 18.6 ± 18.0 | 0 | 0.4366 |
| IFN-β | 0 | 0 | ------ |
| IFN-γ | 0 | 15.67 ± 3.3 | 0.0024 |
| IL-10 | 109 ± 20 | 141 ± 33 | 0.416 |
| IL-12p70 | 0 | 0 | ------ |
| IL-17A | 3.29 ± 3.30 | 0 | 0.1692 |
| IL-1α | 28.4 ± 13.0 | 4.21 ± 2.30 | 0.1801 |
| IL-1β | 19.4 ± 2.63 | 21.0 ± 1.80 | 0.6492 |
| IL-23 | 0 | 0 | ------ |
| IL-27 | 1130 ± 582 | 299 ± 113 | 0.2851 |
| IL-6 | 0 | 137± 99 | 0.1575 |
| MCP-1 | 13.9 ± 2.0 | 107 ± 48 | 0.0691 |
| TNF-α | 1.26 ± 3.3 | 49.4 ± 14.3 | 0.0123 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enriquez, J.; McDaniel Mims, B.; Stroever, S.; dos Santos, A.P.; Jones-Hall, Y.; Furr, K.L.; Grisham, M.B. Influence of Housing Temperature and Genetic Diversity on Allogeneic T Cell-Induced Tissue Damage in Mice. Pathophysiology 2023, 30, 522-547. https://doi.org/10.3390/pathophysiology30040039
Enriquez J, McDaniel Mims B, Stroever S, dos Santos AP, Jones-Hall Y, Furr KL, Grisham MB. Influence of Housing Temperature and Genetic Diversity on Allogeneic T Cell-Induced Tissue Damage in Mice. Pathophysiology. 2023; 30(4):522-547. https://doi.org/10.3390/pathophysiology30040039
Chicago/Turabian StyleEnriquez, Josue, Brianyell McDaniel Mims, Stephanie Stroever, Andrea Pires dos Santos, Yava Jones-Hall, Kathryn L. Furr, and Matthew B. Grisham. 2023. "Influence of Housing Temperature and Genetic Diversity on Allogeneic T Cell-Induced Tissue Damage in Mice" Pathophysiology 30, no. 4: 522-547. https://doi.org/10.3390/pathophysiology30040039
APA StyleEnriquez, J., McDaniel Mims, B., Stroever, S., dos Santos, A. P., Jones-Hall, Y., Furr, K. L., & Grisham, M. B. (2023). Influence of Housing Temperature and Genetic Diversity on Allogeneic T Cell-Induced Tissue Damage in Mice. Pathophysiology, 30(4), 522-547. https://doi.org/10.3390/pathophysiology30040039






