Abstract
Subarachnoid hemorrhage (SAH) is a type of hemorrhagic stroke resulting from the rupture of an arterial vessel within the brain. Unlike other stroke types, SAH affects both young adults (mid-40s) and the geriatric population. Patients with SAH often experience significant neurological deficits, leading to a substantial societal burden in terms of lost potential years of life. This review provides a comprehensive overview of SAH, examining its development across different stages (early, intermediate, and late) and highlighting the pathophysiological and pathohistological processes specific to each phase. The clinical management of SAH is also explored, focusing on tailored treatments and interventions to address the unique pathological changes that occur during each stage. Additionally, the paper reviews current treatment modalities and pharmacological interventions based on the evolving guidelines provided by the American Heart Association (AHA). Recent advances in our understanding of SAH will facilitate clinicians’ improved management of SAH to reduce the incidence of delayed cerebral ischemia in patients.
1. Introduction
Subarachnoid hemorrhage (SAH) is defined as bleeding between the arachnoid and pia mater in the central nervous system (CNS). SAH is classified as either traumatic (an inciting event) or spontaneous (without an immediate identifiable cause). Traumatic SAH (tSAH) is bleeding into the subarachnoid space secondary to head trauma (e.g., falls, gunshot wounds, motor vehicle accidents) [1]. Spontaneous SAH (sSAH) is non-traumatic bleeding, most frequently caused by a ruptured cerebral aneurysm [2]. A further sub-classification of SAH is an aneurysmal SAH (aSAH) [3].
According to the most recent World Health Organization report, aSAH is 10-fold higher in Asia than in Europe [4,5,6]. Furthermore, low- to middle-income countries experience a disproportionately high burden (two-fold) compared to their higher-income counterparts [6]. These figures are disconcerting, as the incidence may be higher than reported since an estimated 12–15% of all aSAHs result in pre-hospital patient death [7,8].
Men have a higher incidence of aSAH between the ages of 25 and 45 or at older than 85 years of age, while women have a peak incidence of aSAH at 50–55 years of age [4,9,10]. Although the incidence of aSAH has decreased in women, they are still 1.24 times more likely than men to experience aSAH [8]. In fact, enhanced sex hormone-binding globulin and low bioavailable testosterone can enhance aSAH risk in women [10]. To further our understanding of the incidence and mechanism(s) of SAH, we have delineated the most important factors, such as inflammation, endothelial dysfunction, and blood within the subarachnoid spaces, followed by clinical implications and current treatment modalities.
2. Loss of Cerebral Autoregulation in SAH
The cerebral vasculature maintains blood flow through modulation of vascular tonicity [11]. This process, called cerebral autoregulation, ensures that perfusion is maintained over a broad range of cerebral perfusion pressures [12]. Cerebral autoregulation is primarily a non-neural, myogenic response to changes in transmural pressure (i.e., pressure across the arterial wall) [13,14]. Specifically, a physiologic increase in transmural pressure produces myogenic vasoconstriction, while a decrease in transmural pressure produces myogenic relaxation and vasodilation [15]. At the molecular level, cerebral autoregulation converts vascular wall tension into membrane depolarization and actin–myosin filament activity [16,17]. In contrast to cerebral autoregulation, neurovascular coupling is neural regulation of perfusion based on local metabolic activity [18]. These two different determinants of cerebral perfusion are often called “pressure” (i.e., cerebral autoregulation) versus “metabolic” (i.e., neurovascular coupling) autoregulation [13]. The function of cerebral autoregulation is the brain’s ability to maintain constant blood flow independent of changes in mean arterial pressure of 60–150 mm Hg [12,19]. Most interestingly, the loss of cerebral autoregulation has been associated with an increase in microvascular spasms [13], which have been correlated with the development of delayed cerebral ischemia (DCI).
A disastrous consequence of SAH is reduction in cerebral blood flow (CBF), which is often followed by DCI. Rupture of blood vessel(s) provokes a devastating cascade of events, which begin within minutes to hours after the initial insult and often lead to permanent disability or death [3]. Following uncontrolled elevations in blood flow, often reflecting high arterial blood pressure in the cranial vault, intracranial pressure (ICP) can quickly exceed physiological limits (ICP greater than 20–25 mm Hg) [20]. Blood pooling from the cranial vault and the expanding hematoma both contribute to elevated ICP, which can then herniate the brain, with life-threatening consequences [21].
The reduction in brain perfusion via aSAH is problematic, as brain tissue relies almost exclusively on aerobic respiration for support of its metabolism [22]. Even brief periods of hypoxia (loss of perfusion > 3–5 min) can lead to permanent neuronal tissue injury [23]. Initially, the hypoperfused tissue will upregulate anaerobic glycolysis, but this process cannot permanently sustain sufficient levels of adenosine triphosphate (ATP) [24], which is required for cellular homeostasis and energy-dependent processes (e.g., maintaining ionic gradients.) The loss of these ionic gradients can lead to cytotoxic edema and neuronal cell death [25].
3. Early Brain Injury and Acute Ischemia (0–3 Days)
Following the initial disruption to CBF via aSAH, blood enters the subarachnoid space and deposits fibrin, fibrinogen, and red blood cells (RBCs) [26]. Blood in the subarachnoid space rapidly disperses into the perivascular space (PVS), which is the area between the outer vascular wall and the glial limitans, which surrounds arterioles and capillaries and/or astrocytic feet [27]. Red blood cells are often lysed during SAH, resulting in elevated hemoglobin levels, which are then released into the brain parenchyma [28]. Since hemoglobin is primarily composed of ferritin, free iron from hemoglobin binds to tissues under oxygenation and generates free radicals, such as superoxide, nitric oxide and its metabolites, hydrogen peroxide, and oxidant heme through the Fenton reaction [29]. As a consequence, free radical production can enhance oxidative stress locally and in the brain penumbra [30,31,32,33,34]. Interestingly, deferoxamine (iron chelator) administered post-SAH can provide neuroprotection [31,33].
3.1. Glycocalyx
The glycocalyx is an important layer lining the internal wall of blood vessels as it can attenuate BBB permeability and is a vasculoprotective [35] barrier between the circulating blood and the endothelium facing the lumen [36,37]. The glycocalyx consists predominantly of proteoglycan and a glycoprotein backbone with glycosaminoglycan connections [35]. It is under constant remodeling due to hemodynamic changes, enzymatic degradation, and shear stress [36]. Interestingly, SAH can disrupt the glycocalyx, often associated with the upregulation of inflammatory cytokine production, reduced nitric oxide production, and neurovascular uncoupling [38].
aSAH can trigger upregulation of pro-inflammatory cytokines (e.g., TNF-alpha, IL-1, IL-6), which can in turn initiate cytokine-induced breakdown of the glycocalyx, leading to endothelium and adhesion molecule (i.e., VCAM, ICAM) dysfunction [38,39,40,41]. While glycocalyx degradation can expose the endothelium to pro-inflammatory cytokine-mediated damage, it also reduces endothelial nitric oxide [38].
During SAH, glycocalyx degradation occurs from the increased expression of inflammatory markers (e.g., IL-1, IL-6, TNF-alpha), atrial natriuretic peptides, and vascular shear stress [37]. Following disruption of the glycocalyx, endothelial NO production is disrupted, leading to vascular smooth muscle cell-mediated vasoconstriction [42]. Additionally, glycocalyx’s breakdown exposes its molecular backbone, which contains the molecules glypican and heparin sulfate, both of which serve to increase neutrophil migration and adhesion and potentiate platelet activation [43,44]. These compounding factors lead to further vascular compromise.
3.2. Endothelial Dysfunction and Neuroinflammation
Endothelial dysfunction in aSAH is characterized by the loss of NO production, unregulated coagulation (via coagulation cascade dysfunction), and enhanced permeability due to glycocalyx impairment [45,46]. Loss of the glycocalyx promotes coagulation since anticoagulant molecules (e.g., anti-thrombin, tissue factor inhibitor pathway, and vWF) are decreased to prevent platelet adhesion and aggregation [38]. aSAH-mediated damage to the endothelium can also decrease prostacyclin release, which prevents platelet adhesion [38]. Furthermore, TNF-α stimulates exposed endothelium to upregulate P-selectin and increase platelet–leukocyte–endothelial cell interactions [47,48].
3.3. Ischemia, Endothelial Dysfunction, and ACE-2: ‘Death in Rigor’
In our recent research, we discovered that ischemic stress to the cerebrum triggers a progressive, significant loss of perfusion, which occurs within the first 24 h, setting off a cascade of events that can have profound implications for patients’ outcomes [49]. Our investigations revealed that the progressive loss of perfusion is closely linked to the disruption of ACE-2-dependent vasodilation and anticoagulation mechanisms [50,51,52]. ACE-2, known for its vital role in regulating blood vessel tone and preventing excessive blood clotting, seems to be compromised under ischemic stress, as may occur during SAH, and post-ischemic vasoconstriction may reflect the constriction and death of microvascular pericytes and intense vasoconstriction, described by Hall et al. as ‘death in rigor’ [49]. Death in rigor may have adaptive value in very small regions of intense ischemia, in which it may control hemorrhage, but globally or regionally, such disruption leads to compromised perfusion, depriving large areas of the brain of essential oxygen and nutrients [53]. The consequences of reduced perfusion are devastating and may mediate injury in SAH, as has been found in ischemic stroke. Large regions of the cerebral volume become susceptible to ‘death in rigor’ [49] and may be sacrificed in the brain due to severe lack of blood flow, resulting in irreversible damage and impaired functionality. An improved understanding of the complex relationships among ischemic stress, ACE-2, perfusion loss, and ‘death in rigor’ may enable innovative approaches that can mitigate the consequences of both SAH and ischemic stroke [49].
3.4. Neuroinflammation
aSAH can lead to infiltration of neutrophils at the site of injury, along with systemic neutrophilia via enhanced IL-6 [54]. This process precipitates neutrophil recruitment to the brain between 12 and 48 h following aSAH [55]. Additionally, microglia activation and monocyte recruitment readily occur within the first 48 h of aSAH to promote neuroinflammation [55]. Neutrophil accumulation on endothelial membranes increases oxidative stress (via myeloperoxidase) and lipid peroxidation, resulting in endothelium damage [53,54,56,57]. Toll-like receptor 4 (TLR4) is enhanced in the activated microglia and macrophages, resulting in the secretion of TNF [58]. Free heme from RBC hemolysis forms reactive oxygen species, resulting in upregulation of metalloproteinase-9 (MMP-9) and breakdown of the vascular basement membrane [59].
3.5. Astrocytes
Naïve astrocyte activation occurs during SAH, transforming the cells into the A1 neurotoxic phenotype [60,61]. The A1 phenotype is characterized by upregulation of glial fibrillary acidic protein (GFAP), S100 calcium binding protein B (S100B), and C3, H2-D1, and serping1 cell markers [62]. A1 astrocytes are a form of reactive astrocyte, promoting cell death by releasing proinflammatory cytokines [61]. A2 astrocytes are another phenotypic variant in reactive astrocytes that play a neuroprotective role, in contrast to the dominant A1 neurotoxic phenotype found in SAH [63,64,65,66]. Reactive astrocytes (RAs) display a spectrum of pro- and anti-inflammatory profiles, rather than the simple A1 and A2 phenotypes based on genetic sequencing [67]. Overall, the pro-inflammatory RAs found in SAH exhibit strong neurotoxicity—forming fewer synapses and killing neurons with detached axons [68,69]. Activated astrocytes also undergo morphological changes after SAH, such as end feet swelling and protrusions compressing the capillary lumen [70].
3.6. Glymphatic System
The glymphatic system (GS) is a fluid exchange and drainage system supported by glial cells [71]. The GS includes the entire peripheral vascular system and consists of periarterial cerebrospinal fluid inlets and perivenous interstitial fluid (ISF) outlets [71]. Astrocytic end plates mediate the transport of metabolites via aquaporin-4 (AQP4) channels [72]. This transport system was confirmed with the use of fluorescent tracers administered into the brain parenchyma, which showed deposition in the meningeal lymphatic vessels and ultimately the deep cervical lymph nodes (dCLNs) [73]. Overall, the glymphatic system facilitates transport and clearance of metabolic waste under normal physiologic conditions [74], while during SAH, the glymphatic system is involved in the clearance of neurotoxic solutes, pro-inflammatory cytokines, and erythrocytes [71,75]. This process can be visualized in Figure 1 below.
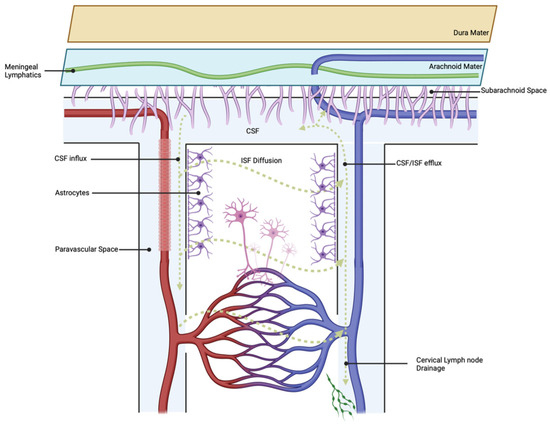
Figure 1.
Glymphatic outflow proceeds via the mechanism described above, resulting in the clearance of toxic waste solutes under normal physiologic conditions.
After the initial SAH event, blood and its metabolites rapidly leak into the PVS. Blood in the PVS diffuses into the perivascular parenchyma via the GS, leading to a cascade of glial activation, neurotoxicity, and widespread microcirculatory dysfunction in the brain [76]. A recent study performed by Chen et al. found that meningeal lymphatics drained extravasated erythrocytes into the cerebral spinal fluid [75]. Following experimental ablation of meningeal lymphatics via the injection of Visudyne, there was a measurable decline in RBCs found in the cervical nymph nodes [75]. Additionally, following meningeal ablation, neuroinflammation and neurologic deficits were increased in a rodent model. Persistent malfunction of glymphatic and meningeal drainage was observed in an SAH mouse model by Pu et al. [77]. Decreased tracer was observed in the SAH mice compared to controls in meningeal lymphatic vessels and dcLNs [77], suggesting that SAH impairs the ability of meningeal lymphatic vessels to drain cellular debris, immune cells, and inflammatory mediators [78,79]. Additionally, glial cells lining the glymphatic regions become activated in SAH mice, as determined by increased levels of IL-1β, IL-6, and TNF-α expression [77]. This process results in AQP4 upregulation surrounding the arteries, while the AQP4 expression remains the same at the drainage sites around the veins [80]. Consequently, impaired PVS and ISF flow reduces metabolite and hemorrhagic clearance, leading to intermediate injury via immune cell accumulation [81], BBB dysfunction [82], neuronal apoptosis [77], vasculitis [76], cerebral edema [83], and acute hydrocephalus [84].
3.7. SAH and the Intramural Periarterial Drainage (IPAD)
In addition to the glymphatic system, there is another drainage system called the intramural periarterial drainage (IPAD), which has been described as functioning in parallel with the glymphatic system. This system can also be affected during subarachnoid hemorrhage (SAH). Interstitial fluid (ISF), which is the fluid found between brain cells, can drain from the brain along the basement membranes of arteries through the IPAD pathway. Disruptions in the IPAD pathway can lead to problems with proteostasis in the brain, such as in cerebral amyloid angiopathy or Alzheimer’s disease. A study by Sun et al. [85] examined disturbances in the IPAD pathway after SAH in rats. In that study, the authors injected dyes of different sizes into the cisterna magna, a fluid-filled compartment at the back of the brain in the posterior cranial fossa. The cisterna magna is one of the subarachnoid spaces, which is filled with cerebrospinal fluid (CSF) and surrounds the brain and spinal cord. Sun’s group observed that these dyes entered the brain through periarterial channels and were cleared through the basement membranes of the brain’s associated capillaries. Interestingly, different molecular weight tracers showed different patterns of clearance. SAH significantly disrupted the IPAD pathway, causing enlargement of spaces around the blood vessels where ISF clearance was reduced. This effect was related to endothelial cell death, activation of astrocytes (a type of brain cell), increased levels of matrix metalloproteinase-9, and loss of type IV collagen in the basement membrane. Consequently, experimental SAH in rats has been found to significantly disrupt the IPAD pathway, which could have important clinical implications for SAH. These implications include problems with proteostasis, amyloid angiopathy, and other forms of abnormal protein accumulation in the brain associated with neurodegeneration. A representation of the IPAD mechanism can be seen in Figure 2 below.
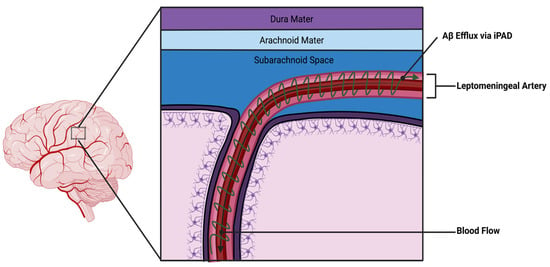
Figure 2.
Pulsatile forces from the leptomeningeal artery drive retrograde CSF outflow along a periarterial path.
4. Intermediate Injury (3–5 Days)
4.1. BBB Dysfunction
A hallmark of SAH is BBB degradation [86] in the form of endothelial and pericyte dysfunction [87]. Extracellular matrix proteins are also degraded following SAH-mediated degradation of endothelial cells [88].
Cytokines are locally released following SAH-mediated neuroinflammation to degrade cell adhesion molecules (i.e., ICAM-VCAM). Moreover, SAH-mediated neuroinflammation can cause a leaky BBB, which has been shown to decrease tight junction proteins such as ZO-1, occludin, and JAMa.
Pericytes are also important regulators in response to the change in cerebral blood flow with respect to local neuronal activity [89,90,91]. Pericytes also regulate and maintain the BBB by supporting tight junctional proteins [92]. Three to five days after brain injury, glycocalyx breakdown and endothelial dysfunction lead to increased BBB permeability [93]. BBB leakage allows for plasma protein extravasation, such as albumin, activating astrocytes, which in turn disrupt the neurovascular coupling [94]. More importantly, due to increased BBB leakage, extravasation of plasma proteins into the brain parenchyma is a major contributor to brain edema [94,95].
Pericytes amplify the inflammatory response by increasing their expression of microglial markers [96], upregulating periarterial AQP4 anchored in astrocytic end feet [97,98] and enabling extravasation of immune cells [99]. Smooth muscle cells (SMCs) undergo phenotypic transformation and migrate to the subepithelial layer [100]. IL-1β stimulates SMC proliferation in cerebral arteries and arterioles [101] and pericyte invasion of capillary networks, producing basement membrane remodeling [102]. Migration of transformed pericytes and SMCs increases the propensity for vessel spasm [103,104,105]. EC apoptosis starts around 24 h following SAH [106].
Astrocytes also modulate local vasoconstriction and vasodilation by releasing vasoactive compounds that stimulate 20-hydroxyeicosa-tetraenoic acid (20-HETE) production in pericytes [107], Intracellular Ca2+ concentrations in pericytes are also regulated by astrocytes promoting contraction [89,108]. Upregulation of AQP4 in astrocytes accelerates the formation of cytotoxic brain edema, leading to intracellular water accumulation [109,110]. Early in SAH, cellular edema in astrocytes produces an influx of ionic and vasogenic edema, leading to parenchymal edema [95]. Ionic edema develops when osmotic forces in the vasculature propel plasma through the vessel wall into the CNS [95]. The trans-endothelial Na+ gradient increases across the endothelium, leading to Na+ accumulation in the brain parenchyma [111]. This effect is magnified with early-onset CSF hypersecretion by the choroid plexi during SAH [112,113] and declining ISF efflux [114]. The large increase in parenchymal influx from extravasation and hydrocephalus exceeds the clearance rate provided by the glymphatic system. Tight junction breakdown and EC dysfunction contribute to vasogenic edema (e.g., extravasation of plasma proteins) [95]. Increased BBB permeability, cell death, and microvascular spasm and decreased ISF waste clearance escalate shifts in ion and water balance [95,115,116]. This process leads to neurovascular uncoupling, resulting in increased neuron susceptibility to terminal injury from cortical spreading depolarizations [117], excitotoxicity [118], neuronal apoptosis [81], and secondary brain injury [119].
4.2. Neurovascular Uncoupling
The neurovascular unit (NVU) is comprised of the BBB (e.g., ECs, pericytes, SMCs, astrocytes) and its communication with neurons and microglia [120]. The NVU serves to modulate pressure to local regions under normal physiologic conditions, such as regions that process reading, arithmetic, languages, etc. The NVU can also respond to pathophysiologic stimuli, such as seizures or CSD. The neurovascular unit is coupled when the local blood supply matches neuronal demand via modulation of vascular diameter [121]. The cellular interactions in the NVU dynamically regulate this activity [122]. Glutamate released from neurons stimulates nearby astrocytes and pericytes, generating vasoactive molecules [123]. The concentration of vasoconstrictor and vasodilator mediators determines the tone of the surrounding vasculature, modulating CBF [123]. In this model, neurons are “pacemakers” within the NVU model in that they regulate CBF [122]. For example, administration of catecholamines (e.g., dopamine, norepinephrine, and epinephrine) to neurons modifies EC tight junction protein production, thus changing BBB permeability [124]. All components in the NVU may modulate BBB maintenance [125]. Similarly, these components all coordinate to tightly control the CNS ionic microenvironment and ensure optimal neuronal functioning [126]. These processes include having specialized functions for neurotransmitters, maintaining low protein concentrations, preventing CNS exposure to neurotoxins, and limiting inflammatory processes [126]. The main function of astrocytes in the NVU is to regulate nutrient exchange by changing the tight junction density [127,128,129].
BBB breakdown following SAH disrupts the NVU, leading to neuronal hyperexcitability [130]. Neuroinflammation occurring in the CNS decreases the seizure threshold due to rapid changes in glutamate and γ-aminobutyric acid (GABA) receptor phosphorylation and channelopathies [131,132]. In rodent and pig models, BBB breakdown synchronized neuronal activity and increased seizure activity [133,134]. Albumin extravasation contributes to neuronal excitotoxicity by disrupting astrocytic K+ buffering capacity [130]. Albumin injection in naïve rats downregulated astrocytic Kir4.1 channels, leading to transient spiking activity [135]. These findings support neuroinflammation and albumin influx as drivers of neuronal network hyperexcitability [127].
During the intermediate phase (3–5 days post-SAH) neurovascular coupling is compromised following BBB dysfunction [136]. The brain tissue is particularly vulnerable during this period (3–5 days post-hemorrhage); cerebral vasospasm (CVS) is likely to occur during this period and further exacerbate damage to the brain parenchyma [137]. Neurovascular uncoupling occurs when there is a mismatch between blood supply and neuronal demand [38]. Uncoupling is exacerbated by neuronal hyperexcitability (e.g., increased metabolic activity), dysregulated vascular contractility, and decreased metabolic waste clearance, thus reducing the neuronal energy supply, leading to apoptosis [138]. The glymphatic system continues to deteriorate as SAH progresses, with loss of gap junctions (GJ) connecting neighboring astrocytes [139,140]. GJ loss limits neurotransmitter uptake, ion buffering, and glucose distribution for stable neuronal activity [141,142,143].
5. Delayed Injury (5–14 Days)
Loss of astrocytic GJs contributes to astroglial network compromise and subsequent astrocytic apoptosis [144,145]. However, loss of the astrocytic GJ loss diminishes the spread of Ca2+ waves, preventing synchronized activity between neurons and vascular SMCs [146]. Concurrently, disconnection between neurons and the supporting astroglial network hinders the neuronal energy supply [147]. In the setting of hypoglycemia or periods of neuronal hyperactivity, astrocytes monopolize the energy supply [148,149]. This cellular strategy provides another energy source (e.g., astrocyte–neuron lactate shuttle) for neurons during periods of excessive energy demand [150]. In SAH, astrocytic apoptosis is favored to precede neuronal apoptosis [145].
The deregulated neuronal activity of astrocytes depletes the neuronal energy supply and disrupts Na+–K+ pumps, which maintain ion homeostasis [151]. The means for neuronal ATP production are also impaired without the supporting astroglial network [151]. Injured neurons incur further injury from reactive Ca2+ influx, leading to large amounts of glutamate release, triggering local depolarizations [152]. Cortical spreading depolarizations (CSDs) consist of recurrent waves of neuronal and glial depolarizations that propagate widely from the onset zone [153]. CSDs begin under hypoxic conditions, strained energy supplies (e.g., glucose), and exposure to oxyhemoglobin following SAH [154,155]. CSDs peak at days 5–7 following aneurysmal SAH [156]. Changes in the neuronal microenvironment lead to massive glutamate release, loss of membrane potential, and CSD throughout the brain parenchyma [157]. If injured neurons cannot restore membrane potentials using Na–K+ pumps, the associated neurons and astrocytes swell and distort the dendritic connections [158]. CSDs eventually evolve into epileptiform discharges or pathologic changes in electrical potentials, silencing brain electrical activity [159]. Epileptiform discharges transmit greater disturbances in ion homeostasis between the intracellular and extracellular environments, resulting in neuronal swelling and glutamate receptor upregulation [159,160]. Underlying epileptiform discharges are neurons stuck in field potentials that are less than the inactivation threshold for channels generating action potentials [154]. Thus, neurons fire at high frequencies, producing moderately sustained depolarizations and less hyperpolarization [154,161].
Cellular Changes
When metabolic demand exceeds the energy supply provided by the neurovascular unit (e.g., glucose delivery, cerebral blood flow), neuronal ischemia and apoptosis occur [162]. Within minutes, neuronal necrosis surpasses apoptosis as the primary form of cell death within the brain’s parenchyma [163]. The neuroinflammatory response is caused by an increase in neuroinflammatory signaling agents, such as IL-6, PAF, T-cells, and macrophages. The cytokines/chemokines involved are p53, Fas/FasL, tumor necrosis factor receptor, caspase 9 and apoptosis inducing factor [164]. These cytokines and chemokines lead to increased cell death of the brain’s parenchyma [165].
During the late or delayed stage (5–14 days post-SAH), CSDs peak in their occurrence. These peaks are associated with loss of neurovascular autoregulation. This loss is due to deranged NO/NOS signaling following changes to eNOS, nNOS, and iNOS [166]. During this phase, RBCs begin to break down/lyse within the subarachnoid spaces and increase oxyhemoglobin and ferritin concentrations within the brain parenchyma, causing further cerebral vasospasm [167].
The mass effect within the cranial vault physically displaces the brain parenchyma. The displaced parenchyma moves from a higher-pressure region (adjacent to the growing hematoma) to lower-pressure/resistance region(s) (e.g., lumens [openings] within the cranial vault). This process is problematic since, within the cranial vault, these openings are occupied by critical structures (e.g., brainstem, cranial nerves, vertebral arteries and veins) [168]. The brainstem controls functions such as respiration, blood pressure regulation, and other autonomic functions required for life. Compression of the brainstem leads to the loss of these critical autonomic functions [169,170,171,172].
Initially described by Ecker and Riemenschneider in 1951 [173], the association of CVS following SAH has been well documented. However, the exact mechanisms of CVS following SAH have been contested within the recent literature [174]. Previously, the accepted model of CVS, as described by Allcock and Drake [175], was that it occurred in the setting of focal ischemia experienced by the brain parenchyma during SAH and with a peak occurrence at 5–14 days post-initial hemorrhagic event [176]. Additionally, there exists a positive correlation between hemorrhage volume (HV) and the severity of CVS; this correlation has been recognized for its clinical utility and is used as the basis for the Fisher scale [177]. Recently, an ongoing clinical trial examined deferoxamine (i-Def), an iron chelating agent that sequesters the iron component of red blood cells. When i-Def was introduced into the brain’s parenchyma during a hemorrhagic event, initial results from second-phase clinical trials [178] were promising for reducing stroke sequalae [179]. If HV was less than 10 mL, the iatrogenic complications can outweigh any benefit that otherwise would be conferred by i-Def [180].
The link between cerebral vasospasm following the accumulation of blood degradation byproducts has been well established for several decades and was described by Toda and Ohta [181]. Recently, it has been shown that a potent inflammatory marker, oxyhemoglobin, is implicated in CVS, replacing the previously targeted methemoglobin and bilirubin as inflammatory markers [182,183]. The exact mechanism by which these substances induce CVS is still not well understood, although several features have been described. When injected intrathecally, oxyhemoglobin has been shown to induce CVS [174]. The mechanisms by which this outcome occurs is through the modification of normal expression of eicosanoids; oxyhemoglobin will increase the production of PGE2 and decrease PGI2 levels. Both eicosanoids are important in maintaining vascular tonicity. When oxyhemoglobin contacts methemoglobin, it spontaneously releases superoxide, which in turn causes lipid peroxidation, as well as vasoconstriction [174]. Additionally, this vasoconstriction is then compounded, as oxyhemoglobin has been shown to decrease the cerebral vasculature’s ability to relax [184]. Although there are ongoing studies to modify and alleviate these neuroinflammatory markers, a consensus has yet to be reached. This consensus has primarily been stymied by our partial understanding of the exact molecular pathways involved within the neuroinflammatory cascade. However, a reduction in the activity of the oxidative pathways [185], as described by the i-DEF trials, is promising in reducing neuronal damage in both the intermediate (within the first 3–5 days following SAH) and delayed (within the first 2 weeks) phases. Given that case fatality rates remain close to a third of patients suffering from intracerebral hemorrhage [186], early recognition and categorization by clinicians are imperative for treatment eligibility (e.g., i-Def), thereby minimizing cognitive and neurological deficits [187,188].
Additional substances released into the brain parenchyma during structural compromise of the vessel wall come from the spillage of intervascular wall components (intracellular enzymes and proteins) [189] that are cytotoxic to surrounding CNS tissue. Within minutes, there are gross anatomical and cellular changes to the CNS vasculature, e.g., mechanical/physical compression of neighboring/adjacent vessels, occluding their internal lumen(s), as well as compression of CNS tissue [190,191]. Regarding the cellular changes, notable is the disruption to chemosignaling agents (vasodilators/vasoconstrictors). These signaling agents under normal physiologic conditions are kept in a delicate balance to maintain the CNS vessels’ ability to autoregulate their tone [192].
Following the loss of cerebrovascular autoregulation, ICP equalizes to diastolic blood pressure (DBP) [191]. Given that DBP is several fold greater than normal CNS pressures (80–100 mm Hg and 7–15 mm Hg, respectively), the pressure differential can cause lateral displacement and subsequent compression of CNS tissue [193].
Ultimately, the compounding anatomical and cellular changes further contribute to a loss of micronutrients (perfusion) to local tissues and lack of clearance of toxic metabolic byproducts [191,194]. Within minutes of changes to perfusion, the cell’s ionic transmembrane gradient, which is crucial for normal neuro-signaling, becomes deranged [195]. This outcome follows the failure of adenosine triphosphatase’s ability to maintain the gradient in hypoxic conditions and thus its reliance on relatively ineffective anerobic pathways.
Following SAH, arterial vasospasm can occur, independently impairing perfusion (excluding non-spasmodic causes, e.g., clots, rupture of an atherosclerotic plaque, or embolus). Traditionally, this outcome has been detected by means of transcranial ultrasound (TCD) performed at the patient’s bedside approximately 2–3 days post-initial insult [196,197]. TCD is performed when vasospasm is suspected [198]. Currently, clinicians rely upon alterations in mentation or focal neurologic changes to escalate to imaging modalities for affected patients. In practice, these include shifts in the patient’s level of consciousness (neurobehavioral changes) or any new-onset focal neurologic defects [199]. However, this process can be challenging in patients who are intubated and/or comatose, especially for junior clinicians [200].
The recent literature has indicated that the link between vasospasm and DCI is tenuous, as the two have yet to be causally linked despite being the focus of many studies over several decades. These studies have utilized both clinical trials and animal models. Statistically, the evidence for a causal association is weak, as a DCI occurs 30–80% of the time following and/or during vasospasm. Additionally, CSD and DCI can occur without any antecedent (local or diffuse) vasospasm. Significant CVS (diffuse and/or involving several large caliber vessels) can occur without the presence of CSD [201].
Historically, transcranial ultrasound has been used to diagnose the presence of CVS [196,197]. Transcranial ultrasound has the benefit of being a noninvasive, relatively inexpensive imaging modality compared to functional MRI. Although TCD can facilitate the activation of a CVS protocol, it is not without its shortcomings. Recent meta-analyses of clinical data have called into question the veracity of transcranial ultrasound as a reliable technique to diagnose the presence of CVS. Critics of this screening modality have cited the low specificity and widely variable sensitivity as the rationale for developing a new mainstay in the diagnosis of CVS [202,203,204]. Clinicians should consider CVS’s limitations; that is, it has higher sensitivity for larger caliber vessels that are more superficial. Clinicians also must consider that the spread of the vasospasm can be segmental in nature. The portion visualized by the TCD could appear as non-spasmodic, thus indicating a false negative, albeit while a distal portion of the vessel is in spasm [205]. Therefore, as an imaging modality, TCD is dependent primarily on the clinician’s experience and expertise to ensure that the entire vessel is swept in a longitudinal manner [196,206,207]. The timeline simplification of aneurysmal rupture, along with expected clinical signs and outcomes, can be seen in Figure 3 below.
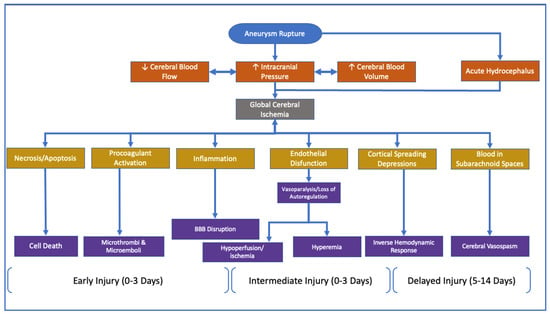
Figure 3.
Chronological depiction of the proposed pathophysiological pathways for the development of DCI following SAH. Based on references: [163,165,208,209,210].
6. Clinical Relevance and Management
aSAH patients present with a variety of symptoms, ranging from stiff neck, photophobia, loss of consciousness, and focal neurological deficits to altered mentation [211]. Approximately 80% of patients will state that they are experiencing “the worst headache of my life” [212]. Although prompt surgical intervention is required, preventing and treating SAH complications prior to surgery are tantamount. These presurgical interventions include the stratification of patients based on SAH severity (e.g., modified Fisher, Hunt Hess scales) and initiating anti-seizure therapy and nicardipine (infusions) for prevention of vasospasm [207].
Following aneurysmal rupture, a clinically observed sequence of events occurs. The presentation of SAH has been well known for decades due to its hallmark of patients reporting experiencing the “worst headache of my life,” clinically described as a “thunderclap headache” due to the rapid onset and the severity of the intensity. The vast majority of SAHs occur during nonstrenuous activities [213,214]. In addition to the thunderclap headache, more than two-thirds of patients will report nausea and/or vomiting, nuchal rigidity, altered level of consciousness, and/or a focal neurological defect. Especially worrisome is the occurrence of an antecedent (sentinel or warning) headache three months before the SAH, as these headaches are associated with a 10-fold increased risk of a rebleed following stabilization of the initial hemorrhagic event [215]. Sentinel or warning headaches are attributed to a minor hemorrhage prior to the major event. These symptoms can persist for several days [216,217] and merit consideration for the effectiveness of greater patient education for at-risk populations. That said, the continual challenge for clinicians is differentiating SAH’s sentinel headache from normal variants or those attributable to other causes because SAHs account for approximately 1% of all headaches presenting to emergency departments [218].
Reducing the time from symptom onset to neurosurgical intervention (i.e., coiling or clipping) is crucial in the reduction of morbidity and mortality [219]. Any patient whose presentation is suspicious for SAH should have radiographic imaging (non-contrast computer tomography or CT) performed. Following diagnosis of SAH, if a patient’s mental status deteriorates, current guidelines support the placement of an external ventricular drain (EVD) [207]. EVD placement is beneficial, as acute hydrocephalus is one of the most common complications in SAH (15–87% of SAH patients) [220]. EVD placement enables clinicians to monitor and reduce ICP via drainage of CSF. Additionally, placement of EVD in SAH-associated hydrocephalus is associated with neurological improvement [221,222,223,224]. EVD insertion serves to both continually monitor ICP and reduce intercompartmental swelling and pressure, thus improving cerebral perfusion pressure and metabolism [225]. If ICP remains elevated by more than 20 mm Hg, neurosurgeons may consider inserting a second EVD on the contralateral side or performing a decompressive craniectomy to reduce intracranial hypertension. Sedation (e.g., propofol, midazolam, fentanyl), osmotic agents (e.g., mannitol, hypertonic saline), barbiturate coma, and hypothermia may also be implemented systematically to lower ICP by 20 mm Hg before performing further surgical interventions [213,226]. In addition to ICP, brain tissue oxygen tension (PbtO2), delivery of oxygen (DO2), and jugular venous oxygen saturation (SjvO2) can also be monitored to optimize brain oxygenation and reduce metabolic stress [227]. The onset of brain hypoxia (PbtO2 < 20 mm Hg) initiates clinical interventions to optimize CPP, hemoglobin levels, intravascular volume, cardiac output, and oxygen saturation to minimize secondary brain injuries [227].
During the acute phase, clinicians optimize parameters to reduce the risk of rebleeding. Rebleeding is associated with a mortality rate as high as 70% and a poor prognosis for functional recovery. Computed tomography angiography (CTA) is performed to determine whether the patient has an aneurysmal subarachnoid hemorrhage. Digital subtraction angiography (DSA) remains the gold standard for detecting cerebral aneurysms.
Patients found to have an aneurysm undergo aggressive blood pressure monitoring and treatment to maintain systolic blood pressure less than 180 mm Hg per the European stroke organization guidelines and less than 160 mm Hg per the American Academy of Neurology guidelines [228]. Management of high blood pressure remains controversial due to the absence of any supporting clinical trials [229]. Data from observational studies suggest that aggressive management of hypertension can reduce rebleeding, but it is also associated with enhanced risk of secondary ischemia [3]. Another factor associated with rebleeding is an increased time between the rupture of the aneurysm and treatment.
Rebleeding occurs at a rate of 4–13.6% in the first 24 h, with maximal risk between 2 to 12 h post-aneurysm rupture [230,231,232,233]. An aneurysm not treated by endovascular embolization or neurosurgical clipping is considered unsecure, and intervention is performed as early as the patient is determined to be medically able, usually within 72 h after SAH. Postoperatively, obliteration of cerebral aneurysms is determined with a repeat DSA. Advantages and disadvantages exist for open versus endovascular treatments. Endovascular embolization is less invasive and was previously associated with a reduction in death and disability, fewer technical complications, reduced postoperative epilepsy, and less cognitive decline compared to clipping [234,235,236]. However, neurosurgical clipping is associated with a reduced incidence of late rebleeding (0.9% clipping vs 2.9% coiled) and a higher rate of aneurysm obliteration (81% clipped vs 58% coiled) [237]. Additionally, endovascular management is limited in its ability to obliterate small aneurysms (<3 mm) and is more costly than microsurgical clipping [238]. Given these differences, clipping is usually the preferred modality for middle cerebral artery aneurysms and patients with large intraparenchymal hematomas > 50 cc, but endovascular embolization is optimal for patients with a poor clinical grade, posterior cerebral artery aneurysms, or vasospasm or who are older in age [220].
Following stabilization of aneurysms, SAH patients are placed on neuromonitoring for vasospasm. This process requires hourly bedside neurological examinations and routine bedside TCD [239]. TCD is widely used as the primary clinical method for vasospasm screening. Detection of vasospasm by TCD activates therapeutic measures sooner and allows clinicians to monitor the patient’s response to treatment. TCD showing a mean flow velocity more than two standard deviations greater than the norm for individual intracranial vessels indicates vasospasm. However, the practitioners must be aware of TCD limitations, such as false positives (e.g., hyperemia, anemia) and false negatives (e.g., distal ACA regions), when interpreting the results [240]. Continuous electroencephalography (cEEG) can augment TCD assessments of vasospasm [241]. Quantification of relative alpha (RA) signals is associated with vasospasm [241]. Combining cEEG with neurological assessments and TCDs increases the sensitivity and specificity for vasospasm, along with surveillance for status epilepticus [242]. Acute neurological changes, in combination with vasospasm detected on TCD, are indications for CTA and CT perfusion (CTP). CTA and CTP are rapid, noninvasive modalities for visualizing vasculature, vasospasm, and the presence of infarction [243].
If symptomatic vasospasms do not respond to medical therapy, interventional cerebral angiography should be considered so that intraarterial therapy can be accomplished. During intraarterial therapy, several commonly used vasodilators, including verapamil, nicardipine, milrinone, and nitroglycerin, can be applied at the site of vasospasm to increase vessel diameters and downstream blood flow. However, if vasospasm remains unresponsive to pharmaceutical interventions, clinicians may escalate to balloon angioplasty (BA), which mechanically dilates the vessels. BA is superior in terms of therapeutic efficacy; however, there is a risk of arterial rupture and hemorrhage. Severe cases of vasospasm have received intrathecal injections of calcium channel blockers via EVD access. Intraventricular drug delivery has been effective in reducing the incidence of vasospasm and improving clinical outcomes [244]. Furthermore, there is not yet a consensus within the body of literature regarding the validity of other non-surgical interventions (triple-H therapy, high dose statins, and prophylactic calcium channel blockers). Triple-H therapy, or hypertension, hypervolemia, and hemodilution therapy, is used to maintain adequate brain perfusion in the setting of symptomatic vasospasm [245]. Randomized, controlled trials have argued against the use of prophylactic triple-H therapy for SAH patients on post-bleed days 0 and 14. Furthermore, there are no data from randomized trials showing improved outcomes with triple-H therapy despite its efficacy in augmenting CBF [246,247,248]. Intra-arterial interventions (e.g., angioplasty, verapamil) are the most effective treatments for large vessel vasospasm, while triple-H therapy is still utilized for mild vasospasm to avoid invasive procedures.
7. Conclusions
Although SAH accounts for a relatively small percentage (~15%) of strokes, those suffering from SAH are younger compared to the mean age of all stroke patients. This fact typically indicates a massive loss of life quality resulting from neurologic deficits experienced by these SAH patients.
The need for newer diagnostic procedures for SAH and the prevention of DCI is imperative, as the limitations of traditional methods (e.g., TCD) have become increasingly apparent. Cerebral vasospasm has proven to be an elusive phenomenon, both in detection and in its prognostic value. Examination of emerging data suggests a shift in focus to alternative detection and treatment modalities. Oxyhemoglobin is the new emerging biomarker implicated in the detection and intervention of CSD, replacing former markers. The exact molecular mechanisms by which oxyhemoglobin exacerbates DCI are currently unknown, but ongoing clinical trials have shown the benefits of curbing its presence. Early surgical intervention is a priority following stabilization of the patient. New findings from ongoing clinical trials merit the inclusion of non-surgical interventions during these early stages. These medical interventions are promising, such as the i-DEF trials.
Last, further appreciation of the contributing factors, as well as the exact mechanisms for reducing neuroinflammation, is crucial, as it plays a significant role in the migration of pericytes and smooth muscle to injured regions. Decreasing inflammation will decrease the migration of pericytes and subsequent DCI.
Author Contributions
Conceptualization, H.W.S. and C.E.S.; methodology, H.W.S. and C.E.S.; software, H.W.S., C.E.S. and P.L.; investigation, H.W.S. and C.E.S.; writing—original draft preparation, H.W.S. and C.E.S.; writing—review and editing, H.W.S., C.E.S., P.L. and K.Y.; visualization, H.W.S. and P.L.; supervision, J.S.A., J.D.J. and B.G.; project administration, J.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AQP4 | aquaporin 4 |
| CBV | cerebral blood volume |
| CSD | cortical spreading depressions |
| CTA | computed tomography angiography |
| CVS | cerebral vasospasm |
| DCI | delayed cerebral ischemia |
| EVD | external ventricular drain |
| GJ | gap junction |
| GS | glymphatic system |
| ICP | intracranial pressure |
| NVU | neurovascular unit |
| PVS | perivascular space |
| SAH | subarachnoid hemorrhage |
| aSAH | aneurysmal subarachnoid hemorrhage |
| sSAH | spontaneous subarachnoid hemorrhage |
| tSAH: | traumatic subarachnoid hemorrhage |
| TCD | transcranial Doppler (ultrasound) |
References
- Griswold, D.P.; Fernandez, L.; Rubiano, A.M. Diagnosis and Management of Traumatic Subarachnoid Hemorrhage: Protocol for a Scoping Review. JMIR Res. Protoc. 2021, 10, e26709. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.L.; Schweizer, T.A. Spontaneous Subarachnoid Haemorrhage. Lancet 2017, 389, 655–666. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S. Aneurysmal Subarachnoid Hemorrhage. J. Neurosurg. Anesth. 2015, 27, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Kozak, N.; Hayashi, M. Trends in the Incidence of Subarachnoid Hemorrhage in Akita Prefecture, Japan. J. Neurosurg. 2007, 106, 234–238. [Google Scholar] [CrossRef]
- Sacco, S.; Totaro, R.; Toni, D.; Marini, C.; Cerone, D.; Carolei, A. Incidence, Case-Fatalities and 10-Year Survival of Subarachnoid Hemorrhage in a Population-Based Registry. Eur. Neurol. 2009, 62, 155–160. [Google Scholar] [CrossRef]
- Feigin, V.L.; Lawes, C.M.; Bennett, D.A.; Barker-Collo, S.L.; Parag, V. Worldwide Stroke Incidence and Early Case Fatality Reported in 56 Population-Based Studies: A Systematic Review. Lancet Neurol. 2009, 8, 355–369. [Google Scholar] [CrossRef]
- Schievink, W.I.; Wijdicks, E.F.; Parisi, J.E.; Piepgras, D.G.; Whisnant, J.P. Sudden Death from Aneurysmal Subarachnoid Hemorrhage. Neurology 1995, 45, 871–874. [Google Scholar] [CrossRef]
- de Rooij, N.K.; Linn, F.H.; van der Plas, J.A.; Algra, A.; Rinkel, G.J. Incidence of Subarachnoid Haemorrhage: A Systematic Review with Emphasis on Region, Age, Gender and Time Trends. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1365–1372. [Google Scholar] [CrossRef]
- Shea, A.M.; Reed, S.D.; Curtis, L.H.; Alexander, M.J.; Villani, J.J.; Schulman, K.A. Characteristics of Nontraumatic Subarachnoid Hemorrhage in the United States in 2003. Neurosurgery 2007, 61, 1131–1137, discussion 1137–1138. [Google Scholar] [CrossRef]
- Molenberg, R.; Thio, C.H.L.; Aalbers, M.W.; Uyttenboogaart, M.; ISGC Intracranial Aneurysm Working Group; Larsson, S.C.; Bakker, M.K.; Ruigrok, Y.M.; Snieder, H.; van Dijk, J.M.C. Sex Hormones and Risk of Aneurysmal Subarachnoid Hemorrhage: A Mendelian Randomization Study. Stroke 2022, 53, 2870–2875. [Google Scholar] [CrossRef]
- Silverman, A.; Petersen, N.H. Physiology, Cerebral Autoregulation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Armstead, W.M. Cerebral Blood Flow Autoregulation and Dysautoregulation. Anesth. Clin. 2016, 34, 465–477. [Google Scholar] [CrossRef]
- Lidington, D.; Wan, H.; Bolz, S.S. Cerebral Autoregulation in Subarachnoid Hemorrhage. Front. Neurol. 2021, 12, 688362. [Google Scholar] [CrossRef] [PubMed]
- Folkow, B. Intravascular Pressure as a Factor Regulating the Tone of the Small Vessels. Acta Physiol. Scand. 1949, 17, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Wallis, S.J.; Firth, J.; Dunn, W.R. Pressure-Induced Myogenic Responses in Human Isolated Cerebral Resistance Arteries. Stroke 1996, 27, 2287–2290, discussion 2291. [Google Scholar] [CrossRef] [PubMed]
- Lidington, D.; Schubert, R.; Bolz, S.S. Capitalizing on Diversity: An Integrative Approach towards the Multiplicity of Cellular Mechanisms Underlying Myogenic Responsiveness. Cardiovasc. Res. 2013, 97, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Cole, W.C.; Welsh, D.G. Role of Myosin Light Chain Kinase and Myosin Light Chain Phosphatase in the Resistance Arterial Myogenic Response to Intravascular Pressure. Arch. Biochem. Biophys. 2011, 510, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Muizelaar, J.P.; Schroder, M.L. Overview of Monitoring of Cerebral Blood Flow and Metabolism after Severe Head Injury. Can. J. Neurol. Sci. 1994, 21, S6–S11. [Google Scholar] [CrossRef]
- Paulson, O.B.; Strandgaard, S.; Edvinsson, L. Cerebral Autoregulation. Cerebrovasc. Brain Metab. Rev. 1990, 2, 161–192. [Google Scholar]
- Zoerle, T.; Lombardo, A.; Colombo, A.; Longhi, L.; Zanier, E.R.; Rampini, P.; Stocchetti, N. Intracranial Pressure after Subarachnoid Hemorrhage. Crit. Care Med. 2015, 43, 168–176. [Google Scholar] [CrossRef]
- Munakomi, S.; Das, J.M. Brain Herniation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bauernfeind, A.L.; Barks, S.K.; Duka, T.; Grossman, L.I.; Hof, P.R.; Sherwood, C.C. Aerobic Glycolysis in the Primate Brain: Reconsidering the Implications for Growth and Maintenance. Brain Struct. Funct. 2014, 219, 1149–1167. [Google Scholar] [CrossRef]
- Thornton, C.; Leaw, B.; Mallard, C.; Nair, S.; Jinnai, M.; Hagberg, H. Cell Death in the Developing Brain after Hypoxia-Ischemia. Front. Cell Neurosci. 2017, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Melkonian, E.A.; Schury, M.P. Biochemistry, Anaerobic Glycolysis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Liang, D.; Bhatta, S.; Gerzanich, V.; Simard, J.M. Cytotoxic Edema: Mechanisms of Pathological Cell Swelling. Neurosurg. Focus. 2007, 22, E2. [Google Scholar] [CrossRef] [PubMed]
- Goulay, R.; Flament, J.; Gauberti, M.; Naveau, M.; Pasquet, N.; Gakuba, C.; Emery, E.; Hantraye, P.; Vivien, D.; Aron-Badin, R.; et al. Subarachnoid Hemorrhage Severely Impairs Brain Parenchymal Cerebrospinal Fluid Circulation in Nonhuman Primate. Stroke 2017, 48, 2301–2305. [Google Scholar] [CrossRef]
- Mathiisen, T.M.; Lehre, K.P.; Danbolt, N.C.; Ottersen, O.P. The Perivascular Astroglial Sheath Provides a Complete Covering of the Brain Microvessels: An Electron Microscopic 3D Reconstruction. Glia 2010, 58, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Akeret, K.; Buzzi, R.M.; Schaer, C.A.; Thomson, B.R.; Vallelian, F.; Wang, S.; Willms, J.; Sebok, M.; Held, U.; Deuel, J.W.; et al. Cerebrospinal Fluid Hemoglobin Drives Subarachnoid Hemorrhage-Related Secondary Brain Injury. J. Cereb. Blood Flow. Metab. 2021, 41, 3000–3015. [Google Scholar] [CrossRef]
- Ayer, R.E.; Zhang, J.H. Oxidative Stress in Subarachnoid Haemorrhage: Significance in Acute Brain Injury and Vasospasm. Acta Neurochir. Suppl. 2008, 104, 33–41. [Google Scholar] [CrossRef]
- Wagner, K.R.; Sharp, F.R.; Ardizzone, T.D.; Lu, A.; Clark, J.F. Heme and Iron Metabolism: Role in Cerebral Hemorrhage. J. Cereb. Blood Flow. Metab. 2003, 23, 629–652. [Google Scholar] [CrossRef]
- Selim, M.; Yeatts, S.; Goldstein, J.N.; Gomes, J.; Greenberg, S.; Morgenstern, L.B.; Schlaug, G.; Torbey, M.; Waldman, B.; Xi, G.; et al. Safety and Tolerability of Deferoxamine Mesylate in Patients with Acute Intracerebral Hemorrhage. Stroke 2011, 42, 3067–3074. [Google Scholar] [CrossRef]
- Wang, J.; Dore, S. Heme Oxygenase 2 Deficiency Increases Brain Swelling and Inflammation after Intracerebral Hemorrhage. Neuroscience 2008, 155, 1133–1141. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Okauchi, M.; Hua, Y.; Keep, R.F.; Xi, G. Deferoxamine Reduces Neuronal Death and Hematoma Lysis after Intracerebral Hemorrhage in Aged Rats. Transl. Stroke Res. 2013, 4, 546–553. [Google Scholar] [CrossRef]
- Haque, M.E.; Gabr, R.E.; Zhao, X.; Hasan, K.M.; Valenzuela, A.; Narayana, P.A.; Ting, S.M.; Sun, G.; Savitz, S.I.; Aronowski, J. Serial Quantitative Neuroimaging of Iron in the Intracerebral Hemorrhage Pig Model. J. Cereb. Blood Flow. Metab. 2018, 38, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; oude Egbrink, M.G. The Endothelial Glycocalyx: Composition, Functions, and Visualization. Pflugers Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Cosgun, Z.C.; Fels, B.; Kusche-Vihrog, K. Nanomechanics of the Endothelial Glycocalyx: From Structure to Function. Am. J. Pathol. 2020, 190, 732–741. [Google Scholar] [CrossRef]
- Schott, U.; Solomon, C.; Fries, D.; Bentzer, P. The Endothelial Glycocalyx and Its Disruption, Protection and Regeneration: A Narrative Review. Scand. J. Trauma. Resusc. Emerg. Med. 2016, 24, 48. [Google Scholar] [CrossRef]
- Schenck, H.; Netti, E.; Teernstra, O.; De Ridder, I.; Dings, J.; Niemela, M.; Temel, Y.; Hoogland, G.; Haeren, R. The Role of the Glycocalyx in the Pathophysiology of Subarachnoid Hemorrhage-Induced Delayed Cerebral Ischemia. Front. Cell Dev. Biol. 2021, 9, 731641. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.C.; Xu, R.; Vajkoczy, P. Inflammatory Events Following Subarachnoid Hemorrhage (SAH). Curr. Neuropharmacol. 2018, 16, 1385–1395. [Google Scholar] [CrossRef]
- Mastorakos, P.; McGavern, D. The Anatomy and Immunology of Vasculature in the Central Nervous System. Sci. Immunol. 2019, 4, eaav0492. [Google Scholar] [CrossRef]
- Choi, S.J.; Lillicrap, D. A Sticky Proposition: The Endothelial Glycocalyx and von Willebrand Factor. J. Thromb. Haemost. 2020, 18, 781–785. [Google Scholar] [CrossRef]
- Blatter, L.A.; Wier, W.G. Nitric Oxide Decreases [Ca2+]i in Vascular Smooth Muscle by Inhibition of the Calcium Current. Cell Calcium 1994, 15, 122–131. [Google Scholar] [CrossRef]
- Battinelli, E.; Willoughby, S.R.; Foxall, T.; Valeri, C.R.; Loscalzo, J. Induction of Platelet Formation from Megakaryocytoid Cells by Nitric Oxide. Proc. Natl. Acad. Sci. USA 2001, 98, 14458–14463. [Google Scholar] [CrossRef]
- Freitas, A.; Alves-Filho, J.C.; Secco, D.D.; Neto, A.F.; Ferreira, S.H.; Barja-Fidalgo, C.; Cunha, F.Q. Heme Oxygenase/Carbon Monoxide-Biliverdin Pathway down Regulates Neutrophil Rolling, Adhesion and Migration in Acute Inflammation. Br. J. Pharmacol. 2006, 149, 345–354. [Google Scholar] [CrossRef]
- Friedrich, V.; Flores, R.; Muller, A.; Sehba, F.A. Escape of Intraluminal Platelets into Brain Parenchyma after Subarachnoid Hemorrhage. Neuroscience 2010, 165, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, B.A.; Pluta, R.M.; Jung, C.; Oldfield, E.H. Endothelial Dysfunction in a Primate Model of Cerebral Vasospasm. J. Neurosurg. 2004, 100, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Gotsch, U.; Jager, U.; Dominis, M.; Vestweber, D. Expression of P-Selectin on Endothelial Cells Is Upregulated by LPS and TNF-Alpha in Vivo. Cell Adhes. Commun. 1994, 2, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Kusaka, G.; Yamaguchi, N.; Sekizuka, E.; Nakadate, H.; Minamitani, H.; Shinoda, S.; Watanabe, E. Platelet and Leukocyte Adhesion in the Microvasculature at the Cerebral Surface Immediately after Subarachnoid Hemorrhage. Neurosurgery 2009, 64, 546–553, discussion 553–554. [Google Scholar] [CrossRef]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary Pericytes Regulate Cerebral Blood Flow in Health and Disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, M.; Stokes, K.Y.; Chernyshev, O.; Kelley, R.E.; Alexander, J.S. The Role of the ACE2/MasR Axis in Ischemic Stroke: New Insights for Therapy. Biomedicines 2021, 9, 1667. [Google Scholar] [CrossRef]
- Barzegar, M.; Vital, S.; Stokes, K.Y.; Wang, Y.; Yun, J.W.; White, L.A.; Chernyshev, O.; Kelley, R.E.; Alexander, J.S. Human Placenta Mesenchymal Stem Cell Protection in Ischemic Stroke Is Angiotensin Converting Enzyme-2 and MasR Receptor-Dependent. Stem Cells Dayt. Ohio 2021, 39, 1335–1348. [Google Scholar] [CrossRef]
- Barzegar, M.; Wang, Y.; Eshaq, R.S.; Yun, J.W.; Boyer, C.J.; Cananzi, S.G.; White, L.A.; Chernyshev, O.; Kelley, R.E.; Minagar, A.; et al. Human Placental Mesenchymal Stem Cells Improve Stroke Outcomes via Extracellular Vesicles-Mediated Preservation of Cerebral Blood Flow. eBioMedicine 2021, 63, 103161. [Google Scholar] [CrossRef]
- Sen, O.; Caner, H.; Aydin, M.V.; Ozen, O.; Atalay, B.; Altinors, N.; Bavbek, M. The Effect of Mexiletine on the Level of Lipid Peroxidation and Apoptosis of Endothelium Following Experimental Subarachnoid Hemorrhage. Neurol. Res. 2006, 28, 859–863. [Google Scholar] [CrossRef]
- Fumoto, T.; Naraoka, M.; Katagai, T.; Li, Y.; Shimamura, N.; Ohkuma, H. The Role of Oxidative Stress in Microvascular Disturbances after Experimental Subarachnoid Hemorrhage. Transl. Stroke Res. 2019, 10, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Gris, T.; Laplante, P.; Thebault, P.; Cayrol, R.; Najjar, A.; Joannette-Pilon, B.; Brillant-Marquis, F.; Magro, E.; English, S.W.; Lapointe, R.; et al. Innate Immunity Activation in the Early Brain Injury Period Following Subarachnoid Hemorrhage. J. Neuroinflam. 2019, 16, 253. [Google Scholar] [CrossRef] [PubMed]
- Sabri, M.; Jeon, H.; Ai, J.; Tariq, A.; Shang, X.; Chen, G.; Macdonald, R.L. Anterior Circulation Mouse Model of Subarachnoid Hemorrhage. Brain Res. 2009, 1295, 179–185. [Google Scholar] [CrossRef]
- Coulibaly, A.P.; Pezuk, P.; Varghese, P.; Gartman, W.; Triebwasser, D.; Kulas, J.A.; Liu, L.; Syed, M.; Tvrdik, P.; Ferris, H.; et al. Neutrophil Enzyme Myeloperoxidase Modulates Neuronal Response in a Model of Subarachnoid Hemorrhage by Venous Injury. Stroke 2021, 52, 3374–3384. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.S.; Woo, S.K.; Kurland, D.B.; Yoon, S.H.; Palmer, A.F.; Banerjee, U.; Iqbal, S.; Ivanova, S.; Gerzanich, V.; Simard, J.M. Methemoglobin Is an Endogenous Toll-like Receptor 4 Ligand-Relevance to Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2015, 16, 5028–5046. [Google Scholar] [CrossRef]
- Seo, K.W.; Lee, S.J.; Kim, Y.H.; Bae, J.U.; Park, S.Y.; Bae, S.S.; Kim, C.D. Mechanical Stretch Increases MMP-2 Production in Vascular Smooth Muscle Cells via Activation of PDGFR-Beta/Akt Signaling Pathway. PLoS ONE 2013, 8, e70437. [Google Scholar] [CrossRef]
- Ma, M.; Li, H.; Wu, J.; Zhang, Y.; Shen, H.; Li, X.; Wang, Z.; Chen, G. Roles of Prokineticin 2 in Subarachnoid Hemorrhage-Induced Early Brain Injury via Regulation of Phenotype Polarization in Astrocytes. Mol. Neurobiol. 2020, 57, 3744–3758. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, K.; Zhou, J.; Zhang, X.; Yin, S.; Peng, J.; Liao, Y.; Jiang, Y. Ponesimod Protects against Neuronal Death by Suppressing the Activation of A1 Astrocytes in Early Brain Injury after Experimental Subarachnoid Hemorrhage. J. Neurochem. 2021, 158, 880–897. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic Analysis of Reactive Astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef]
- Fei, X.; Dou, Y.N.; Wang, L.; Wu, X.; Huan, Y.; Wu, S.; He, X.; Lv, W.; Wei, J.; Fei, Z. Homer1 Promotes the Conversion of A1 Astrocytes to A2 Astrocytes and Improves the Recovery of Transgenic Mice after Intracerebral Hemorrhage. J. Neuroinflam. 2022, 19, 67. [Google Scholar] [CrossRef]
- Hou, J.; Bi, H.; Ge, Q.; Teng, H.; Wan, G.; Yu, B.; Jiang, Q.; Gu, X. Heterogeneity Analysis of Astrocytes Following Spinal Cord Injury at Single-Cell Resolution. FASEB J. 2022, 36, e22442. [Google Scholar] [CrossRef] [PubMed]
- Luchena, C.; Zuazo-Ibarra, J.; Valero, J.; Matute, C.; Alberdi, E.; Capetillo-Zarate, E. A Neuron, Microglia, and Astrocyte Triple Co-Culture Model to Study Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 844534. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, L.; Duan, Y.; Xu, S.; Yang, Y.; Yin, J.; Lang, Y.; Gao, Z.; Wu, C.; Lv, Z.; et al. Phenotype Shifting in Astrocytes Account for Benefits of Intra-Arterial Selective Cooling Infusion in Hypertensive Rats of Ischemic Stroke. Neurotherapeutics 2022, 19, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhang, Y.; Ning, B. Reactive Astrocytes in Central Nervous System Injury: Subgroup and Potential Therapy. Front. Cell Neurosci. 2021, 15, 792764. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Lai, N.; Wu, D.; Liang, T.; Pan, P.; Yuan, G.; Li, X.; Li, H.; Shen, H.; Wang, Z.; Chen, G. Systemic Exosomal MiR-193b-3p Delivery Attenuates Neuroinflammation in Early Brain Injury after Subarachnoid Hemorrhage in Mice. J. Neuroinflam. 2020, 17, 74. [Google Scholar] [CrossRef]
- Anzabi, M.; Ardalan, M.; Iversen, N.K.; Rafati, A.H.; Hansen, B.; Ostergaard, L. Hippocampal Atrophy Following Subarachnoid Hemorrhage Correlates with Disruption of Astrocyte Morphology and Capillary Coverage by AQP4. Front. Cell Neurosci. 2018, 12, 19. [Google Scholar] [CrossRef]
- Lv, T.; Zhao, B.; Hu, Q.; Zhang, X. The Glymphatic System: A Novel Therapeutic Target for Stroke Treatment. Front. Aging Neurosci. 2021, 13, 689098. [Google Scholar] [CrossRef]
- Benveniste, H.; Liu, X.; Koundal, S.; Sanggaard, S.; Lee, H.; Wardlaw, J. The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology 2019, 65, 106–119. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and Functional Features of Central Nervous System Lymphatic Vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Wichmann, T.O.; Damkier, H.H.; Pedersen, M. A Brief Overview of the Cerebrospinal Fluid System and Its Implications for Brain and Spinal Cord Diseases. Front. Hum. Neurosci. 2021, 15, 737217. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, L.; Xu, H.; Xing, L.; Zhuang, Z.; Zheng, Y.; Li, X.; Wang, C.; Chen, S.; Guo, Z.; et al. Meningeal Lymphatics Clear Erythrocytes That Arise from Subarachnoid Hemorrhage. Nat. Commun. 2020, 11, 3159. [Google Scholar] [CrossRef]
- Luo, C.; Yao, X.; Li, J.; He, B.; Liu, Q.; Ren, H.; Liang, F.; Li, M.; Lin, H.; Peng, J.; et al. Paravascular Pathways Contribute to Vasculitis and Neuroinflammation after Subarachnoid Hemorrhage Independently of Glymphatic Control. Cell Death Dis. 2016, 7, e2160. [Google Scholar] [CrossRef]
- Pu, T.; Zou, W.; Feng, W.; Zhang, Y.; Wang, L.; Wang, H.; Xiao, M. Persistent Malfunction of Glymphatic and Meningeal Lymphatic Drainage in a Mouse Model of Subarachnoid Hemorrhage. Exp. Neurobiol. 2019, 28, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Bolte, A.C.; Dutta, A.B.; Hurt, M.E.; Smirnov, I.; Kovacs, M.A.; McKee, C.A.; Ennerfelt, H.E.; Shapiro, D.; Nguyen, B.H.; Frost, E.L.; et al. Meningeal Lymphatic Dysfunction Exacerbates Traumatic Brain Injury Pathogenesis. Nat. Commun. 2020, 11, 4524. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Herz, J.; Alme, M.N.; Salvador, A.F.; Dong, M.Q.; Viar, K.E.; Herod, S.G.; Knopp, J.; Setliff, J.C.; Lupi, A.L.; et al. CNS Lymphatic Drainage and Neuroinflammation Are Regulated by Meningeal Lymphatic Vasculature. Nat. Neurosci. 2018, 21, 1380–1391. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Peng, X.; Ma, H.; Zhang, Y.; Yang, X.; Zhang, Y.; Sun, L.; Yan, J. The Involvement of Aquaporin-4 in the Interstitial Fluid Drainage Impairment Following Subarachnoid Hemorrhage. Front. Aging Neurosci. 2020, 12, 611494. [Google Scholar] [CrossRef]
- El Amki, M.; Dubois, M.; Lefevre-Scelles, A.; Magne, N.; Roussel, M.; Clavier, T.; Guichet, P.O.; Gerardin, E.; Compere, V.; Castel, H. Long-Lasting Cerebral Vasospasm, Microthrombosis, Apoptosis and Paravascular Alterations Associated with Neurological Deficits in a Mouse Model of Subarachnoid Hemorrhage. Mol. Neurobiol. 2018, 55, 2763–2779. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Yang, L.; Qin, W.; Yang, S.; Yuan, J.; Jiang, T.; Hu, W. The Relationship between Blood-Brain Barrier Permeability and Enlarged Perivascular Spaces: A Cross-Sectional Study. Clin. Interv. Aging 2019, 14, 871–878. [Google Scholar] [CrossRef]
- Badaut, J.; Brunet, J.F.; Grollimund, L.; Hamou, M.F.; Magistretti, P.J.; Villemure, J.G.; Regli, L. Aquaporin 1 and Aquaporin 4 Expression in Human Brain after Subarachnoid Hemorrhage and in Peritumoral Tissue. Acta Neurochir. Suppl. 2003, 86, 495–498. [Google Scholar] [CrossRef]
- Bloch, O.; Papadopoulos, M.C.; Manley, G.T.; Verkman, A.S. Aquaporin-4 Gene Deletion in Mice Increases Focal Edema Associated with Staphylococcal Brain Abscess. J. Neurochem. 2005, 95, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, E.; Pei, Y.; Yao, Q.; Ma, H.; Mu, Y.; Wang, Y.; Zhang, Y.; Yang, X.; Wang, X.; et al. The Impairment of Intramural Periarterial Drainage in Brain after Subarachnoid Hemorrhage. Acta Neuropathol. Commun. 2022, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, P.; Fang, Y.; Lenahan, C. The Updated Role of the Blood Brain Barrier in Subarachnoid Hemorrhage: From Basic and Clinical Studies. Curr. Neuropharmacol. 2020, 18, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Q.; Tang, J.; Feng, H.; Zhang, J.H. The Evolving Roles of Pericyte in Early Brain Injury after Subarachnoid Hemorrhage. Brain Res. 2015, 1623, 110–122. [Google Scholar] [CrossRef]
- George, N.; Geller, H.M. Extracellular Matrix and Traumatic Brain Injury. J. Neurosci. Res. 2018, 96, 573–588. [Google Scholar] [CrossRef]
- Mishra, A.; Reynolds, J.P.; Chen, Y.; Gourine, A.V.; Rusakov, D.A.; Attwell, D. Astrocytes Mediate Neurovascular Signaling to Capillary Pericytes but Not to Arterioles. Nat. Neurosci. 2016, 19, 1619–1627. [Google Scholar] [CrossRef]
- Kisler, K.; Nelson, A.R.; Rege, S.V.; Ramanathan, A.; Wang, Y.; Ahuja, A.; Lazic, D.; Tsai, P.S.; Zhao, Z.; Zhou, Y.; et al. Pericyte Degeneration Leads to Neurovascular Uncoupling and Limits Oxygen Supply to Brain. Nat. Neurosci. 2017, 20, 406–416. [Google Scholar] [CrossRef]
- Cai, C.; Fordsmann, J.C.; Jensen, S.H.; Gesslein, B.; Lonstrup, M.; Hald, B.O.; Zambach, S.A.; Brodin, B.; Lauritzen, M.J. Stimulation-Induced Increases in Cerebral Blood Flow and Local Capillary Vasoconstriction Depend on Conducted Vascular Responses. Proc. Natl. Acad. Sci. USA 2018, 115, E5796–E5804. [Google Scholar] [CrossRef]
- Sengillo, J.D.; Winkler, E.A.; Walker, C.T.; Sullivan, J.S.; Johnson, M.; Zlokovic, B.V. Deficiency in Mural Vascular Cells Coincides with Blood-Brain Barrier Disruption in Alzheimer’s Disease. Brain Pathol. 2013, 23, 303–310. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Yin, J.; Hu, Y.; Gu, Y.; Pan, S. Glycocalyx Degradation Leads to Blood-Brain Barrier Dysfunction and Brain Edema after Asphyxia Cardiac Arrest in Rats. J. Cereb. Blood Flow. Metab. 2018, 38, 1979–1992. [Google Scholar] [CrossRef]
- Friedman, A.; Kaufer, D.; Heinemann, U. Blood-Brain Barrier Breakdown-Inducing Astrocytic Transformation: Novel Targets for the Prevention of Epilepsy. Epilepsy Res. 2009, 85, 142–149. [Google Scholar] [CrossRef]
- Stokum, J.A.; Gerzanich, V.; Simard, J.M. Molecular Pathophysiology of Cerebral Edema. J. Cereb. Blood Flow. Metab. 2016, 36, 513–538. [Google Scholar] [CrossRef]
- Ozen, I.; Deierborg, T.; Miharada, K.; Padel, T.; Englund, E.; Genove, G.; Paul, G. Brain Pericytes Acquire a Microglial Phenotype after Stroke. Acta Neuropathol. 2014, 128, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Anderova, M.; Benesova, J.; Mikesova, M.; Dzamba, D.; Honsa, P.; Kriska, J.; Butenko, O.; Novosadova, V.; Valihrach, L.; Kubista, M.; et al. Altered Astrocytic Swelling in the Cortex of Alpha-Syntrophin-Negative GFAP/EGFP Mice. PLoS ONE 2014, 9, e113444. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, G.A.; Vindedal, G.F.; Skare, O.; Nagelhus, E.A. Evidence That Pericytes Regulate Aquaporin-4 Polarization in Mouse Cortical Astrocytes. Brain Struct. Funct. 2014, 219, 2181–2186. [Google Scholar] [CrossRef]
- Wang, S.; Cao, C.; Chen, Z.; Bankaitis, V.; Tzima, E.; Sheibani, N.; Burridge, K. Pericytes Regulate Vascular Basement Membrane Remodeling and Govern Neutrophil Extravasation during Inflammation. PLoS ONE 2012, 7, e45499. [Google Scholar] [CrossRef]
- Shimamura, N.; Ohkuma, H. Phenotypic Transformation of Smooth Muscle in Vasospasm after Aneurysmal Subarachnoid Hemorrhage. Transl. Stroke Res. 2014, 5, 357–364. [Google Scholar] [CrossRef]
- Forsyth, E.A.; Aly, H.M.; Neville, R.F.; Sidawy, A.N. Proliferation and Extracellular Matrix Production by Human Infragenicular Smooth Muscle Cells in Response to Interleukin-1 Beta. J. Vasc. Surg. 1997, 26, 1002–1007, discussion 1007–1008. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kemp, S.S.; Aguera, K.N.; Cha, B.; Davis, G.E. Defining Endothelial Cell-Derived Factors That Promote Pericyte Recruitment and Capillary Network Assembly. Arter. Thromb. Vasc. Biol. 2020, 40, 2632–2648. [Google Scholar] [CrossRef]
- Orlich, M.M.; Dieguez-Hurtado, R.; Muehlfriedel, R.; Sothilingam, V.; Wolburg, H.; Oender, C.E.; Woelffing, P.; Betsholtz, C.; Gaengel, K.; Seeliger, M.; et al. Mural Cell SRF Controls Pericyte Migration, Vessel Patterning and Blood Flow. Circ. Res. 2022, 131, 308–327. [Google Scholar] [CrossRef]
- Zhang, J.H. Vascular Neural Network in Subarachnoid Hemorrhage. Transl. Stroke Res. 2014, 5, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, G.; Liu, L.; He, J.; Darwazeh, R.; Liu, H.; Chen, H.; Zhou, C.; Guo, Z.; Sun, X. Bexarotene Exerts Protective Effects Through Modulation of the Cerebral Vascular Smooth Muscle Cell Phenotypic Transformation by Regulating PPARgamma/FLAP/LTB(4) After Subarachnoid Hemorrhage in Rats. Cell Transpl. 2019, 28, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, V.; Flores, R.; Sehba, F.A. Cell Death Starts Early after Subarachnoid Hemorrhage. Neurosci. Lett. 2012, 512, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Metea, M.R.; Newman, E.A. Glial Cells Dilate and Constrict Blood Vessels: A Mechanism of Neurovascular Coupling. J. Neurosci. 2006, 26, 2862–2870. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the Neurovascular Unit: Key Functions and Signaling Pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Tait, M.J.; Saadoun, S.; Bell, B.A.; Papadopoulos, M.C. Water Movements in the Brain: Role of Aquaporins. Trends Neurosci. 2008, 31, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhan, J.; Cai, Q.; Xu, F.; Chai, R.; Lam, K.; Luan, Z.; Zhou, G.; Tsang, S.; Kipp, M.; et al. The Water Transport System in Astrocytes-Aquaporins. Cells 2022, 11, 2564. [Google Scholar] [CrossRef]
- Lo, W.D.; Betz, A.L.; Schielke, G.P.; Hoff, J.T. Transport of Sodium from Blood to Brain in Ischemic Brain Edema. Stroke 1987, 18, 150–157. [Google Scholar] [CrossRef]
- Karimy, J.K.; Zhang, J.; Kurland, D.B.; Theriault, B.C.; Duran, D.; Stokum, J.A.; Furey, C.G.; Zhou, X.; Mansuri, M.S.; Montejo, J.; et al. Inflammation-Dependent Cerebrospinal Fluid Hypersecretion by the Choroid Plexus Epithelium in Posthemorrhagic Hydrocephalus. Nat. Med. 2017, 23, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ding, Y.; Krafft, P.; Wan, W.; Yan, F.; Wu, G.; Zhang, Y.; Zhan, Q.; Zhang, J.H. Targeting Germinal Matrix Hemorrhage-Induced Overexpression of Sodium-Coupled Bicarbonate Exchanger Reduces Posthemorrhagic Hydrocephalus Formation in Neonatal Rats. J. Am. Heart Assoc. 2018, 7, e007192. [Google Scholar] [CrossRef]
- Thrane, A.S.; Rangroo Thrane, V.; Nedergaard, M. Drowning Stars: Reassessing the Role of Astrocytes in Brain Edema. Trends Neurosci. 2014, 37, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Nedergaard, M. The Neurobiology of Glia in the Context of Water and Ion Homeostasis. Neuroscience 2004, 129, 877–896. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Parpura, V. Astroglial Modulation of Hydromineral Balance and Cerebral Edema. Front. Mol. Neurosci. 2018, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.K.; Chassidim, Y.; Lublinsky, S.; Revankar, G.S.; Major, S.; Kang, E.J.; Oliveira-Ferreira, A.I.; Woitzik, J.; Sandow, N.; Scheel, M.; et al. Impaired Neurovascular Coupling to Ictal Epileptic Activity and Spreading Depolarization in a Patient with Subarachnoid Hemorrhage: Possible Link to Blood-Brain Barrier Dysfunction. Epilepsia 2012, 53 (Suppl. S6), 22–30. [Google Scholar] [CrossRef]
- Santiago, M.F.; Veliskova, J.; Patel, N.K.; Lutz, S.E.; Caille, D.; Charollais, A.; Meda, P.; Scemes, E. Targeting Pannexin1 Improves Seizure Outcome. PLoS ONE 2011, 6, e25178. [Google Scholar] [CrossRef]
- Atangana, E.; Schneider, U.C.; Blecharz, K.; Magrini, S.; Wagner, J.; Nieminen-Kelha, M.; Kremenetskaia, I.; Heppner, F.L.; Engelhardt, B.; Vajkoczy, P. Intravascular Inflammation Triggers Intracerebral Activated Microglia and Contributes to Secondary Brain Injury After Experimental Subarachnoid Hemorrhage (ESAH). Transl. Stroke Res. 2017, 8, 144–156. [Google Scholar] [CrossRef]
- Bell, A.H.; Miller, S.L.; Castillo-Melendez, M.; Malhotra, A. The Neurovascular Unit: Effects of Brain Insults During the Perinatal Period. Front. Neurosci. 2019, 13, 1452. [Google Scholar] [CrossRef]
- Carmignoto, G.; Gomez-Gonzalo, M. The Contribution of Astrocyte Signalling to Neurovascular Coupling. Brain Res. Rev. 2010, 63, 138–148. [Google Scholar] [CrossRef]
- Muoio, V.; Persson, P.B.; Sendeski, M.M. The Neurovascular Unit—Concept Review. Acta Physiol. 2014, 210, 790–798. [Google Scholar] [CrossRef]
- Hendrikx, D.; Smits, A.; Lavanga, M.; De Wel, O.; Thewissen, L.; Jansen, K.; Caicedo, A.; Van Huffel, S.; Naulaers, G. Measurement of Neurovascular Coupling in Neonates. Front. Physiol. 2019, 10, 65. [Google Scholar] [CrossRef]
- Ittner, C.; Burek, M.; Stork, S.; Nagai, M.; Forster, C.Y. Increased Catecholamine Levels and Inflammatory Mediators Alter Barrier Properties of Brain Microvascular Endothelial Cells in Vitro. Front. Cardiovasc. Med. 2020, 7, 73. [Google Scholar] [CrossRef]
- Solar, P.; Zamani, A.; Lakatosova, K.; Joukal, M. The Blood-Brain Barrier and the Neurovascular Unit in Subarachnoid Hemorrhage: Molecular Events and Potential Treatments. Fluids Barriers CNS 2022, 19, 29. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The Blood-Brain Barrier in Systemic Inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Buckwalter, M.; Soreq, H.; Vezzani, A.; Kaufer, D. Blood-Brain Barrier Dysfunction-Induced Inflammatory Signaling in Brain Pathology and Epileptogenesis. Epilepsia 2012, 53 (Suppl. S6), 37–44. [Google Scholar] [CrossRef] [PubMed]
- Dehouck, M.P.; Meresse, S.; Delorme, P.; Fruchart, J.C.; Cecchelli, R. An Easier, Reproducible, and Mass-Production Method to Study the Blood-Brain Barrier in Vitro. J. Neurochem. 1990, 54, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Haseloff, R.F.; Blasig, I.E.; Bauer, H.C.; Bauer, H. In Search of the Astrocytic Factor(s) Modulating Blood-Brain Barrier Functions in Brain Capillary Endothelial Cells In Vitro. Cell Mol. Neurobiol. 2005, 25, 25–39. [Google Scholar] [CrossRef]
- David, Y.; Cacheaux, L.P.; Ivens, S.; Lapilover, E.; Heinemann, U.; Kaufer, D.; Friedman, A. Astrocytic Dysfunction in Epileptogenesis: Consequence of Altered Potassium and Glutamate Homeostasis? J. Neurosci. 2009, 29, 10588–10599. [Google Scholar] [CrossRef]
- Viviani, B.; Gardoni, F.; Marinovich, M. Cytokines and Neuronal Ion Channels in Health and Disease. Int. Rev. Neurobiol. 2007, 82, 247–263. [Google Scholar] [CrossRef]
- Vezzani, A.; Friedman, A. Brain Inflammation as a Biomarker in Epilepsy. Biomark. Med. 2011, 5, 607–614. [Google Scholar] [CrossRef]
- Fieschi, C.; Lenzi, G.L.; Zanette, E.; Orzi, F.; Passero, S. Effects on EEG of the Osmotic Opening of the Blood-Brain Barrier in Rats. Life Sci. 1980, 27, 239–243. [Google Scholar] [CrossRef]
- van Vliet, E.A.; da Costa Araujo, S.; Redeker, S.; van Schaik, R.; Aronica, E.; Gorter, J.A. Blood-Brain Barrier Leakage May Lead to Progression of Temporal Lobe Epilepsy. Brain 2007, 130 Pt 2, 521–534. [Google Scholar] [CrossRef]
- Frigerio, F.; Frasca, A.; Weissberg, I.; Parrella, S.; Friedman, A.; Vezzani, A.; Noe, F.M. Long-Lasting pro-Ictogenic Effects Induced in Vivo by Rat Brain Exposure to Serum Albumin in the Absence of Concomitant Pathology. Epilepsia 2012, 53, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Scholler, K.; Trinkl, A.; Klopotowski, M.; Thal, S.C.; Plesnila, N.; Trabold, R.; Hamann, G.F.; Schmid-Elsaesser, R.; Zausinger, S. Characterization of Microvascular Basal Lamina Damage and Blood-Brain Barrier Dysfunction Following Subarachnoid Hemorrhage in Rats. Brain Res. 2007, 1142, 237–246. [Google Scholar] [CrossRef]
- Ciurea, A.V.; Palade, C.; Voinescu, D.; Nica, D.A. Subarachnoid Hemorrhage and Cerebral Vasospasm—Literature Review. J. Med. Life 2013, 6, 120–125. [Google Scholar] [PubMed]
- Verhoog, Q.P.; Holtman, L.; Aronica, E.; van Vliet, E.A. Astrocytes as Guardians of Neuronal Excitability: Mechanisms Underlying Epileptogenesis. Front. Neurol. 2020, 11, 591690. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.H. Neurovascular Coupling and Epilepsy: Hemodynamic Markers for Localizing and Predicting Seizure Onset. Epilepsy Curr. 2007, 7, 91–94. [Google Scholar] [CrossRef]
- Wong, M. Astrocyte Networks and Epilepsy: When Stars Collide. Epilepsy Curr. 2009, 9, 113–115. [Google Scholar] [CrossRef]
- Gavillet, M.; Allaman, I.; Magistretti, P.J. Modulation of Astrocytic Metabolic Phenotype by Proinflammatory Cytokines. Glia 2008, 56, 975–989. [Google Scholar] [CrossRef]
- Escartin, C.; Rouach, N. Astroglial Networking Contributes to Neurometabolic Coupling. Front. Neuroenergetics 2013, 5, 4. [Google Scholar] [CrossRef]
- Sheldon, A.L.; Robinson, M.B. The Role of Glutamate Transporters in Neurodegenerative Diseases and Potential Opportunities for Intervention. Neurochem. Int. 2007, 51, 333–355. [Google Scholar] [CrossRef]
- Oliveira, J.F.; Araque, A. Astrocyte Regulation of Neural Circuit Activity and Network States. Glia 2022, 70, 1455–1466. [Google Scholar] [CrossRef]
- Li, R.; Zhao, M.; Yao, D.; Zhou, X.; Lenahan, C.; Wang, L.; Ou, Y.; He, Y. The Role of the Astrocyte in Subarachnoid Hemorrhage and Its Therapeutic Implications. Front. Immunol. 2022, 13, 1008795. [Google Scholar] [CrossRef]
- Bazargani, N.; Attwell, D. Astrocyte Calcium Signaling: The Third Wave. Nat. Neurosci. 2016, 19, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Belanger, M.; Allaman, I.; Magistretti, P.J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Ransom, B.R. Astrocyte Glycogen and Brain Energy Metabolism. Glia 2007, 55, 1263–1271. [Google Scholar] [CrossRef]
- Suh, S.W.; Bergher, J.P.; Anderson, C.M.; Treadway, J.L.; Fosgerau, K.; Swanson, R.A. Astrocyte Glycogen Sustains Neuronal Activity during Hypoglycemia: Studies with the Glycogen Phosphorylase Inhibitor CP-316,819 ([R-R*,S*]-5-Chloro-N-[2-Hydroxy-3-(Methoxymethylamino)-3-Oxo-1-(Phenylmethyl)Propyl]-1H-Indole-2-Carboxamide). J. Pharmacol. Exp. Ther. 2007, 321, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Boison, D.; Steinhauser, C. Epilepsy and Astrocyte Energy Metabolism. Glia 2018, 66, 1235–1243. [Google Scholar] [CrossRef]
- Amantea, D.; Bagetta, G. Excitatory and Inhibitory Amino Acid Neurotransmitters in Stroke: From Neurotoxicity to Ischemic Tolerance. Curr. Opin. Pharmacol. 2017, 35, 111–119. [Google Scholar] [CrossRef]
- Choi, D.W. Excitotoxicity: Still Hammering the Ischemic Brain in 2020. Front. Neurosci. 2020, 14, 579953. [Google Scholar] [CrossRef]
- Suzuki, H.; Kanamaru, H.; Kawakita, F.; Asada, R.; Fujimoto, M.; Shiba, M. Cerebrovascular Pathophysiology of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. Histol. Histopathol. 2021, 36, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Dreier, J.P.; Major, S.; Pannek, H.W.; Woitzik, J.; Scheel, M.; Wiesenthal, D.; Martus, P.; Winkler, M.K.; Hartings, J.A.; Fabricius, M.; et al. Spreading Convulsions, Spreading Depolarization and Epileptogenesis in Human Cerebral Cortex. Brain 2012, 135 Pt 1, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, L.; Aamand, R.; Karabegovic, S.; Tietze, A.; Blicher, J.U.; Mikkelsen, I.K.; Iversen, N.K.; Secher, N.; Engedal, T.S.; Anzabi, M.; et al. The Role of the Microcirculation in Delayed Cerebral Ischemia and Chronic Degenerative Changes after Subarachnoid Hemorrhage. J. Cereb. Blood Flow. Metab. 2013, 33, 1825–1837. [Google Scholar] [CrossRef]
- Bosche, B.; Graf, R.; Ernestus, R.I.; Dohmen, C.; Reithmeier, T.; Brinker, G.; Strong, A.J.; Dreier, J.P.; Woitzik, J.; Members of the Cooperative Study of Brain Injury, D. Recurrent Spreading Depolarizations after Subarachnoid Hemorrhage Decreases Oxygen Availability in Human Cerebral Cortex. Ann. Neurol. 2010, 67, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.R.; Fujii, T.; Ohiorhenuan, I.; Liu, C.Y. Cortical Spreading Depolarization: Pathophysiology, Implications, and Future Directions. J. Clin. Neurosci. 2016, 24, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kawakita, F.; Asada, R. Neuroelectric Mechanisms of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2022, 23, 3102. [Google Scholar] [CrossRef]
- Kramer, D.R.; Fujii, T.; Ohiorhenuan, I.; Liu, C.Y. Interplay between Cortical Spreading Depolarization and Seizures. Ster. Funct. Neurosurg. 2017, 95, 1–5. [Google Scholar] [CrossRef]
- Haghir, H.; Kovac, S.; Speckmann, E.J.; Zilles, K.; Gorji, A. Patterns of Neurotransmitter Receptor Distributions Following Cortical Spreading Depression. Neuroscience 2009, 163, 1340–1352. [Google Scholar] [CrossRef]
- Ghadiri, M.K.; Kozian, M.; Ghaffarian, N.; Stummer, W.; Kazemi, H.; Speckmann, E.J.; Gorji, A. Sequential Changes in Neuronal Activity in Single Neocortical Neurons after Spreading Depression. Cephalalgia 2012, 32, 116–124. [Google Scholar] [CrossRef]
- van Lieshout, J.H.; Dibue-Adjei, M.; Cornelius, J.F.; Slotty, P.J.; Schneider, T.; Restin, T.; Boogaarts, H.D.; Steiger, H.J.; Petridis, A.K.; Kamp, M.A. An Introduction to the Pathophysiology of Aneurysmal Subarachnoid Hemorrhage. Neurosurg. Rev. 2018, 41, 917–930. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Suzuki, H.; Sozen, T.; Altay, O.; Zhang, J.H. Apoptotic Mechanisms for Neuronal Cells in Early Brain Injury after Subarachnoid Hemorrhage. Acta Neurochir. Suppl. 2011, 110 Pt 1, 43–48. [Google Scholar] [CrossRef]
- Cahill, J.; Calvert, J.W.; Solaroglu, I.; Zhang, J.H. Vasospasm and P53-Induced Apoptosis in an Experimental Model of Subarachnoid Hemorrhage. Stroke 2006, 37, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Cahill, J.; Calvert, J.W.; Zhang, J.H. Mechanisms of Early Brain Injury after Subarachnoid Hemorrhage. J. Cereb. Blood Flow. Metab. 2006, 26, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Siuta, M.; Zuckerman, S.L.; Mocco, J. Nitric Oxide in Cerebral Vasospasm: Theories, Measurement, and Treatment. Neurol. Res. Int. 2013, 2013, 972417. [Google Scholar] [CrossRef]
- Claassen, J.; Bernardini, G.L.; Kreiter, K.; Bates, J.; Du, Y.E.; Copeland, D.; Connolly, E.S.; Mayer, S.A. Effect of Cisternal and Ventricular Blood on Risk of Delayed Cerebral Ischemia after Subarachnoid Hemorrhage: The Fisher Scale Revisited. Stroke 2001, 32, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Green, D.M.; Burns, J.D.; DeFusco, C.M. ICU Management of Aneurysmal Subarachnoid Hemorrhage. J. Intensive Care Med. 2013, 28, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.; Hinson, H.E.; Baguley, I.J. Autonomic Dysfunction Syndromes after Acute Brain Injury. Handb. Clin. Neurol. 2015, 128, 539–551. [Google Scholar] [CrossRef]
- Khalid, F.; Yang, G.L.; McGuire, J.L.; Robson, M.J.; Foreman, B.; Ngwenya, L.B.; Lorenz, J.N. Autonomic Dysfunction Following Traumatic Brain Injury: Translational Insights. Neurosurg. Focus. 2019, 47, E8. [Google Scholar] [CrossRef]
- Borutta, M.C.; Gerner, S.T.; Moeser, P.; Hoelter, P.; Engelhorn, T.; Doerfler, A.; Huttner, H.B.; Schwab, S.; Kuramatsu, J.B.; Koehn, J. Correlation between Clinical Severity and Extent of Autonomic Cardiovascular Impairment in the Acute Phase of Subarachnoid Hemorrhage. J. Neurol. 2022, 269, 5541–5552. [Google Scholar] [CrossRef]
- Manea, M.M.; Comsa, M.; Minca, A.; Dragos, D.; Popa, C. Brain-Heart Axis--Review Article. J. Med. Life 2015, 8, 266–271. [Google Scholar]
- Ecker, A.; Riemenschneider, P.A. Arteriographic Demonstration of Spasm of the Intracranial Arteries, with Special Reference to Saccular Arterial Aneurysms. J. Neurosurg. 1951, 8, 660–667. [Google Scholar] [CrossRef]
- Budohoski, K.P.; Guilfoyle, M.; Helmy, A.; Huuskonen, T.; Czosnyka, M.; Kirollos, R.; Menon, D.K.; Pickard, J.D.; Kirkpatrick, P.J. The Pathophysiology and Treatment of Delayed Cerebral Ischaemia Following Subarachnoid Haemorrhage. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Allcock, J.M.; Drake, C.G. Postoperative Angiography in Cases of Ruptured Intracranial Aneurysm. J. Neurosurg. 1963, 20, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Harders, A.; Gilsbach, J. Transcranial Doppler Sonography and Its Application in Extracranial-Intracranial Bypass Surgery. Neurol. Res. 1985, 7, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, P.; Runnerstam, M.; Birgander, R.; Koskinen, L.O. The Fisher Grading Correlated to Outcome in Patients with Subarachnoid Haemorrhage. Br. J. Neurosurg. 2009, 23, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Deferoxamine In the Treatment of Aneurysmal Subarachnoid Hemorrhage (aSAH)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04566991 (accessed on 27 June 2023).
- Selim, M.; Foster, L.D.; Moy, C.S.; Xi, G.; Hill, M.D.; Morgenstern, L.B.; Greenberg, S.M.; James, M.L.; Singh, V.; Clark, W.M.; et al. Deferoxamine Mesylate in Patients with Intracerebral Haemorrhage (i-DEF): A Multicentre, Randomised, Placebo-Controlled, Double-Blind Phase 2 Trial. Lancet Neurol. 2019, 18, 428–438. [Google Scholar] [CrossRef]
- Wei, C.; Wang, J.; Foster, L.D.; Yeatts, S.D.; Moy, C.; Mocco, J.; Selim, M.; i-DEF Investigators. Effect of Deferoxamine on Outcome According to Baseline Hematoma Volume: A Post Hoc Analysis of the i-DEF Trial. Stroke 2022, 53, 1149–1156. [Google Scholar] [CrossRef]
- Toda, N.; Shimizu, K.; Ohta, T. Mechanism of Cerebral Arterial Contraction Induced by Blood Constituents. J. Neurosurg. 1980, 53, 312–322. [Google Scholar] [CrossRef]
- Toda, N. Mechanisms of Contracting Action of Oxyhemoglobin in Isolated Monkey and Dog Cerebral Arteries. Am. J. Physiol. 1990, 258 Pt 2, H57–H63. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Weir, B.K.; Grace, M.G.; Martin, T.P.; Doi, M.; Cook, D.A. Morphometric Analysis of Monkey Cerebral Arteries Exposed in Vivo to Whole Blood, Oxyhemoglobin, Methemoglobin, and Bilirubin. Blood Vessels 1991, 28, 498–510. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Weir, B.K. A Review of Hemoglobin and the Pathogenesis of Cerebral Vasospasm. Stroke 1991, 22, 971–982. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Toxicity of Iron and Hydrogen Peroxide: The Fenton Reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Sreekrishnan, A.; Leasure, A.C.; Shi, F.D.; Hwang, D.Y.; Schindler, J.L.; Petersen, N.H.; Gilmore, E.J.; Kamel, H.; Sansing, L.H.; Greer, D.M.; et al. Functional Improvement Among Intracerebral Hemorrhage (ICH) Survivors up to 12 Months Post-Injury. Neurocrit Care 2017, 27, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Hop, J.W.; Rinkel, G.J.; Algra, A.; van Gijn, J. Case-Fatality Rates and Functional Outcome after Subarachnoid Hemorrhage: A Systematic Review. Stroke 1997, 28, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Broderick, J.P.; Brott, T.G.; Duldner, J.E.; Tomsick, T.; Huster, G. Volume of Intracerebral Hemorrhage. A Powerful and Easy-to-Use Predictor of 30-Day Mortality. Stroke 1993, 24, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tsirka, S.E. Neuroprotection by Inhibition of Matrix Metalloproteinases in a Mouse Model of Intracerebral Haemorrhage. Brain 2005, 128 Pt 7, 1622–1633. [Google Scholar] [CrossRef]
- Hayman, L.A.; Pagani, J.J.; Kirkpatrick, J.B.; Hinck, V.C. Pathophysiology of Acute Intracerebral and Subarachnoid Hemorrhage: Applications to MR Imaging. AJR Am. J. Roentgenol. 1989, 153, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Keep, R.F.; Hoff, J.T. Mechanisms of Brain Injury after Intracerebral Haemorrhage. Lancet Neurol. 2006, 5, 53–63. [Google Scholar] [CrossRef]
- Pluta, R.M.; Hansen-Schwartz, J.; Dreier, J.; Vajkoczy, P.; Macdonald, R.L.; Nishizawa, S.; Kasuya, H.; Wellman, G.; Keller, E.; Zauner, A.; et al. Cerebral Vasospasm Following Subarachnoid Hemorrhage: Time for a New World of Thought. Neurol. Res. 2009, 31, 151–158. [Google Scholar] [CrossRef]
- Zazulia, A.R.; Diringer, M.N.; Derdeyn, C.P.; Powers, W.J. Progression of Mass Effect after Intracerebral Hemorrhage. Stroke 1999, 30, 1167–1173. [Google Scholar] [CrossRef]
- Dang, G.; Yang, Y.; Wu, G.; Hua, Y.; Keep, R.F.; Xi, G. Early Erythrolysis in the Hematoma After Experimental Intracerebral Hemorrhage. Transl. Stroke Res. 2017, 8, 174–182. [Google Scholar] [CrossRef]
- Koeppen, A.H.; Dickson, A.C.; McEvoy, J.A. The Cellular Reactions to Experimental Intracerebral Hemorrhage. J. Neurol. Sci. 1995, 134, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Samagh, N.; Bhagat, H.; Jangra, K. Monitoring Cerebral Vasospasm: How Much Can We Rely on Transcranial Doppler. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, 12–18. [Google Scholar] [CrossRef]
- Newell, D.W.; Winn, H.R. Transcranial Doppler in Cerebral Vasospasm. Neurosurg. Clin. N. Am. 1990, 1, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Mascia, L.; Del Sorbo, L. Diagnosis and Management of Vasospasm. F1000 Med. Rep. 2009, 1, 33. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Ai, J.; Sabri, M.; Tariq, A.; Shang, X.; Chen, G.; Macdonald, R.L. Neurological and Neurobehavioral Assessment of Experimental Subarachnoid Hemorrhage. BMC Neurosci. 2009, 10, 103. [Google Scholar] [CrossRef]
- Zakaria, Z.; Abdullah, M.M.; Halim, S.A.; Ghani, A.R.I.; Idris, Z.; Abdullah, J.M. The Neurological Exam of a Comatose Patient: An Essential Practical Guide. Malays. J. Med. Sci. 2020, 27, 108–123. [Google Scholar] [CrossRef]
- Woitzik, J.; Dreier, J.P.; Hecht, N.; Fiss, I.; Sandow, N.; Major, S.; Winkler, M.; Dahlem, Y.A.; Manville, J.; Diepers, M.; et al. Delayed Cerebral Ischemia and Spreading Depolarization in Absence of Angiographic Vasospasm after Subarachnoid Hemorrhage. J. Cereb. Blood Flow. Metab. 2012, 32, 203–212. [Google Scholar] [CrossRef]
- Waziri, A.; Claassen, J.; Stuart, R.M.; Arif, H.; Schmidt, J.M.; Mayer, S.A.; Badjatia, N.; Kull, L.L.; Connolly, E.S.; Emerson, R.G.; et al. Intracortical Electroencephalography in Acute Brain Injury. Ann. Neurol. 2009, 66, 366–377. [Google Scholar] [CrossRef]
- Claassen, J.; Hirsch, L.J.; Frontera, J.A.; Fernandez, A.; Schmidt, M.; Kapinos, G.; Wittman, J.; Connolly, E.S.; Emerson, R.G.; Mayer, S.A. Prognostic Significance of Continuous EEG Monitoring in Patients with Poor-Grade Subarachnoid Hemorrhage. Neurocrit Care 2006, 4, 103–112. [Google Scholar] [CrossRef]
- Claassen, J.; Perotte, A.; Albers, D.; Kleinberg, S.; Schmidt, J.M.; Tu, B.; Badjatia, N.; Lantigua, H.; Hirsch, L.J.; Mayer, S.A.; et al. Nonconvulsive Seizures after Subarachnoid Hemorrhage: Multimodal Detection and Outcomes. Ann. Neurol. 2013, 74, 53–64. [Google Scholar] [CrossRef]
- Soustiel, J.F.; Bruk, B.; Shik, B.; Hadani, M.; Feinsod, M. Transcranial Doppler in Vertebrobasilar Vasospasm after Subarachnoid Hemorrhage. Neurosurgery 1998, 43, 282–291, discussion 291–293. [Google Scholar] [CrossRef]
- Bathala, L.; Mehndiratta, M.M.; Sharma, V.K. Transcranial Doppler: Technique and Common Findings (Part 1). Ann. Indian. Acad. Neurol. 2013, 16, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.S., Jr.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef] [PubMed]
- van Gijn, J.; Kerr, R.S.; Rinkel, G.J. Subarachnoid Haemorrhage. Lancet 2007, 369, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Voldby, B.; Enevoldsen, E.M. Intracranial Pressure Changes Following Aneurysm Rupture. Part 1: Clinical and Angiographic Correlations. J. Neurosurg. 1982, 56, 186–196. [Google Scholar] [CrossRef]
- Nornes, H. Cerebral Arterial Flow Dynamics during Aneurysm Haemorrhage. Acta Neurochir. Wien. 1978, 41, 39–48. [Google Scholar] [CrossRef]
- Fontanarosa, P.B. Recognition of Subarachnoid Hemorrhage. Ann. Emerg. Med. 1989, 18, 1199–1205. [Google Scholar] [CrossRef]
- Bassi, P.; Bandera, R.; Loiero, M.; Tognoni, G.; Mangoni, A. Warning Signs in Subarachnoid Hemorrhage: A Cooperative Study. Acta Neurol. Scand. 1991, 84, 277–281. [Google Scholar] [CrossRef]
- Brisman, J.L.; Song, J.K.; Newell, D.W. Cerebral Aneurysms. N. Engl. J. Med. 2006, 355, 928–939. [Google Scholar] [CrossRef]
- Matsuda, M.; Watanabe, K.; Saito, A.; Matsumura, K.; Ichikawa, M. Circumstances, Activities, and Events Precipitating Aneurysmal Subarachnoid Hemorrhage. J. Stroke Cerebrovasc. Dis. 2007, 16, 25–29. [Google Scholar] [CrossRef]
- Beck, J.; Raabe, A.; Szelenyi, A.; Berkefeld, J.; Gerlach, R.; Setzer, M.; Seifert, V. Sentinel Headache and the Risk of Rebleeding after Aneurysmal Subarachnoid Hemorrhage. Stroke 2006, 37, 2733–2737. [Google Scholar] [CrossRef] [PubMed]
- Juvela, S. Minor Leak before Rupture of an Intracranial Aneurysm and Subarachnoid Hemorrhage of Unknown Etiology. Neurosurgery 1992, 30, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, R. The Minor Leak Preceding Subarachnoid Hemorrhage. J. Neurosurg. 1987, 66, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Edlow, J.A. Diagnosing Headache in the Emergency Department: What Is More Important? Being Right, or Not Being Wrong? Eur. J. Neurol. 2008, 15, 1257–1258. [Google Scholar] [CrossRef]
- Kowalski, R.G.; Claassen, J.; Kreiter, K.T.; Bates, J.E.; Ostapkovich, N.D.; Connolly, E.S.; Mayer, S.A. Initial Misdiagnosis and Outcome after Subarachnoid Hemorrhage. JAMA 2004, 291, 866–869. [Google Scholar] [CrossRef]
- Labovitz, D.L.; Halim, A.X.; Brent, B.; Boden-Albala, B.; Hauser, W.A.; Sacco, R.L. Subarachnoid Hemorrhage Incidence among Whites, Blacks and Caribbean Hispanics: The Northern Manhattan Study. Neuroepidemiology 2006, 26, 147–150. [Google Scholar] [CrossRef]
- Rajshekhar, V.; Harbaugh, R.E. Results of Routine Ventriculostomy with External Ventricular Drainage for Acute Hydrocephalus Following Subarachnoid Haemorrhage. Acta Neurochir. Wien. 1992, 115, 8–14. [Google Scholar] [CrossRef]
- Ransom, E.R.; Mocco, J.; Komotar, R.J.; Sahni, D.; Chang, J.; Hahn, D.K.; Kim, G.H.; Schmidt, J.M.; Sciacca, R.R.; Mayer, S.A.; et al. External Ventricular Drainage Response in Poor Grade Aneurysmal Subarachnoid Hemorrhage: Effect on Preoperative Grading and Prognosis. Neurocrit Care 2007, 6, 174–180. [Google Scholar] [CrossRef]
- Milhorat, T.H. Acute Hydrocephalus after Aneurysmal Subarachnoid Hemorrhage. Neurosurgery 1987, 20, 15–20. [Google Scholar] [CrossRef]
- Hasan, D.; Vermeulen, M.; Wijdicks, E.F.; Hijdra, A.; van Gijn, J. Management Problems in Acute Hydrocephalus after Subarachnoid Hemorrhage. Stroke 1989, 20, 747–753. [Google Scholar] [CrossRef]
- Svedung Wettervik, T.; Engquist, H.; Howells, T.; Hanell, A.; Rostami, E.; Ronne-Engstrom, E.; Lewen, A.; Enblad, P. Higher Intracranial Pressure Variability Is Associated with Lower Cerebrovascular Resistance in Aneurysmal Subarachnoid Hemorrhage. J. Clin. Monit. Comput. 2023, 37, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Tu, X.K.; Chen, Q.; Shi, S.S. Propofol Reduces Inflammatory Brain Injury after Subarachnoid Hemorrhage: Involvement of PI3K/Akt Pathway. J. Stroke Cerebrovasc. Dis. 2019, 28, 104375. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Taccone, F.S.; Citerio, G. Monitoring Cerebral Oxygenation in Acute Brain-Injured Patients. Intensive Care Med. 2022, 48, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Muehlschlegel, S. Subarachnoid Hemorrhage. Contin. Minneap. Minn. 2018, 24, 1623–1657. [Google Scholar] [CrossRef]
- Minhas, J.S.; Moullaali, T.J.; Rinkel, G.J.E.; Anderson, C.S. Blood Pressure Management After Intracerebral and Subarachnoid Hemorrhage: The Knowns and Known Unknowns. Stroke 2022, 53, 1065–1073. [Google Scholar] [CrossRef]
- Hillman, J.; Fridriksson, S.; Nilsson, O.; Yu, Z.; Saveland, H.; Jakobsson, K.E. Immediate Administration of Tranexamic Acid and Reduced Incidence of Early Rebleeding after Aneurysmal Subarachnoid Hemorrhage: A Prospective Randomized Study. J. Neurosurg. 2002, 97, 771–778. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, T.S.; Zhou, L.F. Risk Factors for Rebleeding of Aneurysmal Subarachnoid Hemorrhage: A Meta-Analysis. PLoS ONE 2014, 9, e99536. [Google Scholar] [CrossRef]
- Naidech, A.M.; Janjua, N.; Kreiter, K.T.; Ostapkovich, N.D.; Fitzsimmons, B.F.; Parra, A.; Commichau, C.; Connolly, E.S.; Mayer, S.A. Predictors and Impact of Aneurysm Rebleeding after Subarachnoid Hemorrhage. Arch. Neurol. 2005, 62, 410–416. [Google Scholar] [CrossRef]
- Ohkuma, H.; Tsurutani, H.; Suzuki, S. Incidence and Significance of Early Aneurysmal Rebleeding before Neurosurgical or Neurological Management. Stroke 2001, 32, 1176–1180. [Google Scholar] [CrossRef]
- Huttunen, T.; von und zu Fraunberg, M.; Frosen, J.; Lehecka, M.; Tromp, G.; Helin, K.; Koivisto, T.; Rinne, J.; Ronkainen, A.; Hernesniemi, J.; et al. Saccular Intracranial Aneurysm Disease: Distribution of Site, Size, and Age Suggests Different Etiologies for Aneurysm Formation and Rupture in 316 Familial and 1454 Sporadic Eastern Finnish Patients. Neurosurgery 2010, 66, 631–638, discussion 638. [Google Scholar] [CrossRef]
- Bakker, N.A.; Metzemaekers, J.D.; Groen, R.J.; Mooij, J.J.; Van Dijk, J.M. International Subarachnoid Aneurysm Trial 2009: Endovascular Coiling of Ruptured Intracranial Aneurysms Has No Significant Advantage over Neurosurgical Clipping. Neurosurgery 2010, 66, 961–962. [Google Scholar] [CrossRef] [PubMed]
- Risselada, R.; Lingsma, H.F.; Bauer-Mehren, A.; Friedrich, C.M.; Molyneux, A.J.; Kerr, R.S.; Yarnold, J.; Sneade, M.; Steyerberg, E.W.; Sturkenboom, M.C. Prediction of 60 Day Case-Fatality after Aneurysmal Subarachnoid Haemorrhage: Results from the International Subarachnoid Aneurysm Trial (ISAT). Eur. J. Epidemiol. 2010, 25, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, A.J.; Kerr, R.S.; Yu, L.M.; Clarke, M.; Sneade, M.; Yarnold, J.A.; Sandercock, P.; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of Neurosurgical Clipping versus Endovascular Coiling in 2143 Patients with Ruptured Intracranial Aneurysms: A Randomised Comparison of Effects on Survival, Dependency, Seizures, Rebleeding, Subgroups, and Aneurysm Occlusion. Lancet 2005, 366, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Wiebers, D.O.; Whisnant, J.P.; Huston, J., 3rd; Meissner, I.; Brown, R.D., Jr.; Piepgras, D.G.; Forbes, G.S.; Thielen, K.; Nichols, D.; O’Fallon, W.M.; et al. Unruptured Intracranial Aneurysms: Natural History, Clinical Outcome, and Risks of Surgical and Endovascular Treatment. Lancet 2003, 362, 103–110. [Google Scholar] [CrossRef]
- Vora, Y.Y.; Suarez-Almazor, M.; Steinke, D.E.; Martin, M.L.; Findlay, J.M. Role of Transcranial Doppler Monitoring in the Diagnosis of Cerebral Vasospasm after Subarachnoid Hemorrhage. Neurosurgery 1999, 44, 1237–1247, discussion 1247–1248. [Google Scholar] [PubMed]
- Mastantuono, J.M.; Combescure, C.; Elia, N.; Tramer, M.R.; Lysakowski, C. Transcranial Doppler in the Diagnosis of Cerebral Vasospasm: An Updated Meta-Analysis. Crit. Care Med. 2018, 46, 1665–1672. [Google Scholar] [CrossRef]
- Vespa, P.M.; Nuwer, M.R.; Juhasz, C.; Alexander, M.; Nenov, V.; Martin, N.; Becker, D.P. Early Detection of Vasospasm after Acute Subarachnoid Hemorrhage Using Continuous EEG ICU Monitoring. Electroencephalogr. Clin. Neurophysiol. 1997, 103, 607–615. [Google Scholar] [CrossRef]
- Claassen, J.; Hirsch, L.J.; Kreiter, K.T.; Du, E.Y.; Connolly, E.S.; Emerson, R.G.; Mayer, S.A. Quantitative Continuous EEG for Detecting Delayed Cerebral Ischemia in Patients with Poor-Grade Subarachnoid Hemorrhage. Clin. Neurophysiol. 2004, 115, 2699–2710. [Google Scholar] [CrossRef]
- McKinney, A.M.; Palmer, C.S.; Truwit, C.L.; Karagulle, A.; Teksam, M. Detection of Aneurysms by 64-Section Multidetector CT Angiography in Patients Acutely Suspected of Having an Intracranial Aneurysm and Comparison with Digital Subtraction and 3D Rotational Angiography. AJNR Am. J. Neuroradiol. 2008, 29, 594–602. [Google Scholar] [CrossRef]
- Hafeez, S.; Grandhi, R. Systematic Review of Intrathecal Nicardipine for the Treatment of Cerebral Vasospasm in Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care 2019, 31, 399–405. [Google Scholar] [CrossRef]
- Lee, K.H.; Lukovits, T.; Friedman, J.A. “Triple-H” Therapy for Cerebral Vasospasm Following Subarachnoid Hemorrhage. Neurocrit Care 2006, 4, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Lennihan, L.; Mayer, S.A.; Fink, M.E.; Beckford, A.; Paik, M.C.; Zhang, H.; Wu, Y.C.; Klebanoff, L.M.; Raps, E.C.; Solomon, R.A. Effect of Hypervolemic Therapy on Cerebral Blood Flow after Subarachnoid Hemorrhage: A Randomized Controlled Trial. Stroke 2000, 31, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Jost, S.C.; Diringer, M.N.; Zazulia, A.R.; Videen, T.O.; Aiyagari, V.; Grubb, R.L.; Powers, W.J. Effect of Normal Saline Bolus on Cerebral Blood Flow in Regions with Low Baseline Flow in Patients with Vasospasm Following Subarachnoid Hemorrhage. J. Neurosurg. 2005, 103, 25–30. [Google Scholar] [CrossRef]
- Rosenwasser, R.H.; Armonda, R.A.; Thomas, J.E.; Benitez, R.P.; Gannon, P.M.; Harrop, J. Therapeutic Modalities for the Management of Cerebral Vasospasm: Timing of Endovascular Options. Neurosurgery 1999, 44, 975–979, discussion 979–980. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).