Neurotrophin-3 (NT-3) as a Potential Biomarker of the Peripheral Nervous System Damage Following Breast Cancer Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Neurological Examination
2.3. Enzyme-Linked Immunosorbent Assays
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. Evaluation of BDNF, NT-3, and Gal-3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Francies, F.Z.; Hull, R.; Khanyile, R.; Dlamini, Z. Breast cancer in low-middle income countries: Abnormality in splicing and lack of targeted treatment options. Am. J. Cancer Res. 2020, 10, 1568–1591. [Google Scholar] [PubMed]
- Jonczyk, M.M.; Jean, J.; Graham, R.; Chatterjee, A. Surgical trends in breast cancer: A rise in novel operative treatment options over a 12 year analysis. Breast Cancer Res. Treat. 2019, 173, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Bauer-Nilsen, K.; McNulty, R.H.; Vicini, F. Novel radiation therapy approaches for breast cancer treatment. Semin. Oncol. 2020, 47, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Pondé, N.F.; Zardavas, D.; Piccart, M. Progress in adjuvant systemic therapy for breast cancer. Nat. Rev. Clin. Oncol. 2019, 16, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Mutebi, M.; Anderson, B.O.; Duggan, C.; Adebamowo, C.; Agarwal, G.; Ali, Z.; Bird, P.; Bourque, J.-M.; DeBoer, R.; Gebrim, L.H.; et al. Breast cancer treatment: A phased approach to implementation. Cancer 2020, 126 (Suppl. S10), 2365–2378. [Google Scholar] [CrossRef]
- Villar, R.R.; Fernández, S.P.; Garea, C.C.; Pillado, M.T.S.; Barreiro, V.B.; Martín, C.G. Quality of life and anxiety in women with breast cancer before and after treatment. Rev. Lat. Am. Enferm. 2017, 25, e2958. [Google Scholar] [CrossRef]
- Mokhtari-Hessari, P.; Montazeri, A. Health-related quality of life in breast cancer patients: Review of reviews from 2008 to 2018. Health Qual. Life Outcomes 2020, 18, 338. [Google Scholar] [CrossRef]
- Wang, L.; Guyatt, G.H.; Kennedy, S.A.; Romerosa, B.; Kwon, H.Y.; Kaushal, A.; Chang, Y.; Craigie, S.; de Almeida, C.P.B.; Couban, R.J.; et al. Predictors of persistent pain after breast cancer surgery: A systematic review and meta-analysis of observational studies. CMAJ 2016, 188, E352–E361. [Google Scholar] [CrossRef] [Green Version]
- Delanian, S.; Lefaix, J.-L.; Pradat, P.-F. Radiation-induced neuropathy in cancer survivors. Radiother. Oncol. 2012, 105, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-J.; Chan, Y.-N.; Jheng, Y.-W.; Wu, C.-J.; Lin, M.-W.; Tseng, L.-M.; Tsai, Y.-F.; Liu, L.-C. Chemotherapy-induced peripheral neuropathy in newly diagnosed breast cancer survivors treated with taxane: A prospective longitudinal study. Support Care Cancer 2021, 29, 2959–2971. [Google Scholar] [CrossRef]
- Kim, M.; Jung, M.S. Effects of Chemotherapy-Induced Peripheral Neuropathy in Women With Breast Cancer: A Structural Equation Approach With the Theory of Unpleasant Symptoms. Cancer Nurs. 2021, 44, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Basal, C.; Seluzicki, C.; Li, S.Q.; Seidman, A.D.; Mao, J.J. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: Prevalence, risk factors, and fall risk. Breast Cancer Res. Treat. 2016, 159, 327–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chappell, A.G.; Yuksel, S.; Sasson, D.C.; Wescott, A.B.; Connor, L.M.; Ellis, M.F. Post-Mastectomy Pain Syndrome: An Up-to-Date Review of Treatment Outcomes. JPRAS Open 2021, 30, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Tait, R.C.; Zoberi, K.; Ferguson, M.; Levenhagen, K.; Luebbert, R.A.; Rowland, K.; Salsich, G.B.; Herndon, C. Persistent Post-Mastectomy Pain: Risk Factors and Current Approaches to Treatment. J. Pain 2018, 19, 1367–1383. [Google Scholar] [CrossRef] [PubMed]
- Bortsov, A.V.; Devor, M.; Kaunisto, M.A.; Kalso, E.; Brufsky, A.; Kehlet, H.; Aasvang, E.; Bittner, R.; Diatchenko, L.; Belfer, I. CACNG2 polymorphisms associate with chronic pain after mastectomy. Pain 2019, 160, 561–568. [Google Scholar] [CrossRef]
- Stephens, K.E.; Levine, J.D.; Aouizerat, B.E.; Paul, S.M.; Abrams, G.; Conley, Y.P.; Miaskowski, C. Associations between genetic and epigenetic variations in cytokine genes and mild persistent breast pain in women following breast cancer surgery. Cytokine 2017, 99, 203–213. [Google Scholar] [CrossRef]
- Langford, D.J.; Paul, S.M.; West, C.M.; Dunn, L.B.; Levine, J.D.; Kober, K.M.; Dodd, M.J.; Miaskowski, C.; Aouizerat, B.E. Variations in potassium channel genes are associated with distinct trajectories of persistent breast pain after breast cancer surgery. Pain 2015, 156, 371–380. [Google Scholar] [CrossRef]
- Khan, N.; Smith, M.T. Neurotrophins and Neuropathic Pain: Role in Pathobiology. Molecules 2015, 20, 10657. [Google Scholar] [CrossRef]
- McGregor, C.E.; English, A.W. The Role of BDNF in Peripheral Nerve Regeneration: Activity-Dependent Treatments and Val66Met. Front. Cell Neurosci. 2018, 12, 522. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Han, Q.; Wang, X.; Ai, Z.; Zheng, Y. Galectin-3 Inhibition Is Associated with Neuropathic Pain Attenuation after Peripheral Nerve Injury. PLoS ONE 2016, 11, e0148792. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.J.; Dawbarn, D. Clinical relevance of the neurotrophins and their receptors. Clin. Sci. 2006, 110, 175–191. [Google Scholar] [CrossRef] [Green Version]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R Soc. Lond. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naito, Y.; Lee, A.K.; Takahashi, H. Emerging roles of the neurotrophin receptor TrkC in synapse organization. Neurosci. Res. 2017, 116, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Yalvac, M.E.; Arnold, W.D.; Braganza, C.; Chen, L.; Mendell, J.R.; Sahenk, Z. AAV1.NT-3 gene therapy attenuates spontaneous autoimmune peripheral polyneuropathy. Gene Ther. 2016, 23, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Liao, X.; Shi, B.; Qu, Y.; Huang, Z.; Lin, Q.; Guo, X.; Pei, F. The Effects of Controlled Release of Neurotrophin-3 from PCLA Scaffolds on the Survival and Neuronal Differentiation of Transplanted Neural Stem Cells in a Rat Spinal Cord Injury Model. PLoS ONE 2014, 9, e107517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkman, H.P.; Rao, S.S.C.; Reynolds, J.C.; Schiller, L.R.; Wald, A.; Miner, P.B.; Lembo, A.J.; Gordon, J.M.; Drossman, D.A.; Waltzman, L.; et al. Neurotrophin-3 improves functional constipation. Am. J. Gastroenterol. 2003, 98, 1338–1347. [Google Scholar] [CrossRef]
- Pradat, P.F.; Kennel, P.; Naimi-Sadaoui, S.; Finiels, F.; Orsini, C.; Revah, F.; Delaere, P.; Mallet, J. Continuous delivery of neurotrophin 3 by gene therapy has a neuroprotective effect in experimental models of diabetic and acrylamide neuropathies. Hum. Gene Ther. 2001, 12, 2237–2249. [Google Scholar] [CrossRef]
- Gao, W.Q.; Dybdal, N.; Shinsky, N.; Murnane, A.; Schmelzer, C.; Siegel, M.; Keller, G.; Hefti, F.; Phillips, H.S.; Winslow, J.W. Neurotrophin-3 reverses experimental cisplatin-induced peripheral sensory neuropathy. Ann. Neurol. 1995, 38, 30–37. [Google Scholar] [CrossRef]
- Gandhi, R.; Ryals, J.M.; Wright, D.E. Neurotrophin-3 reverses chronic mechanical hyperalgesia induced by intramuscular acid injection. J. Neurosci. 2004, 24, 9405–9413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson-Gerwing, T.D.; Stucky, C.L.; McComb, G.W.; Verge, V.M.K. Neurotrophin-3 significantly reduces sodium channel expression linked to neuropathic pain states. Exp. Neurol. 2008, 213, 303–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahenk, Z.; Oblinger, J.; Edwards, C. Neurotrophin-3 deficient Schwann cells impair nerve regeneration. Exp. Neurol. 2008, 212, 552–556. [Google Scholar] [CrossRef]
- Cong, Y.; Wang, C.; Wang, J.; Li, H.; Li, Q. NT-3 Promotes Oligodendrocyte Proliferation and Nerve Function Recovery after Spinal Cord Injury by Inhibiting Autophagy Pathway. J. Surg. Res. 2020, 247, 128–135. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 2014, 220, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Walwyn, W.; Ennes, H.S.; Kim, H.; McRoberts, J.A.; Marvizón, J.C.G. BDNF released during neuropathic pain potentiates NMDA receptors in primary afferent terminals. Eur. J. Neurosci. 2014, 39, 1439–1454. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Pain facilitation and activity-dependent plasticity in pain modulatory circuitry: Role of BDNF-TrkB signaling and NMDA receptors. Mol. Neurobiol. 2007, 35, 224–235. [Google Scholar] [CrossRef]
- Huang, L.; Jin, J.; Chen, K.; You, S.; Zhang, H.; Sideris, A.; Norcini, M.; Recio-Pinto, E.; Wang, J.; Gan, W.-B.; et al. BDNF produced by cerebral microglia promotes cortical plasticity and pain hypersensitivity after peripheral nerve injury. PLoS Biol. 2021, 19, e3001337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xie, Z.; Li, C.; Xing, Z.; Xie, S.; Li, M.; Yao, J. Driving effect of BDNF in the spinal dorsal horn on neuropathic pain. Neurosci. Lett. 2021, 756, 135965. [Google Scholar] [CrossRef]
- Ciobanu, C.; Reid, G.; Babes, A. Acute and chronic effects of neurotrophic factors BDNF and GDNF on responses mediated by thermo-sensitive TRP channels in cultured rat dorsal root ganglion neurons. Brain Res. 2009, 1284, 54–67. [Google Scholar] [CrossRef]
- Marcol, W.; Kotulska, K.; Larysz-Brysz, M.; Kowalik, J.L. BDNF contributes to animal model neuropathic pain after peripheral nerve transection. Neurosurg. Rev. 2007, 30, 235–243; discussion 243. [Google Scholar] [CrossRef] [PubMed]
- Karagyaur, M.; Dyikanov, D.; Makarevich, P.; Semina, E.; Stambolsky, D.; Plekhanova, O.; Kalinina, N.; Tkachuk, V. Non-viral transfer of BDNF and uPA stimulates peripheral nerve regeneration. Biomed. Pharmacother. 2015, 74, 63–70. [Google Scholar] [CrossRef]
- Lopes, C.D.F.; Gonçalves, N.P.; Gomes, C.P.; Saraiva, M.J.; Pêgo, A.P. BDNF gene delivery mediated by neuron-targeted nanoparticles is neuroprotective in peripheral nerve injury. Biomaterials 2017, 121, 83–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Turco, S.; De Simone, P.; Ghinolfi, D.; Gaggini, M.; Basta, G. Comparison between galectin-3 and YKL-40 levels for the assessment of liver fibrosis in cirrhotic patients. Arab. J. Gastroenterol. 2021, 22, 187–192. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, P.; Zeng, L.; Zhao, Y.; Xie, L.; Chen, B. Mesenchymal stem cells ameliorate renal fibrosis by galectin-3/Akt/GSK3β/Snail signaling pathway in adenine-induced nephropathy rat. Stem Cell Res. Ther. 2021, 12, 409. [Google Scholar] [CrossRef]
- Argüeso, P.; Panjwani, N. Focus on Molecules: Galectin-3. Exp. Eye Res. 2011, 92, 2–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srejovic, I.; Selakovic, D.; Jovicic, N.; Jakovljević, V.; Lukic, M.L.; Rosic, G. Galectin-3: Roles in Neurodevelopment, Neuroinflammation, and Behavior. Biomolecules 2020, 10, 798. [Google Scholar] [CrossRef]
- Kumar, S.; Ranawat, C.S.; Bhandiwad, C.; Arya, H.; Mali, M.; Singh, C.P.; Sharma, N.; Lathwal, N.; Wasim, S. Galectin-3 as a Potential Biomarker of Microvascular Complications in Patients with Type 2 Diabetes. Indian J. Endocrinol. Metab. 2022, 26, 490–497. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Lo, J.-J.; Wu, S.-H.; Wang, C.-Z.; Chen, R.-F.; Lee, S.-S.; Chai, C.-Y.; Huang, S.-H. Early Hyperbaric Oxygen Treatment Attenuates Burn-Induced Neuroinflammation by Inhibiting the Galectin-3-Dependent Toll-Like Receptor-4 Pathway in a Rat Model. Int. J. Mol. Sci. 2018, 19, 2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyanagi, M.; Imai, S.; Matsumoto, M.; Iguma, Y.; Kawaguchi-Sakita, N.; Kotake, T.; Iwamitsu, Y.; Ntogwa, M.; Hiraiwa, R.; Nagayasu, K.; et al. Pronociceptive Roles of Schwann Cell-Derived Galectin-3 in Taxane-Induced Peripheral Neuropathy. Cancer Res. 2021, 81, 2207–2219. [Google Scholar] [CrossRef]
- Spielberger, C.D. Anxiety and Behavior; Academic Press: Cambridge, MA, USA, 2013; ISBN 978-1-4832-5836-2. [Google Scholar]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-1-4443-3241-4. [Google Scholar]
- Ganz, P.A.; Goodwin, P.J. Breast Cancer Survivorship: Where Are We Today? Adv. Exp. Med. Biol. 2015, 862, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mahanova, A.; Krasnikova, V.; Fionik, O.; Pospelova, M.; Alekseeva, T.; Nikolaeva, A.; Maksimov, A.; Trofimov, N.; Donkov, V.; Bukkieva, T. Clinical and neuropsychological assessment of the condition of patients with post-mastectomy syndrome. Transl. Med. 2022, 9, 50–58. [Google Scholar] [CrossRef]
- Bukreeva, T.A.; Pospelova, M.; Efimtsev, A.; Fionik, O.V.; Konstantin, A.S.; Gorbunova, E.A.; Krasnikova, V.V.; Makanova, A.M. Neurological aspects of postmastectomy syndrome and modern methods for their diagnosis. Med. News North Cauc. 2022, 17, 90–95. [Google Scholar] [CrossRef]
- Pospelova, M.; Krasnikova, V.; Fionik, O.; Alekseeva, T.; Samochernykh, K.; Ivanova, N.; Trofimov, N.; Vavilova, T.; Vasilieva, E.; Topuzova, M.; et al. Adhesion Molecules ICAM-1 and PECAM-1 as Potential Biomarkers of Central Nervous System Damage in Women Breast Cancer Survivors. Pathophysiology 2022, 29, 52–65. [Google Scholar] [CrossRef]
- Huehnchen, P.; Schinke, C.; Bangemann, N.; Dordevic, A.D.; Kern, J.; Maierhof, S.K.; Hew, L.; Nolte, L.; Körtvelyessy, P.; Göpfert, J.C.; et al. Neurofilament proteins as a potential biomarker in chemotherapy-induced polyneuropathy. JCI Insight 2022, 7, e154395. [Google Scholar] [CrossRef]
- Sharma, A.; Johnson, K.B.; Bie, B.; Rhoades, E.E.; Sen, A.; Kida, Y.; Hockings, J.; Gatta, A.; Davenport, J.; Arcangelini, C.; et al. A Multimodal Approach to Discover Biomarkers for Taxane-Induced Peripheral Neuropathy (TIPN): A Study Protocol. Technol. Cancer Res. Treat. 2022, 21, 15330338221127168. [Google Scholar] [CrossRef] [PubMed]
- De Santis, S.; Pace, A.; Bove, L.; Cognetti, F.; Properzi, F.; Fiore, M.; Triaca, V.; Savarese, A.; Simone, M.D.; Jandolo, B.; et al. Patients treated with antitumor drugs displaying neurological deficits are characterized by a low circulating level of nerve growth factor. Clin. Cancer Res. 2000, 6, 90–95. [Google Scholar] [PubMed]
- Baka, P.; Escolano-Lozano, F.; Birklein, F. Systemic inflammatory biomarkers in painful diabetic neuropathy. J. Diabetes Complicat. 2021, 35, 108017. [Google Scholar] [CrossRef]
- Jin, H.Y.; Park, T.S. Role of inflammatory biomarkers in diabetic peripheral neuropathy. J. Diabetes Investig. 2018, 9, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Rossor, A.M.; Reilly, M.M. Blood biomarkers of peripheral neuropathy. Acta Neurol. Scand. 2022, 146, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Alberti, P. A review of novel biomarkers and imaging techniques for assessing the severity of chemotherapy-induced peripheral neuropathy. Expert Opin. Drug Metab. Toxicol. 2020, 16, 1147–1158. [Google Scholar] [CrossRef]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fionik, O.V.; Krasnikova, V.V.; Pokatilo, D.A.; Pospelova, M.L. Changes in the microcirculatory bed in patients with post-mastectomy syndrome. Issues Reconstr. Plast. Surg. 2022, 24, 55–62. [Google Scholar] [CrossRef]
- Omura, T.; Sano, M.; Omura, K.; Hasegawa, T.; Doi, M.; Sawada, T.; Nagano, A. Different expressions of BDNF, NT3, and NT4 in muscle and nerve after various types of peripheral nerve injuries. J. Peripher. Nerv. Syst. 2005, 10, 293–300. [Google Scholar] [CrossRef]
- Uddin, M.S.; Mamun, A.A.; Rahman, M.M.; Jeandet, P.; Alexiou, A.; Behl, T.; Sarwar, M.S.; Sobarzo-Sánchez, E.; Ashraf, G.M.; Sayed, A.A.; et al. Natural Products for Neurodegeneration: Regulating Neurotrophic Signals. Oxidative Med. Cell. Longev. 2021, 2021, 8820406. [Google Scholar] [CrossRef]
- Bukkieva, T.A.; Pospelova, M.L.; Anpilogova, K.S.; Fionik, O.V.; Alekseeva, T.M.; Gorbunova, E.A.; Krasnikova, V.V.; Makanova, A.M.; Tonyan, S.N.; Levchuk, A.G.; et al. Changes in the structural connectom of the brain in patients with postmastectomy syndrome. Transl. Med. 2022, 8, 33–42. [Google Scholar] [CrossRef]
- Siuciak, J.A.; Altar, C.A.; Wiegand, S.J.; Lindsay, R.M. Antinociceptive effect of brain-derived neurotrophic factor and neurotrophin-3. Brain Res. 1994, 633, 326–330. [Google Scholar] [CrossRef]
- Tender, G.C.; Kaye, A.D.; Li, Y.-Y.; Cui, J.-G. Neurotrophin-3 and tyrosine kinase C have modulatory effects on neuropathic pain in the rat dorsal root ganglia. Neurosurgery 2011, 68, 1048–1055; discussion 1055. [Google Scholar] [CrossRef]
- Malcangio, M.; Garrett, N.E.; Cruwys, S.; Tomlinson, D.R. Nerve Growth Factor- and Neurotrophin-3-Induced Changes in Nociceptive Threshold and the Release of Substance P from the Rat Isolated Spinal Cord. J. Neurosci. 1997, 17, 8459–8467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson-Gerwing, T.D.; Dmyterko, M.V.; Zochodne, D.W.; Johnston, J.M.; Verge, V.M.K. Neurotrophin-3 suppresses thermal hyperalgesia associated with neuropathic pain and attenuates transient receptor potential vanilloid receptor-1 expression in adult sensory neurons. J. Neurosci. 2005, 25, 758–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Group Characteristic | Patients after BC Treatment (n = 67) |
|---|---|
| Age (years) | 47 (44, 49) |
| Years after the end of therapy | 3 (2, 5) |
| The structure by stages TNM, UICC | |
| I (T1N0M0) | 8 (12%) |
| II A (T2N1M0) | 46 (68%) |
| II B (T3N1M0) | 3 (5%) |
| III A (T3N2M0) | 2 (3%) |
| III B (T4N2M0) | 8 (12%) |
| The histological type of breast cancer | |
| Ductal carcinoma in situ (DCIS) | 7 (11%) |
| Invasive ductal carcinoma (IDC) | 49 (73%) |
| Invasive lobular carcinoma (ILC) | 11 (16%) |
| Type of breast cancer treatment | |
| Complex therapy of breast cancer (surgical treatment, radiation therapy, chemotherapy) | 37 (55%) |
| Surgical treatment and chemotherapy | 18 (27%) |
| Surgical treatment and radiation therapy | 7 (10%) |
| Only surgical treatment | 5 (7%) |
| Type of surgical treatment | |
| Madden-modified radical mastectomy | 53 (79%) |
| Sectoral resection | 14 (21%) |
| Hormone therapy (Tamoxifen ± GnRH analogue) | |
| Yes | 12 (18%) |
| No | 50 (75%) |
| Completed the course | 5 (7%) |
| Clinical Characteristics | Number of Patients (N, %) |
|---|---|
| Chronic pain syndrome | 46 (69%) |
| Numbness in the armpit | 45 (67%) |
| Polyneuropathy | 34 (51%) |
| Biomarker | BC Survivors (n = 67) | Healthy Volunteers (n = 25) | Mann-Whitney U Test | Significance (p) |
|---|---|---|---|---|
| NT-3 pg/mL | 16.62 [11.18; 20.0] | 5.74 [4.56; 13.7] | 254 | <0.001 * |
| BDNF pg/mL | 31,747.4 [23,068.0; 37,903.0] | 29,281.6 [21,786.4; 35,728.2] | 534.5 | 0.33 |
| Gal-3 ng/mL | 5450.0 [4080.0; 9900.0] | 4660.0 [3240.0; 6380.0] | 521.0 | 0.26 |
| Sing of Separation | Characteristic of the Sing | Number of Patients (and Age) | NT-3 pg/mL | Kruskal–Wallis Test | p | BDNF pg/mL | Kruskal–Wallis Test | p | Gal-3 ng/mL | Kruskal–Wallis Test | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
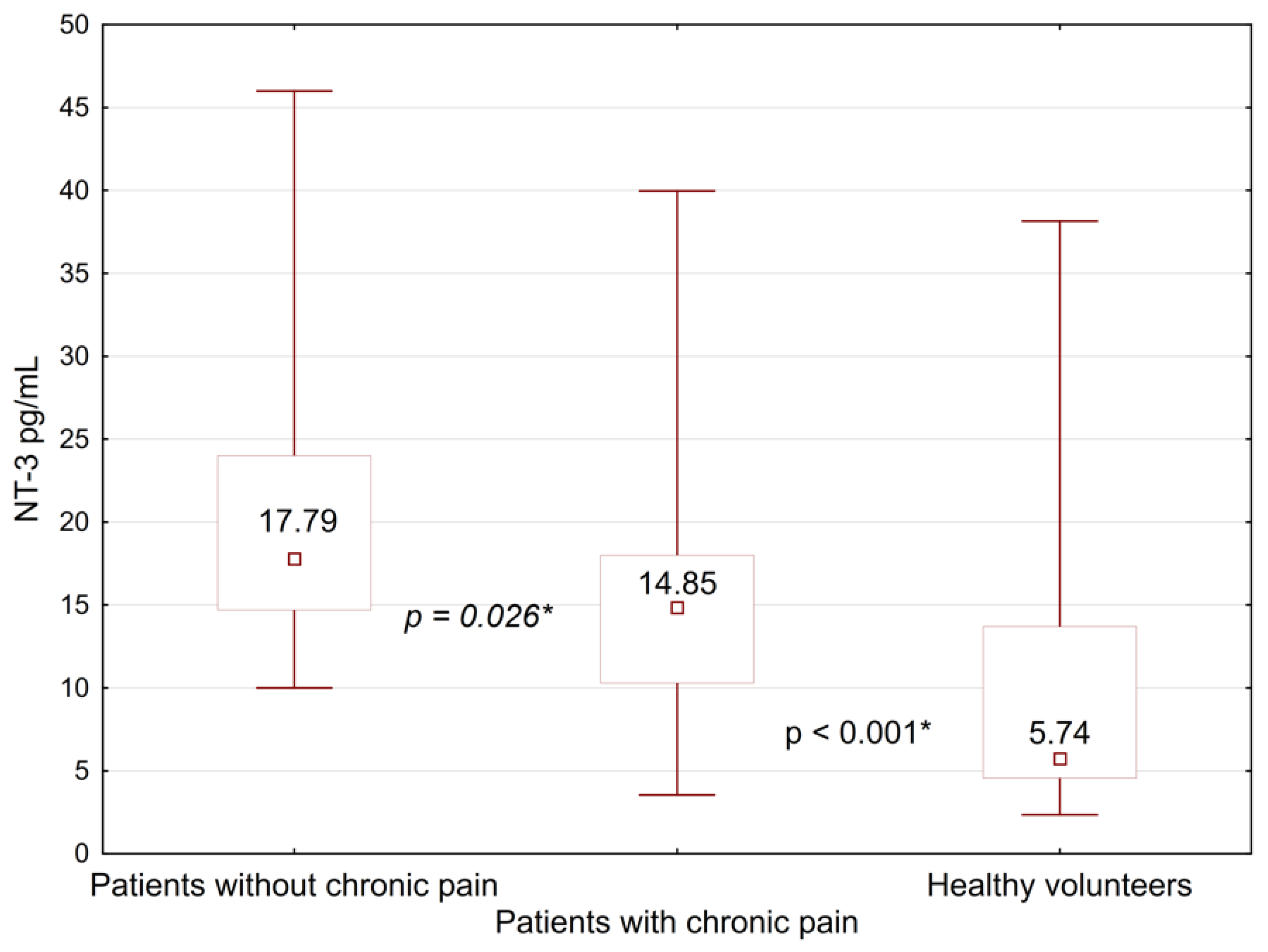
| Chronic pain syndrome | yes | 46 (47 [43; 48]) | 14.85 [10.3; 18.0] | 19.05 | <0.001 * | 32,291.2 [25,359.0; 39,417.4] | 4.45 | 0.11 | 5600.0 [4160.0; 9140.0] | 1.12 | 0.57 |
| no | 21 (47 [40; 50]) | 17.79 [14.7; 24.0] | 29,572.0 [20,621.0; 35,456.0] | 6350.0 [3340.0;13,960.0] | |||||||
| Healthy volunteers | 5.74 [4.56; 13.7] | 29,281.6 [21,786.4; 35,728.2] | 4660.0 [3240.0; 6380.0] | ||||||||
| Hypoesthesia in the armpit | yes | 45 (46 [40; 48]) | 16.0 [11.74; 20.0] | 15.22 | <0.001 * | 31,378.0 [23,689.0; 37,903.0] | 1.13 | 0.57 | 5200.0 [4060.0; 9980.0] | 1.74 | 0.42 |
| no | 22 (48 [44; 49]) | 17.03 [11.18; 20.0] | 32,232.0 [22,757.2; 38,136.0] | 6230.0 [4620.0; 9900.0] | |||||||
| Healthy volunteers | 5.74 [4.56; 13.7] | 29,281.6 [21,786.4; 35,728.2] | 4660.0 [3240.0; 6380.0] | ||||||||
| Polyneuropathy | yes | 34 (46 [42; 48]) | 15.58 [11.75; 17.36] | 16.4 | <0.001 * | 31,164.7 [22,601.5; 39,223.2] | 1.11 | 0.57 | 5090.0 [4070.0; 8760.0] | 1.53 | 0.47 |
| no | 33 (48 [43; 49]) | 17.06 [11.18; 21.46] | 32,077.0 [24,155.4; 36,815.0] | 6340.0 [4620.0; 9900.0] | |||||||
| Healthy volunteers | 5.74 [4.56; 13.7] | 29,281.6 [21,786.4; 35,728.2] | 4660.0 [3240.0; 6380.0] | ||||||||
| Treatment history | Only surgical treatment | 5 (44 [40; 48]) | 17.36 [14.18; 19.12] | 17.03 | 0.0019 * | 30,776.5 [18,225.0; 45,080.8] | 1.87 | 0.75 | 4130.0 [3580.0; 6290.0] | 2.99 | 0.55 |
| Surgical treatment and radiotherapy | 7 (47 [46; 47]) | 16.0 [15.0; 18.82] | 30,951.4 [28,038.0; 32,504.8] | 6340.0 [5140.0; 7080.0] | |||||||
| Surgical treatment and Chemotherapy | 18 (46 [42; 48]) | 17.35 [14.26; 22.35] | 31,203.9 [21,320.1; 39,417.5] | 6110.0 [4070.0; 8360.0] | |||||||
| Complex treatment | 37 (46 [43; 49]) | 13.53 [10.0; 18.96] | 32,386.8 [25,378.5; 37,320.0] | 5350.0 [4220.0; 10,660.0] | |||||||
| Healthy volunteers | 5.74 [4.56; 13.7] | 29,281.6 [21,786.4; 35,728.2] | 4660.0 [3240.0; 6380.0] | ||||||||
| Type of surgery | Modified unilateral mastectomy Madden | 53 (47 [43; 48]) | 16.88 [11.75; 20.0] | 15.84 | <0.001 * | 32,174.7 [22,135.6; 38,407.5] | 1.08 | 0.58 | 5420.0 [4120.0; 10,240.0] | 1.30 | 0.52 |
| Sector mastectomy | 14 (46 [41; 48]) | 15.0 [9.42; 19.4] | 31,456.0 [25,359.0; 37,242.0] | 6160.0 [4060.0; 8140.0] | |||||||
| Healthy volunteers | 5.74 [4.56; 13.7] | 29,281.6 [21,786.4; 35,728.2] | 4660.0 [3240.0; 6380.0] | ||||||||
| Sing of Separation | Compared Groups | Mann-Whitney U Test | p |
|---|---|---|---|
| Chronic pain syndrome | Yes/No | 264 | 0.026 * |
| Healthy volunteers (Healthy)/No | 44 | <0.001 * | |
| (Healthy)/Yes | 208 | <0.001 * | |
| Hypoesthesia in the armpit | Yes/No | 463 | 0.90 |
| (Healthy)/No | 79.5 | <0.001 * | |
| (Healthy)/Yes | 173.5 | <0.001 * | |
| Polyneuropathy | Yes/No | 436 | 0.22 |
| (Healthy)/No | 112.5 | <0.001 * | |
| (Healthy)/Yes | 140.5 | 0.001 * | |
| Type of surgery | Modified unilateral mastectomy Madden (M)/Sector mastectomy (SM) | 294 | 0.48 |
| (Healthy)/(M) | 183 | <0.001 * | |
| (Healthy)/(SM) | 70 | 0.04 * | |
| Breast cancer treatment | Only surgical treatment (OS)/Surgical treatment and radiotherapy (S + R) | 9 | 0.90 |
| Surgical treatment and Chemotherapy (S + Ch)/Complex treatment (CT) | 179 | 0.09 | |
| (OS)/(S + Ch) | 29 | 0.81 | |
| (OS)/(CT) | 50 | 0.50 | |
| (S + R)/(S + Ch) | 34 | 0.65 | |
| (S + R)/(CT) | 71 | 0.71 | |
| (OS)/(Healthy) | 11 | 0.03 * | |
| (S + R)/(Healthy) | 26 | 0.014 * | |
| (S + Ch)/(Healthy) | 46 | <0.001 * | |
| (CT)/(Healthy) | 139 | 0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonyan, S.; Pospelova, M.; Krasnikova, V.; Fionik, O.; Alekseeva, T.; Samochernykh, K.; Ivanova, N.; Vavilova, T.; Vasilieva, E.; Makhanova, A.; et al. Neurotrophin-3 (NT-3) as a Potential Biomarker of the Peripheral Nervous System Damage Following Breast Cancer Treatment. Pathophysiology 2023, 30, 110-122. https://doi.org/10.3390/pathophysiology30020010
Tonyan S, Pospelova M, Krasnikova V, Fionik O, Alekseeva T, Samochernykh K, Ivanova N, Vavilova T, Vasilieva E, Makhanova A, et al. Neurotrophin-3 (NT-3) as a Potential Biomarker of the Peripheral Nervous System Damage Following Breast Cancer Treatment. Pathophysiology. 2023; 30(2):110-122. https://doi.org/10.3390/pathophysiology30020010
Chicago/Turabian StyleTonyan, Samvel, Maria Pospelova, Varvara Krasnikova, Olga Fionik, Tatyana Alekseeva, Konstantin Samochernykh, Nataliya Ivanova, Tatyana Vavilova, Elena Vasilieva, Albina Makhanova, and et al. 2023. "Neurotrophin-3 (NT-3) as a Potential Biomarker of the Peripheral Nervous System Damage Following Breast Cancer Treatment" Pathophysiology 30, no. 2: 110-122. https://doi.org/10.3390/pathophysiology30020010










