Neurotrophin-3 (NT-3) as a Potential Biomarker of the Peripheral Nervous System Damage Following Breast Cancer Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Neurological Examination
2.3. Enzyme-Linked Immunosorbent Assays
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. Evaluation of BDNF, NT-3, and Gal-3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Francies, F.Z.; Hull, R.; Khanyile, R.; Dlamini, Z. Breast cancer in low-middle income countries: Abnormality in splicing and lack of targeted treatment options. Am. J. Cancer Res. 2020, 10, 1568–1591. [Google Scholar] [PubMed]
- Jonczyk, M.M.; Jean, J.; Graham, R.; Chatterjee, A. Surgical trends in breast cancer: A rise in novel operative treatment options over a 12 year analysis. Breast Cancer Res. Treat. 2019, 173, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Bauer-Nilsen, K.; McNulty, R.H.; Vicini, F. Novel radiation therapy approaches for breast cancer treatment. Semin. Oncol. 2020, 47, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Pondé, N.F.; Zardavas, D.; Piccart, M. Progress in adjuvant systemic therapy for breast cancer. Nat. Rev. Clin. Oncol. 2019, 16, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Mutebi, M.; Anderson, B.O.; Duggan, C.; Adebamowo, C.; Agarwal, G.; Ali, Z.; Bird, P.; Bourque, J.-M.; DeBoer, R.; Gebrim, L.H.; et al. Breast cancer treatment: A phased approach to implementation. Cancer 2020, 126 (Suppl. S10), 2365–2378. [Google Scholar] [CrossRef]
- Villar, R.R.; Fernández, S.P.; Garea, C.C.; Pillado, M.T.S.; Barreiro, V.B.; Martín, C.G. Quality of life and anxiety in women with breast cancer before and after treatment. Rev. Lat. Am. Enferm. 2017, 25, e2958. [Google Scholar] [CrossRef]
- Mokhtari-Hessari, P.; Montazeri, A. Health-related quality of life in breast cancer patients: Review of reviews from 2008 to 2018. Health Qual. Life Outcomes 2020, 18, 338. [Google Scholar] [CrossRef]
- Wang, L.; Guyatt, G.H.; Kennedy, S.A.; Romerosa, B.; Kwon, H.Y.; Kaushal, A.; Chang, Y.; Craigie, S.; de Almeida, C.P.B.; Couban, R.J.; et al. Predictors of persistent pain after breast cancer surgery: A systematic review and meta-analysis of observational studies. CMAJ 2016, 188, E352–E361. [Google Scholar] [CrossRef]
- Delanian, S.; Lefaix, J.-L.; Pradat, P.-F. Radiation-induced neuropathy in cancer survivors. Radiother. Oncol. 2012, 105, 273–282. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Chan, Y.-N.; Jheng, Y.-W.; Wu, C.-J.; Lin, M.-W.; Tseng, L.-M.; Tsai, Y.-F.; Liu, L.-C. Chemotherapy-induced peripheral neuropathy in newly diagnosed breast cancer survivors treated with taxane: A prospective longitudinal study. Support Care Cancer 2021, 29, 2959–2971. [Google Scholar] [CrossRef]
- Kim, M.; Jung, M.S. Effects of Chemotherapy-Induced Peripheral Neuropathy in Women With Breast Cancer: A Structural Equation Approach With the Theory of Unpleasant Symptoms. Cancer Nurs. 2021, 44, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Basal, C.; Seluzicki, C.; Li, S.Q.; Seidman, A.D.; Mao, J.J. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: Prevalence, risk factors, and fall risk. Breast Cancer Res. Treat. 2016, 159, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Chappell, A.G.; Yuksel, S.; Sasson, D.C.; Wescott, A.B.; Connor, L.M.; Ellis, M.F. Post-Mastectomy Pain Syndrome: An Up-to-Date Review of Treatment Outcomes. JPRAS Open 2021, 30, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Tait, R.C.; Zoberi, K.; Ferguson, M.; Levenhagen, K.; Luebbert, R.A.; Rowland, K.; Salsich, G.B.; Herndon, C. Persistent Post-Mastectomy Pain: Risk Factors and Current Approaches to Treatment. J. Pain 2018, 19, 1367–1383. [Google Scholar] [CrossRef] [PubMed]
- Bortsov, A.V.; Devor, M.; Kaunisto, M.A.; Kalso, E.; Brufsky, A.; Kehlet, H.; Aasvang, E.; Bittner, R.; Diatchenko, L.; Belfer, I. CACNG2 polymorphisms associate with chronic pain after mastectomy. Pain 2019, 160, 561–568. [Google Scholar] [CrossRef]
- Stephens, K.E.; Levine, J.D.; Aouizerat, B.E.; Paul, S.M.; Abrams, G.; Conley, Y.P.; Miaskowski, C. Associations between genetic and epigenetic variations in cytokine genes and mild persistent breast pain in women following breast cancer surgery. Cytokine 2017, 99, 203–213. [Google Scholar] [CrossRef]
- Langford, D.J.; Paul, S.M.; West, C.M.; Dunn, L.B.; Levine, J.D.; Kober, K.M.; Dodd, M.J.; Miaskowski, C.; Aouizerat, B.E. Variations in potassium channel genes are associated with distinct trajectories of persistent breast pain after breast cancer surgery. Pain 2015, 156, 371–380. [Google Scholar] [CrossRef]
- Khan, N.; Smith, M.T. Neurotrophins and Neuropathic Pain: Role in Pathobiology. Molecules 2015, 20, 10657. [Google Scholar] [CrossRef]
- McGregor, C.E.; English, A.W. The Role of BDNF in Peripheral Nerve Regeneration: Activity-Dependent Treatments and Val66Met. Front. Cell Neurosci. 2018, 12, 522. [Google Scholar] [CrossRef]
- Ma, Z.; Han, Q.; Wang, X.; Ai, Z.; Zheng, Y. Galectin-3 Inhibition Is Associated with Neuropathic Pain Attenuation after Peripheral Nerve Injury. PLoS ONE 2016, 11, e0148792. [Google Scholar] [CrossRef]
- Allen, S.J.; Dawbarn, D. Clinical relevance of the neurotrophins and their receptors. Clin. Sci. 2006, 110, 175–191. [Google Scholar] [CrossRef]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R Soc. Lond. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Lee, A.K.; Takahashi, H. Emerging roles of the neurotrophin receptor TrkC in synapse organization. Neurosci. Res. 2017, 116, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Yalvac, M.E.; Arnold, W.D.; Braganza, C.; Chen, L.; Mendell, J.R.; Sahenk, Z. AAV1.NT-3 gene therapy attenuates spontaneous autoimmune peripheral polyneuropathy. Gene Ther. 2016, 23, 95–102. [Google Scholar] [CrossRef]
- Tang, S.; Liao, X.; Shi, B.; Qu, Y.; Huang, Z.; Lin, Q.; Guo, X.; Pei, F. The Effects of Controlled Release of Neurotrophin-3 from PCLA Scaffolds on the Survival and Neuronal Differentiation of Transplanted Neural Stem Cells in a Rat Spinal Cord Injury Model. PLoS ONE 2014, 9, e107517. [Google Scholar] [CrossRef] [PubMed]
- Parkman, H.P.; Rao, S.S.C.; Reynolds, J.C.; Schiller, L.R.; Wald, A.; Miner, P.B.; Lembo, A.J.; Gordon, J.M.; Drossman, D.A.; Waltzman, L.; et al. Neurotrophin-3 improves functional constipation. Am. J. Gastroenterol. 2003, 98, 1338–1347. [Google Scholar] [CrossRef]
- Pradat, P.F.; Kennel, P.; Naimi-Sadaoui, S.; Finiels, F.; Orsini, C.; Revah, F.; Delaere, P.; Mallet, J. Continuous delivery of neurotrophin 3 by gene therapy has a neuroprotective effect in experimental models of diabetic and acrylamide neuropathies. Hum. Gene Ther. 2001, 12, 2237–2249. [Google Scholar] [CrossRef]
- Gao, W.Q.; Dybdal, N.; Shinsky, N.; Murnane, A.; Schmelzer, C.; Siegel, M.; Keller, G.; Hefti, F.; Phillips, H.S.; Winslow, J.W. Neurotrophin-3 reverses experimental cisplatin-induced peripheral sensory neuropathy. Ann. Neurol. 1995, 38, 30–37. [Google Scholar] [CrossRef]
- Gandhi, R.; Ryals, J.M.; Wright, D.E. Neurotrophin-3 reverses chronic mechanical hyperalgesia induced by intramuscular acid injection. J. Neurosci. 2004, 24, 9405–9413. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Gerwing, T.D.; Stucky, C.L.; McComb, G.W.; Verge, V.M.K. Neurotrophin-3 significantly reduces sodium channel expression linked to neuropathic pain states. Exp. Neurol. 2008, 213, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Sahenk, Z.; Oblinger, J.; Edwards, C. Neurotrophin-3 deficient Schwann cells impair nerve regeneration. Exp. Neurol. 2008, 212, 552–556. [Google Scholar] [CrossRef]
- Cong, Y.; Wang, C.; Wang, J.; Li, H.; Li, Q. NT-3 Promotes Oligodendrocyte Proliferation and Nerve Function Recovery after Spinal Cord Injury by Inhibiting Autophagy Pathway. J. Surg. Res. 2020, 247, 128–135. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 2014, 220, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Walwyn, W.; Ennes, H.S.; Kim, H.; McRoberts, J.A.; Marvizón, J.C.G. BDNF released during neuropathic pain potentiates NMDA receptors in primary afferent terminals. Eur. J. Neurosci. 2014, 39, 1439–1454. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Pain facilitation and activity-dependent plasticity in pain modulatory circuitry: Role of BDNF-TrkB signaling and NMDA receptors. Mol. Neurobiol. 2007, 35, 224–235. [Google Scholar] [CrossRef]
- Huang, L.; Jin, J.; Chen, K.; You, S.; Zhang, H.; Sideris, A.; Norcini, M.; Recio-Pinto, E.; Wang, J.; Gan, W.-B.; et al. BDNF produced by cerebral microglia promotes cortical plasticity and pain hypersensitivity after peripheral nerve injury. PLoS Biol. 2021, 19, e3001337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xie, Z.; Li, C.; Xing, Z.; Xie, S.; Li, M.; Yao, J. Driving effect of BDNF in the spinal dorsal horn on neuropathic pain. Neurosci. Lett. 2021, 756, 135965. [Google Scholar] [CrossRef]
- Ciobanu, C.; Reid, G.; Babes, A. Acute and chronic effects of neurotrophic factors BDNF and GDNF on responses mediated by thermo-sensitive TRP channels in cultured rat dorsal root ganglion neurons. Brain Res. 2009, 1284, 54–67. [Google Scholar] [CrossRef]
- Marcol, W.; Kotulska, K.; Larysz-Brysz, M.; Kowalik, J.L. BDNF contributes to animal model neuropathic pain after peripheral nerve transection. Neurosurg. Rev. 2007, 30, 235–243; discussion 243. [Google Scholar] [CrossRef] [PubMed]
- Karagyaur, M.; Dyikanov, D.; Makarevich, P.; Semina, E.; Stambolsky, D.; Plekhanova, O.; Kalinina, N.; Tkachuk, V. Non-viral transfer of BDNF and uPA stimulates peripheral nerve regeneration. Biomed. Pharmacother. 2015, 74, 63–70. [Google Scholar] [CrossRef]
- Lopes, C.D.F.; Gonçalves, N.P.; Gomes, C.P.; Saraiva, M.J.; Pêgo, A.P. BDNF gene delivery mediated by neuron-targeted nanoparticles is neuroprotective in peripheral nerve injury. Biomaterials 2017, 121, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Del Turco, S.; De Simone, P.; Ghinolfi, D.; Gaggini, M.; Basta, G. Comparison between galectin-3 and YKL-40 levels for the assessment of liver fibrosis in cirrhotic patients. Arab. J. Gastroenterol. 2021, 22, 187–192. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, P.; Zeng, L.; Zhao, Y.; Xie, L.; Chen, B. Mesenchymal stem cells ameliorate renal fibrosis by galectin-3/Akt/GSK3β/Snail signaling pathway in adenine-induced nephropathy rat. Stem Cell Res. Ther. 2021, 12, 409. [Google Scholar] [CrossRef]
- Argüeso, P.; Panjwani, N. Focus on Molecules: Galectin-3. Exp. Eye Res. 2011, 92, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Srejovic, I.; Selakovic, D.; Jovicic, N.; Jakovljević, V.; Lukic, M.L.; Rosic, G. Galectin-3: Roles in Neurodevelopment, Neuroinflammation, and Behavior. Biomolecules 2020, 10, 798. [Google Scholar] [CrossRef]
- Kumar, S.; Ranawat, C.S.; Bhandiwad, C.; Arya, H.; Mali, M.; Singh, C.P.; Sharma, N.; Lathwal, N.; Wasim, S. Galectin-3 as a Potential Biomarker of Microvascular Complications in Patients with Type 2 Diabetes. Indian J. Endocrinol. Metab. 2022, 26, 490–497. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Lo, J.-J.; Wu, S.-H.; Wang, C.-Z.; Chen, R.-F.; Lee, S.-S.; Chai, C.-Y.; Huang, S.-H. Early Hyperbaric Oxygen Treatment Attenuates Burn-Induced Neuroinflammation by Inhibiting the Galectin-3-Dependent Toll-Like Receptor-4 Pathway in a Rat Model. Int. J. Mol. Sci. 2018, 19, 2195. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, M.; Imai, S.; Matsumoto, M.; Iguma, Y.; Kawaguchi-Sakita, N.; Kotake, T.; Iwamitsu, Y.; Ntogwa, M.; Hiraiwa, R.; Nagayasu, K.; et al. Pronociceptive Roles of Schwann Cell-Derived Galectin-3 in Taxane-Induced Peripheral Neuropathy. Cancer Res. 2021, 81, 2207–2219. [Google Scholar] [CrossRef]
- Spielberger, C.D. Anxiety and Behavior; Academic Press: Cambridge, MA, USA, 2013; ISBN 978-1-4832-5836-2. [Google Scholar]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-1-4443-3241-4. [Google Scholar]
- Ganz, P.A.; Goodwin, P.J. Breast Cancer Survivorship: Where Are We Today? Adv. Exp. Med. Biol. 2015, 862, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mahanova, A.; Krasnikova, V.; Fionik, O.; Pospelova, M.; Alekseeva, T.; Nikolaeva, A.; Maksimov, A.; Trofimov, N.; Donkov, V.; Bukkieva, T. Clinical and neuropsychological assessment of the condition of patients with post-mastectomy syndrome. Transl. Med. 2022, 9, 50–58. [Google Scholar] [CrossRef]
- Bukreeva, T.A.; Pospelova, M.; Efimtsev, A.; Fionik, O.V.; Konstantin, A.S.; Gorbunova, E.A.; Krasnikova, V.V.; Makanova, A.M. Neurological aspects of postmastectomy syndrome and modern methods for their diagnosis. Med. News North Cauc. 2022, 17, 90–95. [Google Scholar] [CrossRef]
- Pospelova, M.; Krasnikova, V.; Fionik, O.; Alekseeva, T.; Samochernykh, K.; Ivanova, N.; Trofimov, N.; Vavilova, T.; Vasilieva, E.; Topuzova, M.; et al. Adhesion Molecules ICAM-1 and PECAM-1 as Potential Biomarkers of Central Nervous System Damage in Women Breast Cancer Survivors. Pathophysiology 2022, 29, 52–65. [Google Scholar] [CrossRef]
- Huehnchen, P.; Schinke, C.; Bangemann, N.; Dordevic, A.D.; Kern, J.; Maierhof, S.K.; Hew, L.; Nolte, L.; Körtvelyessy, P.; Göpfert, J.C.; et al. Neurofilament proteins as a potential biomarker in chemotherapy-induced polyneuropathy. JCI Insight 2022, 7, e154395. [Google Scholar] [CrossRef]
- Sharma, A.; Johnson, K.B.; Bie, B.; Rhoades, E.E.; Sen, A.; Kida, Y.; Hockings, J.; Gatta, A.; Davenport, J.; Arcangelini, C.; et al. A Multimodal Approach to Discover Biomarkers for Taxane-Induced Peripheral Neuropathy (TIPN): A Study Protocol. Technol. Cancer Res. Treat. 2022, 21, 15330338221127168. [Google Scholar] [CrossRef] [PubMed]
- De Santis, S.; Pace, A.; Bove, L.; Cognetti, F.; Properzi, F.; Fiore, M.; Triaca, V.; Savarese, A.; Simone, M.D.; Jandolo, B.; et al. Patients treated with antitumor drugs displaying neurological deficits are characterized by a low circulating level of nerve growth factor. Clin. Cancer Res. 2000, 6, 90–95. [Google Scholar] [PubMed]
- Baka, P.; Escolano-Lozano, F.; Birklein, F. Systemic inflammatory biomarkers in painful diabetic neuropathy. J. Diabetes Complicat. 2021, 35, 108017. [Google Scholar] [CrossRef]
- Jin, H.Y.; Park, T.S. Role of inflammatory biomarkers in diabetic peripheral neuropathy. J. Diabetes Investig. 2018, 9, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Rossor, A.M.; Reilly, M.M. Blood biomarkers of peripheral neuropathy. Acta Neurol. Scand. 2022, 146, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Alberti, P. A review of novel biomarkers and imaging techniques for assessing the severity of chemotherapy-induced peripheral neuropathy. Expert Opin. Drug Metab. Toxicol. 2020, 16, 1147–1158. [Google Scholar] [CrossRef]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef] [PubMed]
- Fionik, O.V.; Krasnikova, V.V.; Pokatilo, D.A.; Pospelova, M.L. Changes in the microcirculatory bed in patients with post-mastectomy syndrome. Issues Reconstr. Plast. Surg. 2022, 24, 55–62. [Google Scholar] [CrossRef]
- Omura, T.; Sano, M.; Omura, K.; Hasegawa, T.; Doi, M.; Sawada, T.; Nagano, A. Different expressions of BDNF, NT3, and NT4 in muscle and nerve after various types of peripheral nerve injuries. J. Peripher. Nerv. Syst. 2005, 10, 293–300. [Google Scholar] [CrossRef]
- Uddin, M.S.; Mamun, A.A.; Rahman, M.M.; Jeandet, P.; Alexiou, A.; Behl, T.; Sarwar, M.S.; Sobarzo-Sánchez, E.; Ashraf, G.M.; Sayed, A.A.; et al. Natural Products for Neurodegeneration: Regulating Neurotrophic Signals. Oxidative Med. Cell. Longev. 2021, 2021, 8820406. [Google Scholar] [CrossRef]
- Bukkieva, T.A.; Pospelova, M.L.; Anpilogova, K.S.; Fionik, O.V.; Alekseeva, T.M.; Gorbunova, E.A.; Krasnikova, V.V.; Makanova, A.M.; Tonyan, S.N.; Levchuk, A.G.; et al. Changes in the structural connectom of the brain in patients with postmastectomy syndrome. Transl. Med. 2022, 8, 33–42. [Google Scholar] [CrossRef]
- Siuciak, J.A.; Altar, C.A.; Wiegand, S.J.; Lindsay, R.M. Antinociceptive effect of brain-derived neurotrophic factor and neurotrophin-3. Brain Res. 1994, 633, 326–330. [Google Scholar] [CrossRef]
- Tender, G.C.; Kaye, A.D.; Li, Y.-Y.; Cui, J.-G. Neurotrophin-3 and tyrosine kinase C have modulatory effects on neuropathic pain in the rat dorsal root ganglia. Neurosurgery 2011, 68, 1048–1055; discussion 1055. [Google Scholar] [CrossRef]
- Malcangio, M.; Garrett, N.E.; Cruwys, S.; Tomlinson, D.R. Nerve Growth Factor- and Neurotrophin-3-Induced Changes in Nociceptive Threshold and the Release of Substance P from the Rat Isolated Spinal Cord. J. Neurosci. 1997, 17, 8459–8467. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Gerwing, T.D.; Dmyterko, M.V.; Zochodne, D.W.; Johnston, J.M.; Verge, V.M.K. Neurotrophin-3 suppresses thermal hyperalgesia associated with neuropathic pain and attenuates transient receptor potential vanilloid receptor-1 expression in adult sensory neurons. J. Neurosci. 2005, 25, 758–767. [Google Scholar] [CrossRef] [PubMed]
| Group Characteristic | Patients after BC Treatment (n = 67) |
|---|---|
| Age (years) | 47 (44, 49) |
| Years after the end of therapy | 3 (2, 5) |
| The structure by stages TNM, UICC | |
| I (T1N0M0) | 8 (12%) |
| II A (T2N1M0) | 46 (68%) |
| II B (T3N1M0) | 3 (5%) |
| III A (T3N2M0) | 2 (3%) |
| III B (T4N2M0) | 8 (12%) |
| The histological type of breast cancer | |
| Ductal carcinoma in situ (DCIS) | 7 (11%) |
| Invasive ductal carcinoma (IDC) | 49 (73%) |
| Invasive lobular carcinoma (ILC) | 11 (16%) |
| Type of breast cancer treatment | |
| Complex therapy of breast cancer (surgical treatment, radiation therapy, chemotherapy) | 37 (55%) |
| Surgical treatment and chemotherapy | 18 (27%) |
| Surgical treatment and radiation therapy | 7 (10%) |
| Only surgical treatment | 5 (7%) |
| Type of surgical treatment | |
| Madden-modified radical mastectomy | 53 (79%) |
| Sectoral resection | 14 (21%) |
| Hormone therapy (Tamoxifen ± GnRH analogue) | |
| Yes | 12 (18%) |
| No | 50 (75%) |
| Completed the course | 5 (7%) |
| Clinical Characteristics | Number of Patients (N, %) |
|---|---|
| Chronic pain syndrome | 46 (69%) |
| Numbness in the armpit | 45 (67%) |
| Polyneuropathy | 34 (51%) |
| Biomarker | BC Survivors (n = 67) | Healthy Volunteers (n = 25) | Mann-Whitney U Test | Significance (p) |
|---|---|---|---|---|
| NT-3 pg/mL | 16.62 [11.18; 20.0] | 5.74 [4.56; 13.7] | 254 | <0.001 * |
| BDNF pg/mL | 31,747.4 [23,068.0; 37,903.0] | 29,281.6 [21,786.4; 35,728.2] | 534.5 | 0.33 |
| Gal-3 ng/mL | 5450.0 [4080.0; 9900.0] | 4660.0 [3240.0; 6380.0] | 521.0 | 0.26 |
| Sing of Separation | Characteristic of the Sing | Number of Patients (and Age) | NT-3 pg/mL | Kruskal–Wallis Test | p | BDNF pg/mL | Kruskal–Wallis Test | p | Gal-3 ng/mL | Kruskal–Wallis Test | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
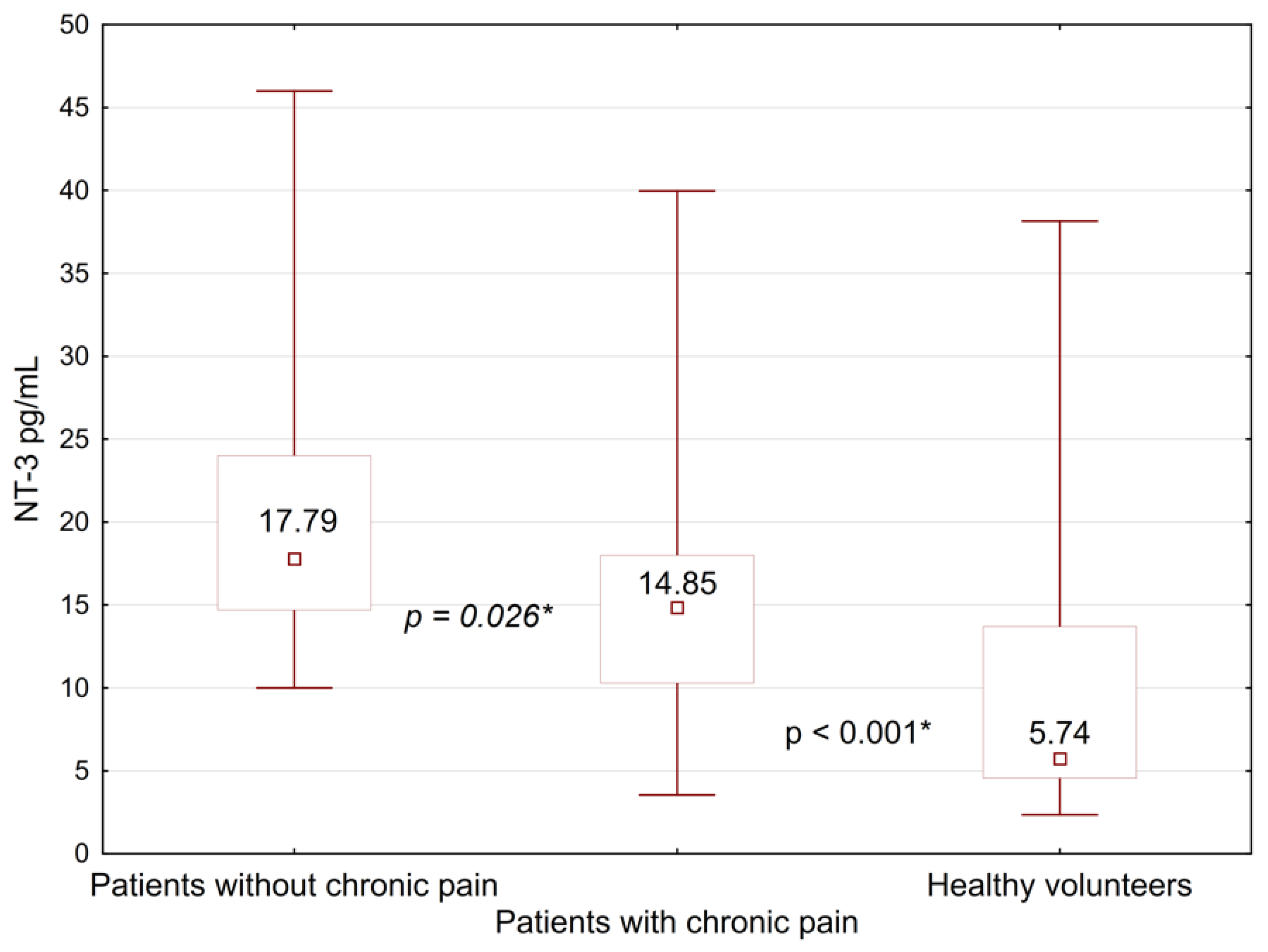
| Chronic pain syndrome | yes | 46 (47 [43; 48]) | 14.85 [10.3; 18.0] | 19.05 | <0.001 * | 32,291.2 [25,359.0; 39,417.4] | 4.45 | 0.11 | 5600.0 [4160.0; 9140.0] | 1.12 | 0.57 |
| no | 21 (47 [40; 50]) | 17.79 [14.7; 24.0] | 29,572.0 [20,621.0; 35,456.0] | 6350.0 [3340.0;13,960.0] | |||||||
| Healthy volunteers | 5.74 [4.56; 13.7] | 29,281.6 [21,786.4; 35,728.2] | 4660.0 [3240.0; 6380.0] | ||||||||
| Hypoesthesia in the armpit | yes | 45 (46 [40; 48]) | 16.0 [11.74; 20.0] | 15.22 | <0.001 * | 31,378.0 [23,689.0; 37,903.0] | 1.13 | 0.57 | 5200.0 [4060.0; 9980.0] | 1.74 | 0.42 |
| no | 22 (48 [44; 49]) | 17.03 [11.18; 20.0] | 32,232.0 [22,757.2; 38,136.0] | 6230.0 [4620.0; 9900.0] | |||||||
| Healthy volunteers | 5.74 [4.56; 13.7] | 29,281.6 [21,786.4; 35,728.2] | 4660.0 [3240.0; 6380.0] | ||||||||
| Polyneuropathy | yes | 34 (46 [42; 48]) | 15.58 [11.75; 17.36] | 16.4 | <0.001 * | 31,164.7 [22,601.5; 39,223.2] | 1.11 | 0.57 | 5090.0 [4070.0; 8760.0] | 1.53 | 0.47 |
| no | 33 (48 [43; 49]) | 17.06 [11.18; 21.46] | 32,077.0 [24,155.4; 36,815.0] | 6340.0 [4620.0; 9900.0] | |||||||
| Healthy volunteers | 5.74 [4.56; 13.7] | 29,281.6 [21,786.4; 35,728.2] | 4660.0 [3240.0; 6380.0] | ||||||||
| Treatment history | Only surgical treatment | 5 (44 [40; 48]) | 17.36 [14.18; 19.12] | 17.03 | 0.0019 * | 30,776.5 [18,225.0; 45,080.8] | 1.87 | 0.75 | 4130.0 [3580.0; 6290.0] | 2.99 | 0.55 |
| Surgical treatment and radiotherapy | 7 (47 [46; 47]) | 16.0 [15.0; 18.82] | 30,951.4 [28,038.0; 32,504.8] | 6340.0 [5140.0; 7080.0] | |||||||
| Surgical treatment and Chemotherapy | 18 (46 [42; 48]) | 17.35 [14.26; 22.35] | 31,203.9 [21,320.1; 39,417.5] | 6110.0 [4070.0; 8360.0] | |||||||
| Complex treatment | 37 (46 [43; 49]) | 13.53 [10.0; 18.96] | 32,386.8 [25,378.5; 37,320.0] | 5350.0 [4220.0; 10,660.0] | |||||||
| Healthy volunteers | 5.74 [4.56; 13.7] | 29,281.6 [21,786.4; 35,728.2] | 4660.0 [3240.0; 6380.0] | ||||||||
| Type of surgery | Modified unilateral mastectomy Madden | 53 (47 [43; 48]) | 16.88 [11.75; 20.0] | 15.84 | <0.001 * | 32,174.7 [22,135.6; 38,407.5] | 1.08 | 0.58 | 5420.0 [4120.0; 10,240.0] | 1.30 | 0.52 |
| Sector mastectomy | 14 (46 [41; 48]) | 15.0 [9.42; 19.4] | 31,456.0 [25,359.0; 37,242.0] | 6160.0 [4060.0; 8140.0] | |||||||
| Healthy volunteers | 5.74 [4.56; 13.7] | 29,281.6 [21,786.4; 35,728.2] | 4660.0 [3240.0; 6380.0] | ||||||||
| Sing of Separation | Compared Groups | Mann-Whitney U Test | p |
|---|---|---|---|
| Chronic pain syndrome | Yes/No | 264 | 0.026 * |
| Healthy volunteers (Healthy)/No | 44 | <0.001 * | |
| (Healthy)/Yes | 208 | <0.001 * | |
| Hypoesthesia in the armpit | Yes/No | 463 | 0.90 |
| (Healthy)/No | 79.5 | <0.001 * | |
| (Healthy)/Yes | 173.5 | <0.001 * | |
| Polyneuropathy | Yes/No | 436 | 0.22 |
| (Healthy)/No | 112.5 | <0.001 * | |
| (Healthy)/Yes | 140.5 | 0.001 * | |
| Type of surgery | Modified unilateral mastectomy Madden (M)/Sector mastectomy (SM) | 294 | 0.48 |
| (Healthy)/(M) | 183 | <0.001 * | |
| (Healthy)/(SM) | 70 | 0.04 * | |
| Breast cancer treatment | Only surgical treatment (OS)/Surgical treatment and radiotherapy (S + R) | 9 | 0.90 |
| Surgical treatment and Chemotherapy (S + Ch)/Complex treatment (CT) | 179 | 0.09 | |
| (OS)/(S + Ch) | 29 | 0.81 | |
| (OS)/(CT) | 50 | 0.50 | |
| (S + R)/(S + Ch) | 34 | 0.65 | |
| (S + R)/(CT) | 71 | 0.71 | |
| (OS)/(Healthy) | 11 | 0.03 * | |
| (S + R)/(Healthy) | 26 | 0.014 * | |
| (S + Ch)/(Healthy) | 46 | <0.001 * | |
| (CT)/(Healthy) | 139 | 0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonyan, S.; Pospelova, M.; Krasnikova, V.; Fionik, O.; Alekseeva, T.; Samochernykh, K.; Ivanova, N.; Vavilova, T.; Vasilieva, E.; Makhanova, A.; et al. Neurotrophin-3 (NT-3) as a Potential Biomarker of the Peripheral Nervous System Damage Following Breast Cancer Treatment. Pathophysiology 2023, 30, 110-122. https://doi.org/10.3390/pathophysiology30020010
Tonyan S, Pospelova M, Krasnikova V, Fionik O, Alekseeva T, Samochernykh K, Ivanova N, Vavilova T, Vasilieva E, Makhanova A, et al. Neurotrophin-3 (NT-3) as a Potential Biomarker of the Peripheral Nervous System Damage Following Breast Cancer Treatment. Pathophysiology. 2023; 30(2):110-122. https://doi.org/10.3390/pathophysiology30020010
Chicago/Turabian StyleTonyan, Samvel, Maria Pospelova, Varvara Krasnikova, Olga Fionik, Tatyana Alekseeva, Konstantin Samochernykh, Nataliya Ivanova, Tatyana Vavilova, Elena Vasilieva, Albina Makhanova, and et al. 2023. "Neurotrophin-3 (NT-3) as a Potential Biomarker of the Peripheral Nervous System Damage Following Breast Cancer Treatment" Pathophysiology 30, no. 2: 110-122. https://doi.org/10.3390/pathophysiology30020010
APA StyleTonyan, S., Pospelova, M., Krasnikova, V., Fionik, O., Alekseeva, T., Samochernykh, K., Ivanova, N., Vavilova, T., Vasilieva, E., Makhanova, A., Nikolaeva, A., Bukkieva, T., Combs, S., & Shevtsov, M. (2023). Neurotrophin-3 (NT-3) as a Potential Biomarker of the Peripheral Nervous System Damage Following Breast Cancer Treatment. Pathophysiology, 30(2), 110-122. https://doi.org/10.3390/pathophysiology30020010











