Autoimmune Autonomic Dysfunction Syndromes: Potential Involvement and Pathophysiology Related to Complex Regional Pain Syndrome, Fibromyalgia, Chronic Fatigue Syndrome, Silicone Breast Implant–Related Symptoms and Post-COVID Syndrome
Abstract
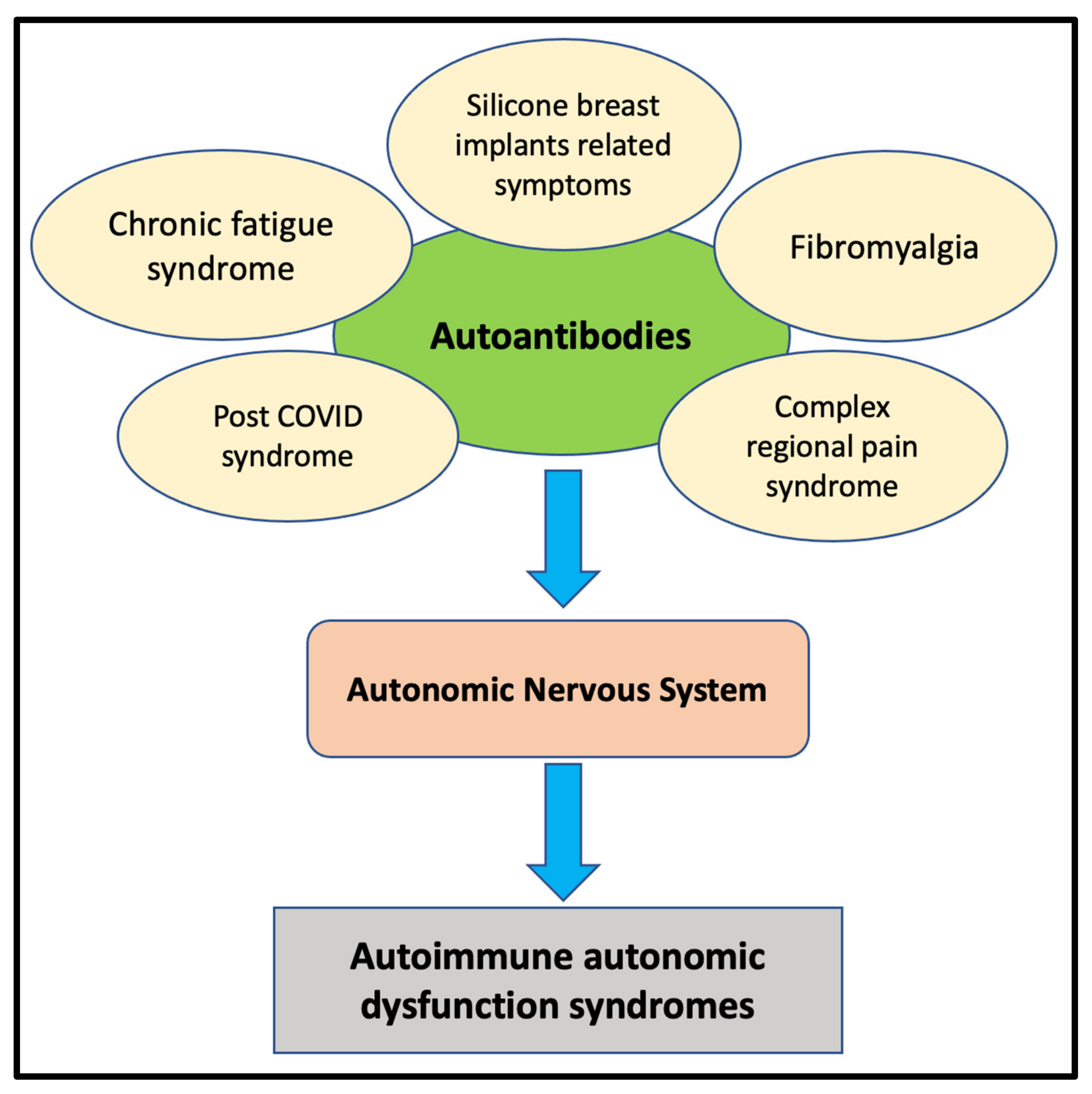
:1. Introduction
2. Complex Regional Pain Syndrome (CRPS)
3. Fibromyalgia
4. Chronic Fatigue Syndrome
5. Silicone Breast Implant (SBI)–Related Symptoms
6. Post-COVID Syndrome
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garland, E.L. Pain processing in the human nervous system: A selective review of nociceptive and biobehavioral pathways. Prim. Care 2012, 39, 561–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merskey, H.E. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986, 3, S1–S226. [Google Scholar]
- Aydede, M. Does the IASP definition of pain need updating? Pain Rep. 2019, 4, e777. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.A.; George, S.Z. A New Definition of Pain: Update and Implications for Physical Therapist Practice and Rehabilitation Science. Phys. Ther. 2021, 101, pzab019. [Google Scholar] [CrossRef]
- Williams, A.C.C.; Craig, K.D. Updating the definition of pain. Pain 2016, 157, 2420–2423. [Google Scholar] [CrossRef]
- Alcock, M.M. Defining pain: Past, present, and future. Pain 2017, 158, 761–762. [Google Scholar] [CrossRef]
- Goebel, A. Autoantibody pain. Autoimmun Rev. 2016, 15, 552–557. [Google Scholar] [CrossRef]
- Cuhadar, U.; Gentry, C.; Vastani, N.; Sensi, S.; Bevan, S.; Goebel, A.; Andersson, D.A. Autoantibodies produce pain in complex regional pain syndrome by sensitizing nociceptors. Pain 2019, 160, 2855–2865. [Google Scholar] [CrossRef]
- Cohen Tervaert, J.W.; Mohazab, N.; Redmond, D.; van Eeden, C.; Osman, M. Breast implant illness: Scientific evidence of its existence. Expert Rev. Clin. Immunol. 2021, 18, 15–29. [Google Scholar] [CrossRef]
- Wong, T.L.; Weitzer, D.J. Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)-A Systemic Review and Comparison of Clinical Presentation and Symptomatology. Medicina 2021, 57, 418. [Google Scholar] [CrossRef]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef]
- Wallukat, G.; Hohberger, B.; Wenzel, K.; Furst, J.; Schulze-Rothe, S.; Wallukat, A.; Hönicke, A.-S.; Müller, J. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J. Transl. Autoimmun. 2021, 4, 100100. [Google Scholar] [CrossRef]
- Bruehl, S. Complex regional pain syndrome. BMJ 2015, 351, h2730. [Google Scholar] [CrossRef] [Green Version]
- Ott, S.; Maihofner, C. Signs and Symptoms in 1043 Patients with Complex Regional Pain Syndrome. J. Pain 2018, 19, 599–611. [Google Scholar] [CrossRef]
- Harden, R.N.; Bruehl, S.; Stanton-Hicks, M.; Wilson, P.R. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007, 8, 326–331. [Google Scholar] [CrossRef]
- Birklein, F.; Schlereth, T. Complex regional pain syndrome-significant progress in understanding. Pain 2015, 156 (Suppl. S1), S94–S103. [Google Scholar] [CrossRef]
- Parkitny, L.; McAuley, J.H.; Di Pietro, F.; Stanton, T.R.; O’Connell, N.E.; Marinus, J.; van Hilten, J.J.; Moseley, G.L. Inflammation in complex regional pain syndrome: A systematic review and meta-analysis. Neurology 2013, 80, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Alexander, G.M.; van Rijn, M.A.; van Hilten, J.J.; Perreault, M.J.; Schwartzman, R.J. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain 2005, 116, 213–219. [Google Scholar] [CrossRef]
- Birklein, F.; Riedl, B.; Claus, D.; Neundorfer, B. Pattern of autonomic dysfunction in time course of complex regional pain syndrome. Clin Auton Res. 1998, 8, 79–85. [Google Scholar] [CrossRef]
- Knudsen, L.F.; Terkelsen, A.J.; Drummond, P.D.; Birklein, F. Complex regional pain syndrome: A focus on the autonomic nervous system. Clin. Auton. Res. 2019, 29, 457–467. [Google Scholar] [CrossRef]
- Blaes, F.; Schmitz, K.; Tschernatsch, M.; Kaps, M.; Krasenbrink, I.; Hempelmann, G.; Brau, M.E. Autoimmune etiology of complex regional pain syndrome (M. Sudeck). Neurology 2004, 63, 1734–1736. [Google Scholar] [CrossRef]
- Kohr, D.; Singh, P.; Tschernatsch, M.; Kaps, M.; Pouokam, E.; Diener, M.; Kummer, W.; Birklein, F.; Vincent, A.; Goebel, A.; et al. Autoimmunity against the beta2 adrenergic receptor and muscarinic-2 receptor in complex regional pain syndrome. Pain 2011, 152, 2690–2700. [Google Scholar] [CrossRef]
- Baerlecken, N.T.; Gaulke, R.; Pursche, N.; Witte, T.; Karst, M.; Bernateck, M. Autoantibodies against P29ING4 are associated with complex regional pain syndrome. Immunol. Res. 2019, 67, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Goebel, A.; Blaes, F. Complex regional pain syndrome, prototype of a novel kind of autoimmune disease. Autoimmun Rev. 2013, 12, 682–686. [Google Scholar] [CrossRef]
- Helyes, Z.; Tekus, V.; Szentes, N.; Pohoczky, K.; Botz, B.; Kiss, T.; Kemény, Á.; Környei, Z.; Tóth, K.; Lénárt, N.; et al. Transfer of complex regional pain syndrome to mice via human autoantibodies is mediated by interleukin-1-induced mechanisms. Proc. Natl. Acad. Sci. USA 2019, 116, 13067–13076. [Google Scholar] [CrossRef] [Green Version]
- Sahbaie, P.; Li, W.W.; Guo, T.Z.; Shi, X.Y.; Kingery, W.S.; Clark, J.D. Autonomic Regulation of Nociceptive and Immunologic Changes in a Mouse Model of Complex Regional Pain Syndrome. J. Pain 2021, 23, 472–486. [Google Scholar] [CrossRef]
- Clauw, D.J. Fibromyalgia: A clinical review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef]
- Wormser, G.P.; Weitzner, E.; McKenna, D.; Nadelman, R.B.; Scavarda, C.; Farber, S.; Prakash, P.; Ash, J.; Nowakowski, J. Long-Term Assessment of Fibromyalgia in Patients with Culture-Confirmed Lyme Disease. Arthritis. Rheumatol. 2015, 67, 837–839. [Google Scholar] [CrossRef]
- Krumina, A.; Chapenko, S.; Kenina, V.; Mihailova, M.; Logina, I.; Rasa, S.; Gintere, S.; Viksna, L.; Svirskis, S.; Murovska, M. The role of HHV-6 and HHV-7 infections in the development of fibromyalgia. J. Neurovirol. 2019, 25, 194–207. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, M.; Asprusten, T.T.; Godang, K.; Leegaard, T.M.; Osnes, L.T.; Skovlund, E.; Tjade, T.; Øie, M.G.; Wyller, V.B.B. Predictors of chronic fatigue in adolescents six months after acute Epstein-Barr virus infection: A prospective cohort study. Brain Behav. Immun. 2019, 75, 94–100. [Google Scholar] [CrossRef]
- Clauw, D.J.; Arnold, L.M.; McCarberg, B.H.; FibroCollaborative. The science of fibromyalgia. Mayo Clin. Proc. 2011, 86, 907–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cagnie, B.; Coppieters, I.; Denecker, S.; Six, J.; Danneels, L.; Meeus, M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin. Arthritis. Rheum. 2014, 44, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Buskila, D.; Sarzi-Puttini, P. Fibromyalgia and autoimmune diseases: The pain behind autoimmunity. Isr. Med. Assoc. J. 2008, 10, 77–78. [Google Scholar] [PubMed]
- Goebel, A.; Krock, E.; Gentry, C.; Israel, M.R.; Jurczak, A.; Urbina, C.M.; Sandor, K.; Vastani, N.; Maurer, M.; Cuhadar, U.; et al. Passive transfer of fibromyalgia symptoms from patients to mice. J. Clin. Invest. 2021, 13, 131. [Google Scholar] [CrossRef]
- Martinez-Lavin, M. Is fibromyalgia an autoimmune illness? Clin. Rheumatol. 2021, 40, 3865–3866. [Google Scholar] [CrossRef]
- Sharpe, M.; Wilks, D. Fatigue. BMJ 2002, 325, 480–483. [Google Scholar] [CrossRef]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Brurberg, K.G.; Fonhus, M.S.; Larun, L.; Flottorp, S.; Malterud, K. Case definitions for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): A systematic review. BMJ Open 2014, 4, e003973. [Google Scholar] [CrossRef]
- Cortes Rivera, M.; Mastronardi, C.; Silva-Aldana, C.T.; Arcos-Burgos, M.; Lidbury, B.A. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Comprehensive Review. Diagnostics 2019, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Sharif, K.; Watad, A.; Bragazzi, N.L.; Lichtbroun, M.; Martini, M.; Perricone, C.; Amital, H.; Shoenfeld, Y. On chronic fatigue syndrome and nosological categories. Clin. Rheumatol. 2018, 37, 1161–1170. [Google Scholar] [CrossRef]
- Meeus, M.; Nijs, J.; Meirleir, K.D. Chronic musculoskeletal pain in patients with the chronic fatigue syndrome: A systematic review. Eur. J. Pain 2007, 11, 377–386. [Google Scholar] [CrossRef]
- Afari, N.; Buchwald, D. Chronic fatigue syndrome: A review. Am. J. Psychiatry 2003, 160, 221–236. [Google Scholar] [CrossRef]
- Rasa, S.; Nora-Krukle, Z.; Henning, N.; Eliassen, E.; Shikova, E.; Harrer, T.; Scheibenbogen, C.; Murovska, M.; Prusty, B.K. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2018, 16, 268. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 1 April 2022).
- Kamal, M.; Abo Omirah, M.; Hussein, A.; Saeed, H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 2020, 75, e13746. [Google Scholar] [CrossRef]
- Xiong, Q.; Xu, M.; Li, J.; Liu, Y.; Zhang, J.; Xu, Y.; Dong, W. Clinical sequelae of COVID-19 survivors in Wuhan, China: A single-centre longitudinal study. Clin. Microbiol. Infect. 2021, 27, 89–95. [Google Scholar] [CrossRef]
- Freeman, R.; Komaroff, A.L. Does the chronic fatigue syndrome involve the autonomic nervous system? Am. J. Med. 1997, 102, 357–364. [Google Scholar] [CrossRef]
- Newton, J.L.; Okonkwo, O.; Sutcliffe, K.; Seth, A.; Shin, J.; Jones, D.E. Symptoms of autonomic dysfunction in chronic fatigue syndrome. QJM 2007, 100, 519–526. [Google Scholar] [CrossRef] [Green Version]
- Matsui, T.; Hara, K.; Iwata, M.; Hojo, S.; Shitara, N.; Endo, Y.; Fukuoka, H.; Matsui, M.; Kawaguchi, H. Possible involvement of the autonomic nervous system in cervical muscles of patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). BMC Musculoskelet. Disord. 2021, 22, 419. [Google Scholar] [CrossRef]
- Sotzny, F.; Blanco, J.; Capelli, E.; Castro-Marrero, J.; Steiner, S.; Murovska, M.; Scheibenbogen, C. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome—Evidence for an autoimmune disease. Autoimmun. Rev. 2018, 17, 601–609. [Google Scholar] [CrossRef]
- Press, R.I.; Peebles, C.L.; Kumagai, Y.; Ochs, R.L.; Tan, E.M. Antinuclear autoantibodies in women with silicone breast implants. Lancet 1992, 340, 1304–1307. [Google Scholar] [CrossRef]
- Bar-Meir, E.; Teuber, S.S.; Lin, H.C.; Alosacie, I.; Goddard, G.; Terybery, J.; Barka, N.; Shen, B.; Peter, J.; Blank, M.; et al. Multiple autoantibodies in patients with silicone breast implants. J. Autoimmun. 1995, 8, 267–277. [Google Scholar] [CrossRef]
- Zandman-Goddard, G.; Blank, M.; Ehrenfeld, M.; Gilburd, B.; Peter, J.; Shoenfeld, Y. A comparison of autoantibody production in asymptomatic and symptomatic women with silicone breast implants. J. Rheumatol. 1999, 26, 73–77. [Google Scholar]
- Halpert, G.; Watad, A.; Tsur, A.M.; Dotan, A.; Quiros-Lim, H.E.; Heidecke, H.; Gilburd, B.; Haik, J.; Levy, Y.; Blank, M.; et al. Autoimmune dysautonomia in women with silicone breast implants. J. Autoimmun. 2021, 120, 102631. [Google Scholar] [CrossRef]
- Anaya, J.M.; Rojas, M.; Salinas, M.L.; Rodriguez, Y.; Roa, G.; Lozano, M.; Rodríguez-Jiménez, M.; Montoya, N.; Zapata, E.; Monsalve, D.M.; et al. Post-COVID syndrome. A case series and comprehensive review. Autoimmun. Rev. 2021, 20, 102947. [Google Scholar] [CrossRef]
- Salamanna, F.; Veronesi, F.; Martini, L.; Landini, M.P.; Fini, M. Post-COVID-19 Syndrome: The Persistent Symptoms at the Post-viral Stage of the Disease. A Systematic Review of the Current Data. Front. Med. 2021, 8, 653516. [Google Scholar] [CrossRef]
- Oronsky, B.; Larson, C.; Hammond, T.C.; Oronsky, A.; Kesari, S.; Lybeck, M.; Reid, T.R. A Review of Persistent Post-COVID Syndrome (PPCS). Clin. Rev. Allergy Immunol. 2021, 1–9. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Chu, H.; Han, S.; Shuai, H.; Deng, J.; Hu, Y.F.; Gong, H.-R.; Lee, A.C.-Y.; Zou, Z.; Yau, T.; et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020, 30, 928–931. [Google Scholar] [CrossRef]
- Kempuraj, D.; Selvakumar, G.P.; Ahmed, M.E.; Raikwar, S.P.; Thangavel, R.; Khan, A.; Zaheer, S.A.; Iyer, S.S.; Burton, C.; James, D.; et al. COVID-19, Mast Cells, Cytokine Storm, Psychological Stress, and Neuroinflammation. Neuroscientist 2020, 26, 402–414. [Google Scholar] [CrossRef]
- Mahroum, N.; Alghory, A.; Kiyak, Z.; Alwani, A.; Seida, R.; Alrais, M.; Shoenfeld, Y. Ferritin—From iron, through inflammation and autoimmunity, to COVID-19. J. Autoimmun. 2021, 126, 102778. [Google Scholar] [CrossRef]
- Colafrancesco, S.; Alessandri, C.; Conti, F.; Priori, R. COVID-19 gone bad: A new character in the spectrum of the hyperferritinemic syndrome? Autoimmun. Rev. 2020, 19, 102573. [Google Scholar] [CrossRef]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H.; et al. Covid-19 and autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar] [CrossRef] [PubMed]
- Lyons-Weiler, J. Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. J. Transl. Autoimmun. 2020, 3, 100051. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.; Dotan, A.; Fritzler, M.J.; Bogdanos, D.P.; Meroni, P.L.; Roggenbuck, D.; Goldman, M.; Landegren, N.; Bastard, P.; Shoenfield, Y.; et al. Autoantibodies and SARS-CoV2 infection: The spectrum from association to clinical implication: Report of the 15th Dresden Symposium on Autoantibodies. Autoimmun. Rev. 2021, 21, 103012. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef] [PubMed]
| Syndrome | Authors | Name of study | Conclusion |
|---|---|---|---|
| CRPS | Birkelin et al. [19] | Pattern of autonomic dysfunction in time course of complex regional pain syndrome. | Alterations of ANS differ in acute and chronic stages of CRPS |
| Knudsen et al. [20] | Complex regional pain syndrome: a focus on the autonomic nervous system. | Alterations in the sympathetic nervous system contribute to CRPS pathology. | |
| Blaes at al [21] | Autoimmune etiology of complex regional pain syndrome. | Autoantibodies against receptors in the autonomic nervous system were found in 40% of patients with CRPS, suggesting an autoimmune nature of the disease | |
| Kohr et al. [22] | Autoimmunity against the beta2 adrenergic receptor and muscarinic-2 receptor in complex regional pain syndrome. | functional autoantibodies of IgG directed against beta-2 and muscarinic-2 receptors were detected in the serum of patients with CRPS. The autoantibodies isolated showed pain-like properties | |
| Goebel et al. [24] | Complex regional pain syndrome, prototype of a novel kind of autoimmune disease. | The term IRAM” indicating injury-triggered, regionally restricted, autoantibody-mediated autoimmune disorder with minimally destructive course, was proposed. | |
| Helyes et al. [25] | Transfer of complex regional pain syndrome to mice via human autoantibodies is mediated by interleukin-1-induced mechanisms. | The skin of mice treated with IgG isolated from patients with longstanding CRPS showed prolonged swelling and hyperalgesia in comparison to the control group. In addition, mice treated with CRPS IgG manifested cellular changes as microglia and astrocyte activation at the dorsal horn as well as pain regions in the brain. | |
| Sahbaie et al. [26] | Autonomic Regulation of Nociceptive and Immunologic Changes in a Mouse Model of Complex Regional Pain Syndrome. | The ANS regulates adaptive immune system activation and nociceptive sensitization in a mouse model of chronic post-traumatic pain with features of CRPS. | |
| Fibromyalgia | Clauw et al. [31] | The science of fibromyalgia | Abnormalities in pain processing and neurochemical imbalances in the CNS were found to result in central amplification of pain in patients with fibromyalgia. |
| Cagnie et al. [32] | Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI | Structural and functional brain magnetic resonance imaging (MRI) in patients with fibromyalgia demonstrated several abnormalities in grey matter volume, pain-modulating system, and pain matrix. | |
| Buskila et al. [33] | Fibromyalgia and autoimmune diseases: the pain behind autoimmunity | An association and possible correlation between fibromyalgia and autoimmunity exist. Patients with fibromyalgia were found to have a variety of autoantibodies including antinuclear antibody (ANA) as well as antithyroid antibodies. In addition, high incidence of fibromyalgia in patients with autoimmune and rheumatic diseases was illustrated. | |
| Goebel et al. [34] | Passive transfer of fibromyalgia symptoms from patients to mice | Mice injected with IgG isolated from patients with fibromyalgia showed amplified response to mechanical and cold stimulation compared to IgG from healthy people and IgG-depleted fibromyalgia patients. According to the study, the IgG isolated was found to hyperstimulate nociceptive afferents in the dorsal root ganglia which are responsible for the hypersensitivity to pain stimuli seen in patients with fibromyalgia. | |
| CFS | Freeman et al. [47] | Does the chronic fatigue syndrome involve the autonomic nervous system? | Significant alterations in the sympathetic and parasympathetic nervous system functions in 23 patients with CFS compared to healthy controls was registered. During tilt tests conducted in the patients, alterations were recorded in expiratory to inspiratory ratio, systolic and diastolic blood pressure, and heart rate. |
| Newton et al. [48] | Symptoms of autonomic dysfunction in chronic fatigue syndrome | Autonomic dysfunction was strongly associated with fatigue in 40 patients with CFS compared to healthy age- and sex-matched controls. | |
| Matsui et al. [49] | Possible involvement of the autonomic nervous system in cervical muscles of patients with myalgic encephalomyelitis / chronic fatigue syndrome (ME/CFS) | The recovery of 1226 patients with CFS suffering from cervical muscle-related complaints was found to be linked to the amelioration of the ANS function. | |
| Sotzny et al. [50] | Myalgic Encephalomyelitis/Chronic Fatigue Syndrome - Evidence for an autoimmune disease | Autoimmune etiology of CFS, including autoantibodies against neurotransmitters and their role in severe metabolic disturbances seen in patients with CFS. | |
| SBIs | Press et al. [51] | Antinuclear autoantibodies in women with silicone breast implants | High titers of antinuclear antibodies (ANA) were found in 10 out of 11 symptomatic women with silicone breast implants. |
| Bar-Meir et al. [52] | Multiple autoantibodies in patients with silicone breast implants | A thorough analysis of 20 autoantibodies in 116 women with silicone breast implants compared to 134 controls. A significantly increased incidence of autoantibodies against 15 of the 20 autoantigens studied was detected in the breast implants group. Around 20% of the breast implants group had 4 autoantibodies, and 8% had 6 autoantibodies. | |
| Zandman-Goddard et al. [53] | A comparison of autoantibody production in asymptomatic and symptomatic women with silicone breast implants | An increased incidence of autoantibody production in women with silicone breast implants particularly of anti-SSB/La and anticollagen II types, both symptomatic and asymptomatic women. Polyclonality of the autoantibodies was more prominent in the symptomatic group. | |
| Halpert et al. [54] | Autoimmune dysautonomia in women with silicone breast implants | Chronic immune stimulation by silicone material may lead to an autoimmune dysautonomia in a subgroup of potentially genetically susceptible women with SBIs. The appearance of autoantibodies against GPCRs of the autonomic nervous system serve as an explanation for the subjective autonomic-related manifestations reported in women with SBIs. | |
| Post-COVID | Zhang et al. [58] | SARS-CoV-2 infects human neural progenitor cells and brain organoids | Brain tissues were found to be highly permeable to SARS-CoV-2 shedding lights on the neurological presentation of COVID-19 and proposing a possible explanation for the neurological manifestations of the post-COVID syndrome. |
| Kempuraj et al. [59] | COVID-19, Mast Cells, Cytokine Storm, Psychological Stress, and Neuroinflammation | COVID-19 can activate mast cells, neurons, glial cells, and endothelial cells. SARS-CoV-2 infection can cause psychological stress and neuroinflammation. In conclusion, COVID-19 can induce mast cell activation, psychological stress, cytokine storm, and neuroinflammation. | |
| Ehrenfeld et al. [62] | COVID-19 and autoimmunity | A strong relation between COVID-19 and autoimmunity in terms of pathophysiology, presentation, complications, and treatment. | |
| Lyons-Weiler J [63] | Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity | ||
| Damoiseaux et al. [64] | Autoantibodies and SARS-CoV2 infection: The spectrum from association to clinical implication | The relation between infections and autoimmune diseases has been suggest with molecular mimicry, hyperstimulation and dysregulation of the immune system as plausible mechanisms. The recent pandemic with a new virus, i.e., SARS-CoV-2, has resulted in numerous studies addressing the potential of this virus to induce autoimmunity and, eventually, autoimmune disease. In addition, it has also revealed that pre-existing auto-immunity (auto-Abs neutralizing type I IFNs) could cause life-threatening disease. | |
| Wang et al. [65] | Diverse functional autoantibodies in patients with COVID-19 | Our analysis of autoantibodies against tissue-associated antigens revealed associations with specific clinical characteristics. Our findings suggest a pathological role for exoproteome-directed autoantibodies in COVID-19, with diverse effects on immune functionality and associations with clinical outcomes. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahroum, N.; Shoenfeld, Y. Autoimmune Autonomic Dysfunction Syndromes: Potential Involvement and Pathophysiology Related to Complex Regional Pain Syndrome, Fibromyalgia, Chronic Fatigue Syndrome, Silicone Breast Implant–Related Symptoms and Post-COVID Syndrome. Pathophysiology 2022, 29, 414-425. https://doi.org/10.3390/pathophysiology29030033
Mahroum N, Shoenfeld Y. Autoimmune Autonomic Dysfunction Syndromes: Potential Involvement and Pathophysiology Related to Complex Regional Pain Syndrome, Fibromyalgia, Chronic Fatigue Syndrome, Silicone Breast Implant–Related Symptoms and Post-COVID Syndrome. Pathophysiology. 2022; 29(3):414-425. https://doi.org/10.3390/pathophysiology29030033
Chicago/Turabian StyleMahroum, Naim, and Yehuda Shoenfeld. 2022. "Autoimmune Autonomic Dysfunction Syndromes: Potential Involvement and Pathophysiology Related to Complex Regional Pain Syndrome, Fibromyalgia, Chronic Fatigue Syndrome, Silicone Breast Implant–Related Symptoms and Post-COVID Syndrome" Pathophysiology 29, no. 3: 414-425. https://doi.org/10.3390/pathophysiology29030033
APA StyleMahroum, N., & Shoenfeld, Y. (2022). Autoimmune Autonomic Dysfunction Syndromes: Potential Involvement and Pathophysiology Related to Complex Regional Pain Syndrome, Fibromyalgia, Chronic Fatigue Syndrome, Silicone Breast Implant–Related Symptoms and Post-COVID Syndrome. Pathophysiology, 29(3), 414-425. https://doi.org/10.3390/pathophysiology29030033







