High Sucrose and Cholic Acid Diet Triggers PCOS-like Phenotype and Reduces Enterobacteriaceae Colonies in Female Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
- Animals
- b.
- Study Design
- c.
- Bacterial Culture
- d.
- HOMA-IR and Testosterone Level Measurement
- e.
- Hematoxylin and Eosin Staining
- f.
- Statistical Analysis
3. Results
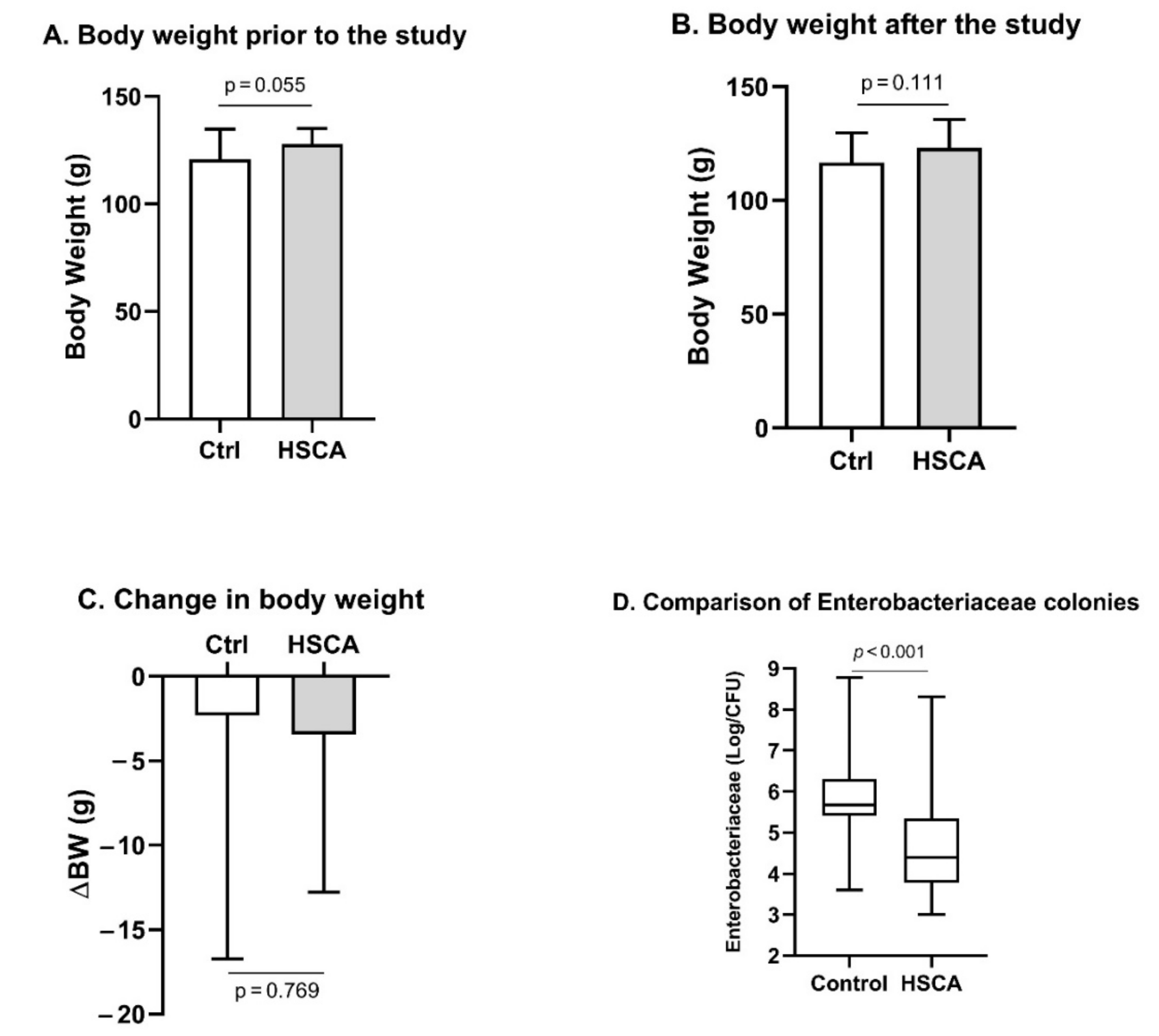
- HSCA did not lead to an overweight but reduced composition of the Enterobacteriaceae family in the gastrointestinal tract of female rats
- b.
- HSCA induced insulin resistance and hyperandrogenism in female Wistar rats
- c.
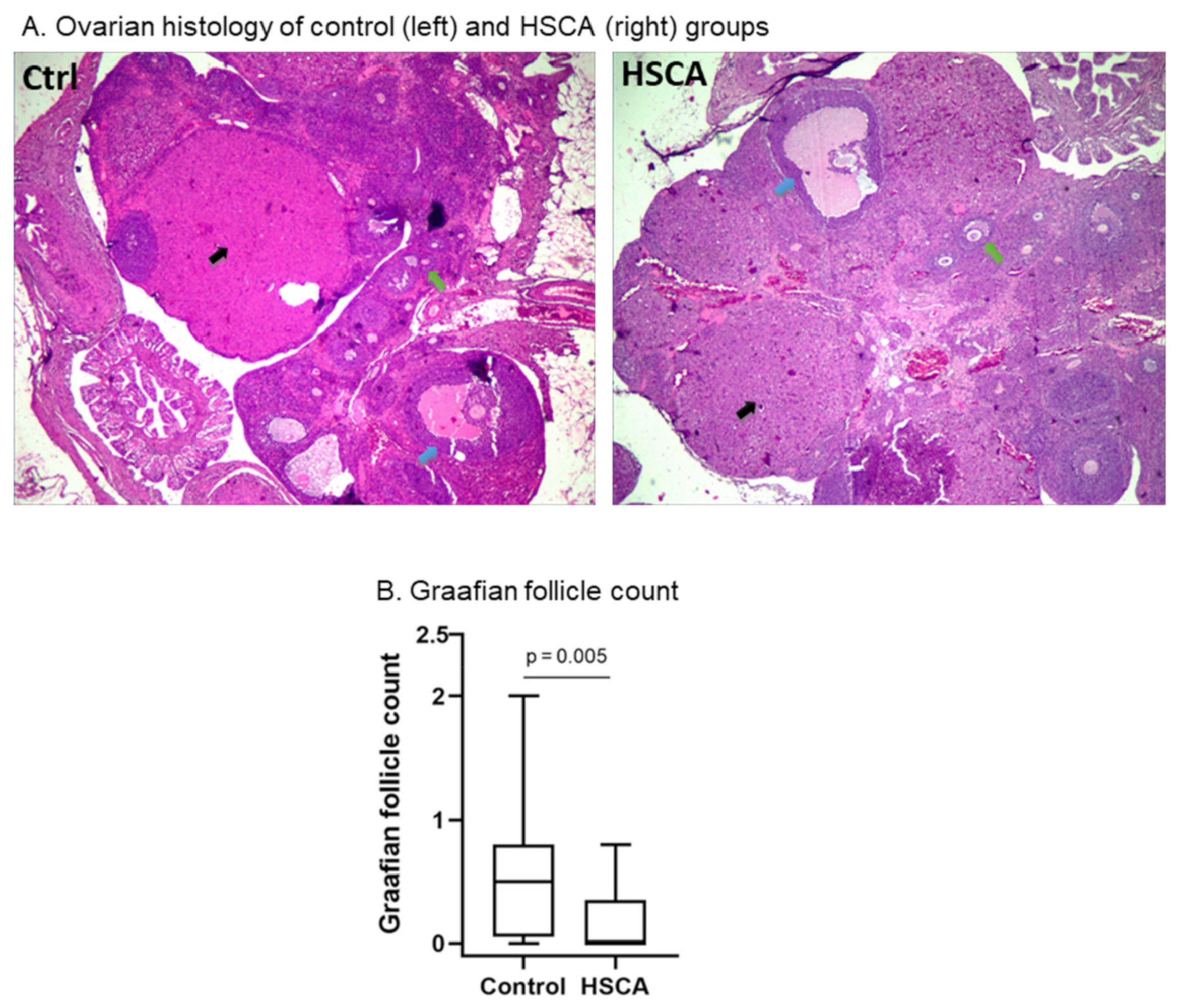
- HSCA exposure had no effect on ovulation, but induced impaired follicular maturation
- d.
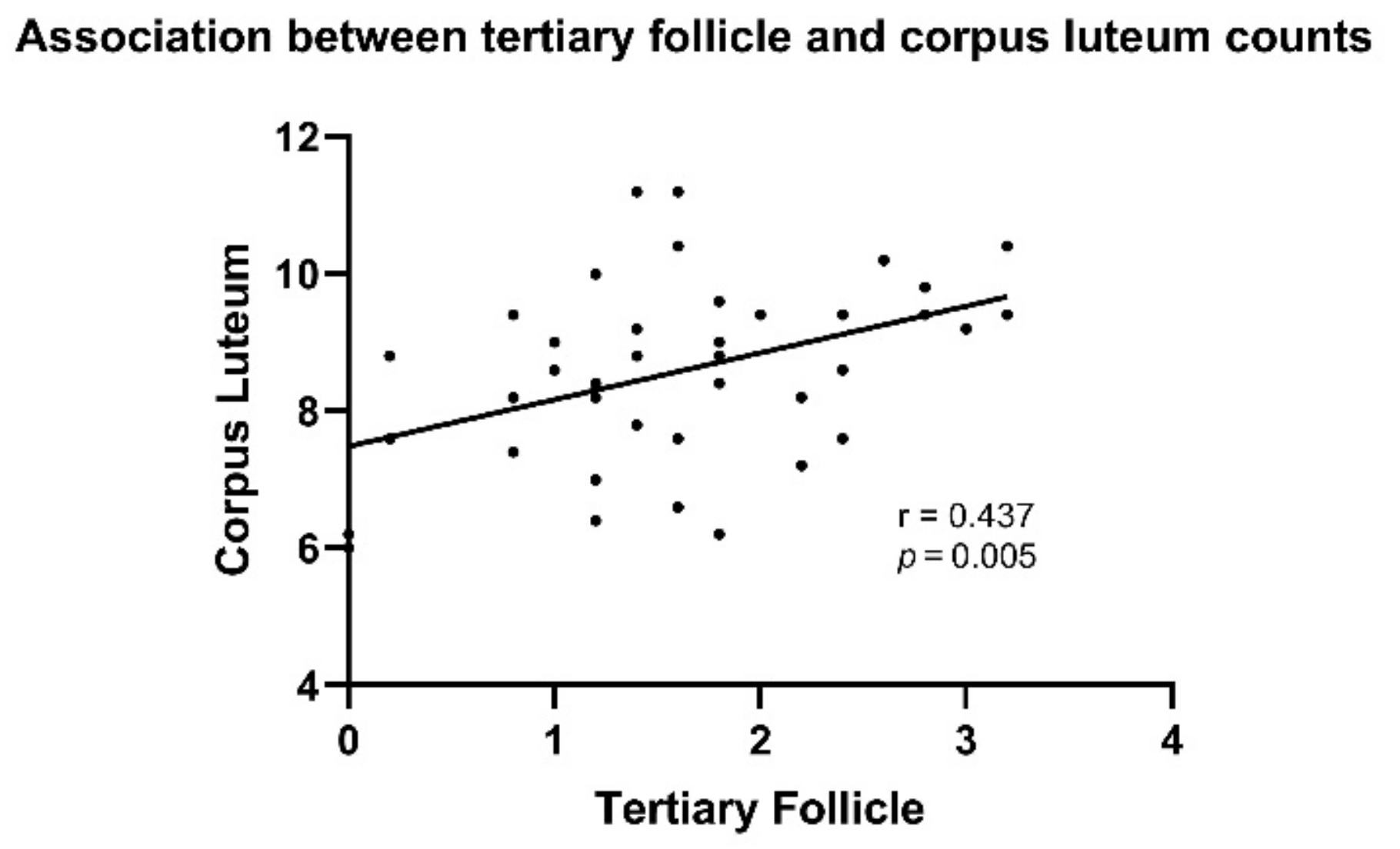
- Numbers of tertiary follicles positively associated with corpus luteum countation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, T.; Mortada, R.; Porter, S. Diagnosis and Treatment of Polycystic Ovary Syndrome. Am. Fam. Physician 2016, 94, 106–113. [Google Scholar] [PubMed]
- Franks, S. Polycystic Ovary Syndrome. In Encyclopedia of Hormones; Henry, H.L., Norman, A.W., Eds.; Academic Press: New York, NY, USA, 2003; pp. 237–243. [Google Scholar]
- Tabassum, F.; Jyoti, C.; Sinha, H.H.; Dhar, K.; Akhtar, M.S. Impact of polycystic ovary syndrome on quality of life of women in correlation to age, basal metabolic index, education and marriage. PLoS ONE 2021, 16, e0247486. [Google Scholar] [CrossRef] [PubMed]
- Wolf, W.M.; Wattick, R.A.; Kinkade, O.N.; Olfert, M.D. Geographical Prevalence of Polycystic Ovary Syndrome as Determined by Region and Race/Ethnicity. Int. J. Environ. Res. Public Health 2018, 15, 2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deswal, R.; Narwal, V.; Dang, A.; Pundir, C.S. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Hum. Reprod. Sci. 2020, 13, 261–271. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Q.; Hao, Y.; Jiao, M.; Wang, X.; Jiang, S.; Han, L. Measuring the global disease burden of polycystic ovary syndrome in 194 countries: Global Burden of Disease Study 2017. Hum. Reprod. 2021, 36, 1108–1119. [Google Scholar] [CrossRef]
- Belenkaia, L.V.; Lazareva, L.M.; Walker, W.; Lizneva, D.V.; Suturina, L.V. Criteria, phenotypes and prevalence of polycystic ovary syndrome. Minerva Ginecol. 2019, 71, 211–223. [Google Scholar] [CrossRef]
- Giampaolino, P.; Foreste, V.; Di Filippo, C.; Gallo, A.; Mercorio, A.; Serafino, P.; Improda, F.; Verrazzo, P.; Zara, G.; Buonfantino, C.; et al. Microbiome and PCOS: State-of-Art and Future Aspects. Int. J. Mol. Sci. 2021, 22, 2048. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Merkin, S.S.; Phy, J.L.; Sites, C.K.; Yang, D. Environmental determinants of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Barrea, L.; Arnone, A.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Salzano, C.; Pugliese, G.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients 2019, 11, 2278. [Google Scholar] [CrossRef] [Green Version]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [Green Version]
- De Melo, G.B.; Soares, J.F.; Costa, T.C.L.; Benevides, R.O.A.; Vale, C.C.; Paes, A.M.D.A.; Gaspar, R.S. Early Exposure to High-Sucrose Diet Leads to Deteriorated Ovarian Health. Front. Endocrinol. 2021, 12, 656831. [Google Scholar] [CrossRef]
- Yang, X.; Wu, R.; Qi, D.; Fu, L.; Song, T.; Wang, Y.; Bian, Y.; Shi, Y. Profile of Bile Acid Metabolomics in the Follicular Fluid of PCOS Patients. Metabolites 2021, 11, 845. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, S.; Gu, T.; Hong, T.; Liu, J.; Sun, J.; Wang, H.; Bi, Y.; Zhu, D. Increased circulating conjugated primary bile acids are associated with hyperandrogenism in women with polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 2019, 189, 171–175. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Gonzalez, F.J.; et al. Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019, 25, 1225–1233. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Jiang, Y.; Zhang, J.; Duan, R.; Liu, L.; Liu, C.; Xu, X.; Yu, L.; Wang, Q.; et al. Prediction of Gut Microbial Community Structure and Function in Polycystic Ovary Syndrome with High Low-Density Lipoprotein Cholesterol. Front. Cell. Infect. Microbiol. 2021, 11, 651. [Google Scholar] [CrossRef]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pr. 2020, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Ghiasi, R.; Soufi, F.G.; Somi, M.H.; Mohaddes, G.; Bavil, F.M.; Naderi, R.; Alipour, M.R. Swim Training Improves HOMA-IR in Type 2 Diabetes Induced by High Fat Diet and Low Dose of Streptozotocin in Male Rats. Adv. Pharm. Bull. 2015, 5, 379–384. [Google Scholar] [CrossRef]
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014, 2014, 655–658. [Google Scholar] [CrossRef]
- Kang, E.; Crouse, A.; Chevallier, L.; Pontier, S.M.; Alzahrani, A.; Silué, N.; Campbell-Valois, F.-X.; Montagutelli, X.; Gruenheid, S.; Malo, D. Enterobacteria and host resistance to infection. Mamm. Genome 2018, 29, 558–576. [Google Scholar] [CrossRef]
- Dunaif, A.; Segal, K.R.; Shelley, D.R.; Green, G.; Dobrjansky, A.; Licholai, T. Evidence for Distinctive and Intrinsic Defects in Insulin Action in Polycystic Ovary Syndrome. Diabetes 1992, 41, 1257–1266. [Google Scholar] [CrossRef]
- Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome: Mechanism and Implications for Pathogenesis. Endocr. Rev. 1997, 18, 774–800. [Google Scholar] [CrossRef] [Green Version]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2013, 346, e7492. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, M.; Xue, C.Y.; Kageyama, H.; Suzuki, E.; Ito, R.; Namba, Y.; Osaka, T.; Kimura, S.; Inoue, S. Effects of a high-sucrose diet on body weight, plasma triglycerides, and stress tolerance. Nutr. Rev. 2003, 61 Pt 2, S27–S33. [Google Scholar] [CrossRef]
- Do, H.J.; Lee, Y.S.; Ha, M.J.; Cho, Y.; Yi, H.; Hwang, Y.-J.; Hwang, G.-S.; Shin, M.-J. Beneficial effects of voglibose administration on body weight and lipid metabolism via gastrointestinal bile acid modification. Endocr. J. 2016, 63, 691–702. [Google Scholar] [CrossRef]
- Moghetti, P. Insulin Resistance and Polycystic Ovary Syndrome. Curr. Pharm. Des. 2016, 22, 5526–5534. [Google Scholar] [CrossRef]
- Neves, V.G.d.O.; de Oliveira, D.T.; Oliveira, D.C.; Perucci, L.O.; dos Santos, T.A.P.; Fernandes, I.d.C.; de Sousa, G.G.; Barboza, N.R.; Guerra-Sá, R. High-sugar diet intake, physical activity, and gut microbiota crosstalk: Implications for obesity in rats. Food Sci. Nutr. 2020, 8, 5683–5695. [Google Scholar] [CrossRef]
- Wei, S.; Bahl, M.I.; Baunwall, S.M.D.; Hvas, C.L.; Licht, T.R. Determining Gut Microbial Dysbiosis: A Review of Applied Indexes for Assessment of Intestinal Microbiota Imbalances. Appl. Environ. Microbiol. 2021, 87, e00395-21. [Google Scholar] [CrossRef]
- Li, A.; Zhang, L.; Jiang, J.; Yang, N.; Liu, Y.; Cai, L.; Cui, Y.; Diao, F.; Han, X.; Liu, J.; et al. Follicular hyperandrogenism and insulin resistance in polycystic ovary syndrome patients with normal circulating testosterone levels. J. Biomed. Res. 2017, 32, 208–214. [Google Scholar] [CrossRef]
- Poretsky, L. On the Paradox of Insulin-Induced Hyperandrogenism in Insulin-Resistant States. Endocr. Rev. 1991, 12, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, S.H.; Bouchard, T.P.; Leiva, R.A.; Boyle, P.; Iwaz, J.; Ecochard, R. Hormonal Predictors of Abnormal Luteal Phases in Normally Cycling Women. Front. Public Health 2018, 6, 144. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juliawan, I.M.P.; Suwana, F.P.; Annas, J.Y.; Akbar, M.F.; Widjiati, W. High Sucrose and Cholic Acid Diet Triggers PCOS-like Phenotype and Reduces Enterobacteriaceae Colonies in Female Wistar Rats. Pathophysiology 2022, 29, 344-353. https://doi.org/10.3390/pathophysiology29030026
Juliawan IMP, Suwana FP, Annas JY, Akbar MF, Widjiati W. High Sucrose and Cholic Acid Diet Triggers PCOS-like Phenotype and Reduces Enterobacteriaceae Colonies in Female Wistar Rats. Pathophysiology. 2022; 29(3):344-353. https://doi.org/10.3390/pathophysiology29030026
Chicago/Turabian StyleJuliawan, I Made Putra, Febie Putra Suwana, Jimmy Yanuar Annas, Muhammad Firman Akbar, and Widjiati Widjiati. 2022. "High Sucrose and Cholic Acid Diet Triggers PCOS-like Phenotype and Reduces Enterobacteriaceae Colonies in Female Wistar Rats" Pathophysiology 29, no. 3: 344-353. https://doi.org/10.3390/pathophysiology29030026
APA StyleJuliawan, I. M. P., Suwana, F. P., Annas, J. Y., Akbar, M. F., & Widjiati, W. (2022). High Sucrose and Cholic Acid Diet Triggers PCOS-like Phenotype and Reduces Enterobacteriaceae Colonies in Female Wistar Rats. Pathophysiology, 29(3), 344-353. https://doi.org/10.3390/pathophysiology29030026







