Pathogenesis of Autoimmune Male Infertility: Juxtacrine, Paracrine, and Endocrine Dysregulation
Abstract
1. Introduction
2. Sperm Antigens and Antisperm Antibodies (ASAs): Past and Present
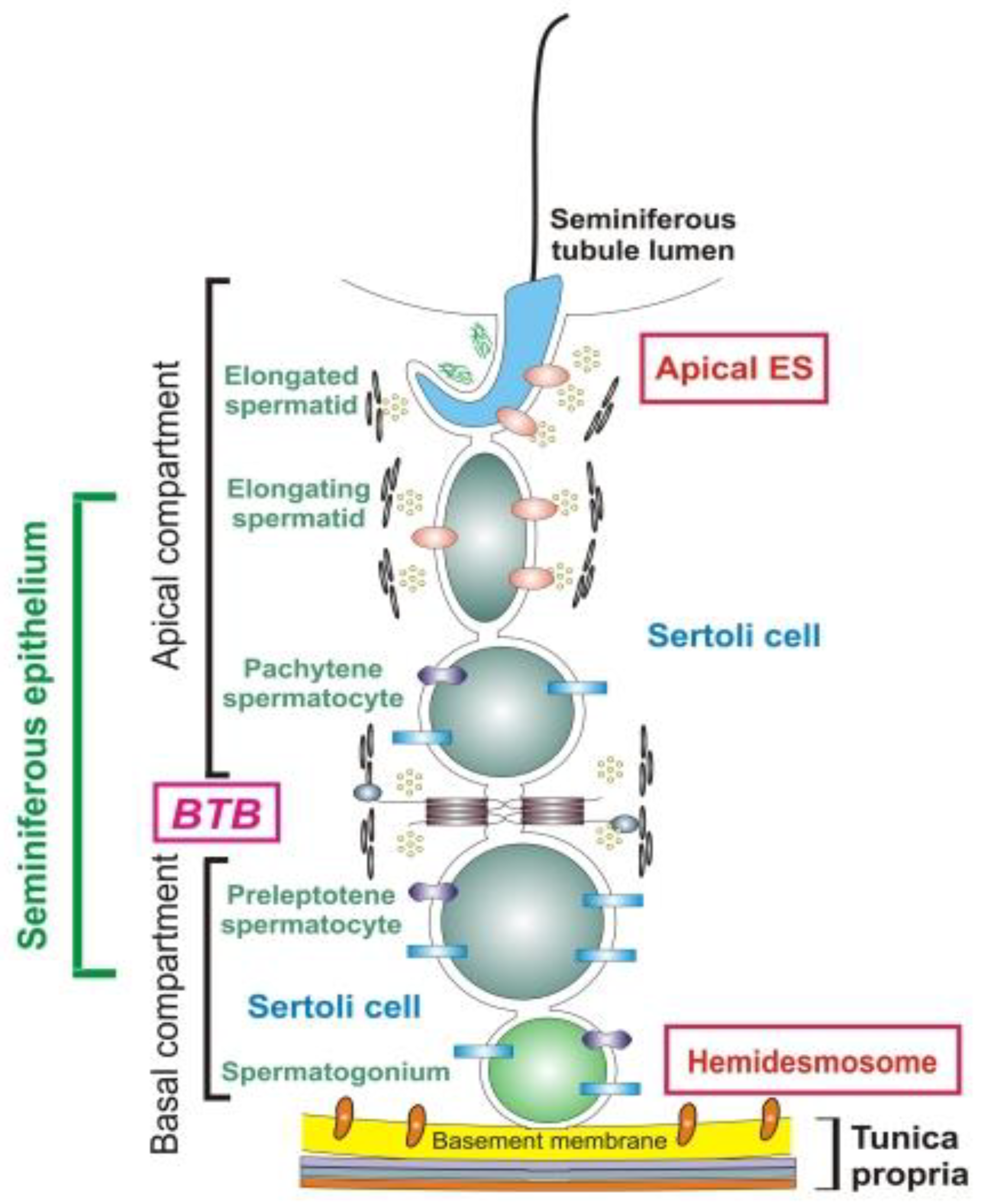
3. Immune Privilege of Testes: Informational, Not Only Anatomical Barrier
4. Role of Epididymis
5. Targets of ASAs: Assorted Mosaic
6. ASAs and Varicocele
7. Sperm Autoimmunity and Varicocelectomy: Conflicting Data
8. Anti-Sperm Autoimmunity and Infection: Essential Trigger
9. Systemic and Multiorgan Autoimmune Diseases and ASAs
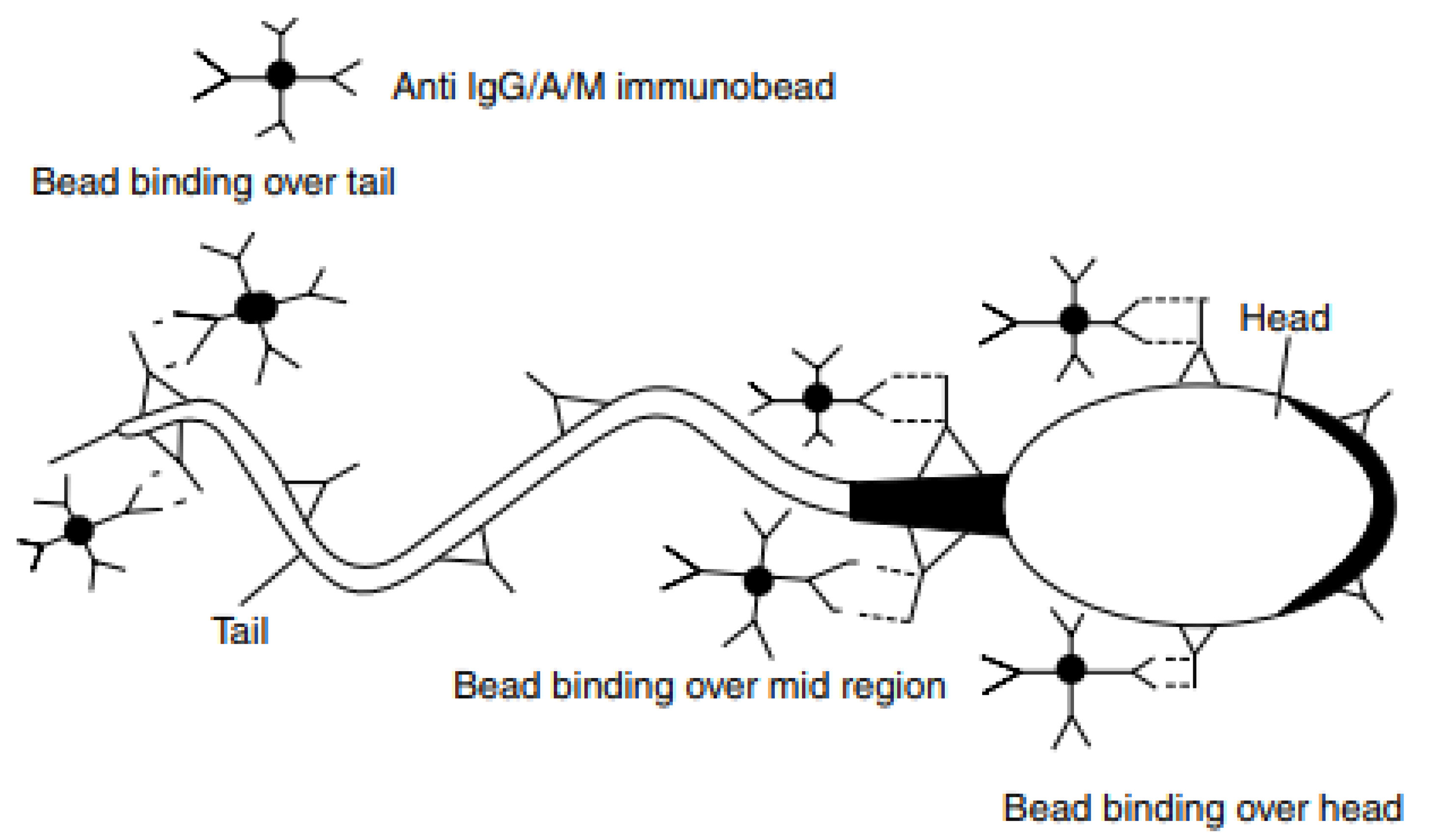
10. Methods of ASAs Detection
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.C.; Jing, L.I.; Liang, W.B.; Zhu, W.J. Evaluation on antisperm antibody in infertile men with oligoasthenoteratozoospermia. J. Reprod. Contracept. 2014, 25, 49–53. [Google Scholar] [CrossRef]
- Shevyrin, A.A. Modern view on treatment of male infertility. Russ. Med Rev. 2018, 12, 30–35. (In Russian) [Google Scholar]
- Kalinichenko, S.Y.; Tyuzikov, I.A. Oxidative stress and male infertility are interrelated pandemics of the 21st century. Modern pharmacotherapeutic possibilities of pathogenetic correction of spermatogenesis disorders with L-carnitine/acetyl-L-carnitine preparations. Urol. Nephrol. Spec. Issue Men’s Health 2017, 22, 6–19. (In Russian) [Google Scholar]
- Baskaran, S.; Agarwal, A.; Selvam, M.K.P.; Finelli, R.; Robert, K.A.; Iovine, C.; Pushparaj, P.N.; Samanta, L.; Harlev, A.; Henkel, R. Tracking Research Trends and Hotspots in Sperm DNA Fragmentation Testing for the Evaluation of Male Infertility: A Scientometric Analysis. Reprod. Biol. Endocrinol. 2019, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Vybornov, S.V.; Asfandiyarov, F.R.; Seidov, K.S.; Kruglov, V.A. Antioxidants in the treatment of patients with inflammatory diseases of the male reproductive system, complicated by excretory toxic form of infertility. Exp. Clin. Urol. 2018, 3, 74–78. (In Russian) [Google Scholar]
- Kirilenko, E.A.; Onopko, V.F. Oxidative stress and male fertility: A modern view on the problem. Acta Biomed. Sci. 2017, 2, 102–108. (In Russian) [Google Scholar] [CrossRef]
- Vickram, A.S.; Dhama, K.; Chakraborty, S.; Samad, H.A.; Latheef, S.K.; Sharun, K.; Khurana, S.K.; Tiwari, R.; Bhatt, P.; Chaicumpa, W. Role of Antisperm Antibodies in Infertility, Pregnancy, and Potential for Contraceptive and Antifertility Vaccine Designs: Research Progress and Pioneering Vision. Vaccines 2019, 17, 116. [Google Scholar] [CrossRef]
- Shirshov, V.N. Current state of the male infertility problem: The review of European Association of Urology clinical guidelines. J. Clin. Pract. 2016, 7, 39–50. [Google Scholar] [CrossRef][Green Version]
- Epanchintseva, E.A.; Selyatitskaya, V.G.; Mitrofanov, I.M.; Pinkhasov, B.B.; Sviridova, M.A. Antisperm antibodies under infertility in males, connection with abdominal obesity. Adv. Curr. Nat. Sci. 2015, 4, 24–27. (In Russian) [Google Scholar]
- Dorofeev, S.D.; Efremov, E.A.; Simakov, V.V. Management of idiopathic pathospermia. Effective pharmacotherapy. Urol. Nefrol. 2015, 2, 24–30. (In Russian) [Google Scholar]
- Xu, F.; Ye, L.; Hu, Y.; Cai, C.; Wang, Z.; Fan, L.; Song, L.; Xu, Z.; Du, W. A novel protein biochip screening serum anti-sperm antibody expression and natural pregnancy rate in a follow-up study in Chinese infertility. Biosci. Rep. 2020, 40, BSR20191769. [Google Scholar] [CrossRef] [PubMed]
- Potekhina, E.S.; Mikhailyuk, E.V.; Nepomnyashchikh, A.S. Spermogram as an instrument for assessing male fertility. Sci. Rev. 2020, 1, 11–14. [Google Scholar] [CrossRef]
- Khairutdinov, K.N.; Sitdykova, M.E.; Zubkov, A. Male infertility is the problem of XXI century. Obstet. Gynecol. 2018, 6, 185–189. (In Russian) [Google Scholar]
- Silva, C.A.; Cocuzza, M.; Carvalho, J.F.; Bonfá, E. Diagnosis and classification of autoimmune orchitis. Autoimmun. Rev. 2014, 13, 431–434. [Google Scholar] [CrossRef]
- Wald, M. Male infertility: Causes and cures. Sex. Reprod. Menopause 2005, 3, 83–87. [Google Scholar] [CrossRef]
- Schmidt, A.A.; Zamyatnin, S.A.; Gonchar, I.S.; Korovin, A.E. Risk factors of the development of male infertility. Clin. Pathophysiol. 2019, 25, 56–60. (In Russian) [Google Scholar]
- Avadieva, N.E. The use of DNA semen fragmentation in andrological practice. Vestn. Urol. 2019, 7, 7–11. [Google Scholar] [CrossRef]
- Likhonosov, N.P.; Ayub, A.K.; Babenko, A.; Borovets, S. The role of inhibin B in the regulation of spermatogenesis and its clinical significance in male infertility. Urol. Vedom. 2019, 9, 39–45. [Google Scholar] [CrossRef]
- Apolikhin, O.I.; Moskaleva, N.G.; Komarova, V.A. Contemporary demographic situation and problems of improving the reproductive health of Russian population. Exp. Clin. Urol. 2015, 4, 4–14. (In Russian) [Google Scholar]
- Lutov, Y.V.; Selyatitskaya, V.G.; Epanchintseva, E.A.; Ryabinchenko, T.I. The relationship between the andrological status of adolescents with anthropometric and hormonal indicators. Hum. Physiol. 2014, 40, 124–131. [Google Scholar] [CrossRef]
- Jiang, Y.; Cui, D.; Du, Y.; Lu, J.; Yang, L.; Li, J.; Zhang, J.; Bai, X. Association of anti-sperm antibodies with chronic prostatitis: A systematic review and meta-analysis. J. Reprod. Immunol. 2016, 118, 85–91. [Google Scholar] [CrossRef]
- Paoli, D.; Gilio, B.; Piroli, E.; Gallo, M.; Lombardo, F.; Dondero, F.; Lenzi, A.; Gandini, L. Testicular tumors as a possible cause of antisperm autoimmune response. Fertil. Steril. 2009, 91, 414–419. [Google Scholar] [CrossRef]
- Joki-Korpela, P.; Sahrakorpi, N.; Halttunen, M.; Surcel, H.M.; Paavonen, J.; Tiitinen, A. The role of Chlamydia trachomatis infection in male infertility. Fertil. Steril. 2009, 91, 1448–1450. [Google Scholar] [CrossRef] [PubMed]
- Hulusi, B.Z.; Hakan, Y. Antisperm antibodies: Fact or fiction? Immunol. Allergy Clin. N. Am. 2002, 22, 471–501. [Google Scholar] [CrossRef]
- Kurpisz, M.; Havryluk, A.; Nakonechnyj, A.; Chopyak, V.; Kamieniczna, M. Cryptorchidism and long-term consequences. Reprod. Biol. 2010, 10, 19–35. [Google Scholar] [CrossRef]
- London, E. On the doctrine of spermolysins. Proceeding 1 (with 1 phototypic table). Arkh. Biol. Nauk. (St. Petersburg). 1901, 9, 82–125. (In Russian) [Google Scholar]
- London, E.S. Contribution a l’étude des spermolysines. Arch. Sci. Biol. 1903, 9, 171. [Google Scholar]
- Metalnikoff, S. Etudes sur la spermotoxine. Ann. Inst. Pasteur 1900, 14, 577–589. [Google Scholar]
- Parida, R. Human MOSPD2: A bacterial Lmb mimicked auto-antigen is involved in immune infertility. J. Transl. Autoimmun. 2019, 1, 100002. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, W.; Cardona Maya, B. Anticuerpos antiespermatozoides y su asociación con la fertilidad. Actas Urol. Esp. 2013, 37, 571–578. [Google Scholar] [CrossRef]
- Wasilewski, T.; Łukaszewicz-Zając, M.; Wasilewska, J.; Mroczko, B. Biochemistry of infertility. Clin. Chim. Acta 2020, 508, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Kutteh, W.H. Autoimmune factors and their influence on assisted reproduction. Immunol. Allergy Clin. N. Am. 2002, 22, 643–661. [Google Scholar] [CrossRef]
- Wilson, L. Sperm agglutinins in human semen and blood. Proc. Soc. Exp. Biol. Med. (N. Y.) 1954, 85, 652–655. [Google Scholar] [CrossRef]
- Rümke, P. The presence of sperm antibodies in the serum of two patients with oligozoospermia. Vox Sang. 1954, 4, 135–140. [Google Scholar]
- Wakimoto, Y.; Fukui, A.; Kojima, T.; Hasegawa, A.; Shigeta, M.; Shibahara, H. Application of computer-aided sperm analysis (CASA) for detecting sperm-immobilizing antibody. Am. J. Reprod. Immunol. 2018, 79. [Google Scholar] [CrossRef] [PubMed]
- Rümke, P. Autospermagglutinins: A cause of infertility in men. Ann. N. Y. Acad. Sci. 1965, 124, 696–701. [Google Scholar] [CrossRef]
- Emin, A.; Konova, E.; Lichev, D.; Aĭvazova, N.; Popov, I.; Radev, R. The importance of the presence of antisperm antibodies in serum and ejaculate of men with infertility. Akush Ginekol (Sofiia) 2008, 47, 26–30. [Google Scholar]
- Chamley, L.W.; Clarke, G.N. Antisperm antibodies and conception. Semin. Immunopathol. 2007, 29, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, B.; Cardona-Maya, W. Antisperm antibodies and fertility association. Actas Urol. Esp. 2013, 37, 571–578. [Google Scholar] [CrossRef]
- Cui, D.; Han, G.; Shang, Y.; Liu, C.; Xia, L.; Li, L.; Yi, S. Antisperm antibodies in infertile men and their effect on semen parameters: A systematic review and meta-analysis. Clin. Chim. Acta 2015, 15, 29–36. [Google Scholar] [CrossRef]
- Lu, S.M.; Li, X.; Wang, S.L.; Yang, X.L.; Xu, Y.-Z.; Huang, L.-L.; Liu, J.-L.; Cai, F.-F.; Chen, Z.-J. Success rates of in vitro fertilization versus intracytoplasmic sperm injection in men with serum anti-sperm antibodies: A consecutive cohort study. Asian J. Androl. 2019, 21, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Yasin, A.L.; Basha, W.S. The Epidemiology of Anti-Sperm Antibodies Among Couples with Unexplained Infertility in North West Bank, Palestine. J. Clin. Diagn. Res. 2016, 10, QC01-3. [Google Scholar] [CrossRef] [PubMed]
- Bieniek, J.M.; Drabovich, A.P.; Lo, K.C. Seminal biomarkers for the evaluation of male infertility. Asian J. Androl. 2016, 18, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Westerman, R. Biomarkers for demographic research: Sperm counts and other male infertility biomarkers. Biodemogr. Soc. Biol. 2020, 65, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Tyuzikov, I.A.; Kalinichenko, S.; Vorslov, L.O.; Tishova, A. Male infertility and insulin resistance: Are there pathogenetic links and who, when and how should diagnose and treat? Exp. Clin. Urol. 2014, 2, 68–75. (In Russian) [Google Scholar]
- McLachlan, R.I. Basis, diagnosis and treatment of immunological infertility in men. J. Reprod. Immunol. 2002, 57, 35–45. [Google Scholar] [CrossRef]
- Dörr, H.; Bohring, C.; Krause, W. Are antisperm antibodies indeed sperm-specific? Andrologia 2005, 37, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Piroozmand, A.; Nasab, S.D.M.; Erami, M.; Hashemi, S.M.A.; Khodabakhsh, E.; Ahmadi, N.; Vahedpoor, Z. Distribution of Human Papillomavirus and Antisperm Antibody in Semen and Its Association with Semen Parameters Among Infertile Men. J. Reprod. Infertil. 2020, 21, 183–188. [Google Scholar] [PubMed]
- Tchiokadze, S.; Galdava, G. Clinical and anamnestic characteristics of development of antispermimmunity in infertile men. Georgian Med. News 2015, 246, 18–22. [Google Scholar]
- Patel, A.S.; Leong, J.Y.; Ranjith, R. Prediction of male infertility by the World Health Organization laboratory manual for assessment of semen analysis: A systematic review. Arab. J. Urol. 2017, 16, 96–102. [Google Scholar] [CrossRef]
- Adams, C.E.; Wald, M. Risks and complications of vasectomy. Urol. Clin. North Am. 2009, 36, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Amory, J.K. Male contraception. Fertil. Steril. 2016, 106, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, P. The danger model: A renewed sense of self. Science 2002, 296, 301–305. [Google Scholar] [CrossRef]
- Fijak, M.; Meinhardt, A. The testis in immune privilege. Immunol. Rev. 2006, 213, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, A.; Esteves, S.C.; Gosálvez, J.; Agarwal, A. Specialized sperm function tests in varicocele and the future of andrology laboratory. Asian J. Androl. 2016, 18, 205–212. [Google Scholar] [CrossRef]
- Jiang, H.; Zhu, W.J. Testicular microlithiasis is not a risk factor for the production of antisperm antibody in infertile males. Andrologia 2013, 45, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Bozhedomov, V.A.; Teodorovich, O.V. Epidemiology and causes of autoimmune male infertility. Urologiia 2005, 1, 35–44. [Google Scholar]
- Al-Adi, A.M.; El-Karamany, T.; Issa, H.; Zaazaa, M. The influence of antisperm antibodies, intratesticular haemodynamics and the surgical approach to varicocelectomy on seminal variables. Arab. J. Urol. 2014, 4, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Archana, S.S.; Selvaraju, S.; Binsila, B.K.; Arangasamy, A.; Krawetz, S.A. Immune regulatory molecules as modifiers of semen and fertility: A review. Mol. Reprod. Dev. 2019, 86, 1485–1504. [Google Scholar] [CrossRef] [PubMed]
- Fijak, M.; Pilatz, A.; Hedger, M.P.; Nicolas, N.; Bhushan, S.; Michel, V.; Tung, K.S.K.; Schuppe, H.-C.; Meinhardt, A. Infectious, inflammatory and ‘autoimmune’ male factor infertility: How do rodent models inform clinical practice? Hum. Reprod. Update 2018, 24, 416–441. [Google Scholar] [CrossRef] [PubMed]
- Nagaria, T.; Sirmor, N. Misoprostol vs. mifepristone and misoprostol in second trimester termination of pregnancy. J. Obstet. Gynaecol. 2011, 61, 659. [Google Scholar] [CrossRef]
- Ombelet, W.; Bosmans, E.; Cox, A.; Janssen, M.; Mestdagh, G.; Nijs, M. In search for the general population’s semen profile: The study of sperm parameters in partners of women with chronic anovulation. Facts Views Vis Obgyn. 2009, 1, 18–26. [Google Scholar] [PubMed]
- Leushuis, E.; van der Steeg, J.W.; Steures, P.; Repping, S.; Schols, W.; van der Veen, F.; Mol, B.W.; Hompes, P.G. Immunoglobulin G antisperm antibodies and prediction of spontaneous pregnancy. Fertil. Steril. 2009, 92, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Nishlag, E.; Bere, G.M. Andrology. In Male Reproductive Health and Dysfunction; Nishlag, E., Bere, G.M., Eds.; Medical News Agency: Moscow, Russia, 2005; p. 551. (In Russian) [Google Scholar]
- Cheng, C.Y.; Mruk, D.D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zeng, Q.; Yu, D.; Duan, Y.G. T Lymphocytes and Testicular Immunity: A New Insight into Immune Regulation in Testes. Int. J. Mol. Sci. 2020, 22, 57. [Google Scholar] [CrossRef]
- Duan, Y.G.; Gong, J.; Yeung, W.S.B.; Haidl, G.; Allam, J.P. Natural killer and NKT cells in the male reproductive tract. J. Reprod. Immunol. 2020, 142, 103178. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.M.; Lord, S.J.; Kin, T.; Rayat, G.R.; Dixon, D.E.; Bleackley, R.C.; Korbutt, G.S.; Rajotte, R.V. Comparison of successful and unsuccessful islet/Sertoli cell cotransplant grafts in streptozotocin-induced diabetic mice. Cell Transplant. 2008, 16, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Guazzone, V.A.; Jacobo, P.; Theas, M.S.; Lustig, L. Cytokines and chemokines in testicular inflammation: A brief review. Microsc. Res. Tech. 2009, 72, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Lotti, F.; Baldi, E.; Corona, G.; Lombardo, F.; Maseroli, E.; Degl’Innocenti, S.; Bartoli, L.; Maggi, M. Epididymal more than testicular abnormalities are associated with the occurrence of antisperm antibodies as evaluated by the MAR test. Hum. Reprod. 2018, 33, 1417–1429. [Google Scholar] [CrossRef]
- Friend, D.S.; Gilula, N.B. Variations in tight and gap junctions in mammalian tissues. J. Cell Biol. 1972, 53, 758–776. [Google Scholar] [CrossRef] [PubMed]
- Chereshnev, V.A.; Rybina, I.V.; Beikin, Y.B.; Oboskalova, T.A. Immunological and Genetic Factors of Reproductive Disorders; UB RAS Publisher: Ekaterinburg, Russia, 2005; p. 175. (In Russian) [Google Scholar]
- Clark, S.; Naz, R.K. Presence and incidence of IZUMO antibodies in sera of immunoinfertile women and men. Am. J. Reprod. Immunol. 2013, 69, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Nowicka-Bauer, K.; Kamieniczna, M.; Cibulka, J.; Ulcova-Gallova, Z.; Kurpisz, M. Proteomic identification of sperm antigens using serum samples from individuals with and without antisperm antibodies. Andrologia 2016, 48, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-C.; Huang, Y.-F.; Lu, N.-Q. Antisperm immunity and infertility. Expert Rev. Clin. Immunol. 2008, 4, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.; Ronquist, G.; Nilsson, B.O.; Larsson, A. Dominant prostasome immunogens for sperm-agglutinating autoantibodies of infertile men. J. Androl. 2004, 25, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Pavlásek, J.; Peknicová, J.; Ulcová-Gallová, Z.; Nováková, P.; Reischig, J.; Micanová, Z.; Rokyta, Z. Significance of determination of intra-acrosomal proteins and sperm antibodies in human reproduction. Ceska Gynekol. 2004, 69, 306–311. [Google Scholar] [PubMed]
- Bonyadi, M.R.; Madaen, S.K.; Saghafi, M. Effects of Varicocelectomy on Anti-sperm Antibody in Patients with Varicocele. J. Reprod. Infertil. 2013, 2, 73–78. [Google Scholar]
- Shibahara, H.; Tsunoda, T.; Taneichi, A.; Hirano, Y.; Ohno, A.; Takamizawa, S.; Yamaguchi, C.; Tsunoda, H.; Sato, I. Diversity of antisperm antibodies bound to sperm surface in male immunological infertility. Am. J. Reprod. Immunol. 2002, 47, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.S.; Cury, V.N.; Cha, J.D.; Junior, V.M.L.; Marques, J.L.; Mizrahi, F.E.; Figueiredo, F.W.D.S.; Barbosa, C.; Glina, S. Do assisted reproduction outcomes differ according to aetiology of obstructive azoospermia? Andrologia 2020, 52, e13425. [Google Scholar] [CrossRef]
- Bozhedomov, V.A.; Lipatova, N.A.; Alexeev, R.A.; Alexandrova, L.M.; Nikolaeva, M.A.; Sukhikh, G.T. The role of the antisperm antibodies in male infertility assessment after microsurgical varicocelectomy. Andrology 2014, 6, 847–855. [Google Scholar] [CrossRef]
- Bozhedomov, V.A.; Lipatova, N.A.; Rokhlikov, I.M.; Alexeev, R.A.; Ushakova, I.V.; Sukhikh, G.T. Male fertility and varicocoele: Role of immune factors. Andrology 2014, 1, 51–58. [Google Scholar] [CrossRef]
- Al-Daghistani, H.I.; Hamad, A.W.; Abdel-Dayem, M.; Al-Swaifi, M.; Abu Zaid, M. Evaluation of Serum Testosterone, Progesterone, Seminal Antisperm Antibody, and Fructose Levels among Jordanian Males with a History of Infertility. Biochem. Res. Int. 2010, 2010, 409640. [Google Scholar] [CrossRef] [PubMed]
- Bohring, C.; Krause, W. Differences in the antigen pattern recognized by antisperm antibodies in patients with infertility and vasectomy. J. Urol. 2001, 166, 1178–1180. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, E.; Zhang, C.; Zhang, H. Study of the Changes of Acrosomal Enzyme, Nitric Oxide Synthase, and Superoxide Dismutase of Infertile Patients with Positive Antisperm Antibody in Seminal Plasma. Cell Biochem. Biophys. 2015, 3, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Gatimel, N.; Moreau, J.; Isus, F.; Moinard, N.; Parinaud, J.; Leandri, R.D. Anti-sperm antibodies detection by a modified MAR test: Towards a better definition of its indications. Reprod. Biomed. Online 2018, 37, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, F.A.; Grzechocinska, B.; Wielgos, M. The role of vitamin D in reproductive health--a Trojan Horse or the Golden Fleece? Nutrients 2015, 7, 4139–4153. [Google Scholar] [CrossRef]
- Silva, A.F.; Ramalho-Santos, J.; Amaral, S. The impact of antisperm antibodies on human male reproductive function: An update. Reproduction 2021, 162, R55–R71. [Google Scholar] [CrossRef]
- Clarke, G.N. Association between sperm autoantibodies and enhanced embryo implantation rates during in vitro fertilization. Fertil. Steril. 2006, 86, 753. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C.; Schneider, D.T.; Verza, S., Jr. Influence of antisperm antibodies in the semen on intracytoplasmic sperm injection outcome. Int. Braz. J. Urol. 2007, 33, 795–802. [Google Scholar] [CrossRef]
- Lombardo, F.; Gandini, L.; Lenzi, A.; Dondero, F. Antisperm immunity in assisted reproduction. J. Reprod. Immunol. 2004, 62, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.N. Etiology of sperm immunity in women. Fertil. Steril. 2009, 91, 639–643. [Google Scholar] [CrossRef]
- Bahraminejad, R.; Kadanali, S.; Erten, O.; Bahar, H. Reproductive failure and antisperm-antibody production among prostitutes. Acta Obstet. Gynecol. Scand. 1999, 70, 483–485. [Google Scholar] [CrossRef]
- Yang, Y.X.; Zhu, W.J.; Ren, X. Evaluation of Circulating ASAs in Women with Secondary Infertility. J. Reprod. Contracept. 2011, 22, 195–200. [Google Scholar] [CrossRef]
- Witkin, S.S.; David, S.S. Effect of sperm antibodies on pregnancy outcome in a subfertile population. Am. J. Obstet. Gynecol. 1988, 158, 59–62. [Google Scholar] [CrossRef]
- Naz, R.K. Effects of Antisperm Antibodies on Early Cleavage of Fertilized Ova1. Biol. Reprod. 1992, 46, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Zhu, W.J.; Li, J. Evaluation of Circulating Antisperm Antibody in Infertile Women with Polycystic Ovary Syndrome. J. Reprod. Contracept. 2012, 23, 25–28. [Google Scholar] [CrossRef]
- Zhu, W.; Jiang, H. Circulating Antisperm Antibody is not Related to the Pathogenesis of Infertility in Women with Endometriosis. J. Reprod. Contracept. 2012, 23, 75–80. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhu, W.J. Circulating antisperm antibody (ASAs) in women is not associated with missed abortions at the first-trimester pregnancy. J. Reprod. Contracept. 2011, 22, 139–143. [Google Scholar] [CrossRef]
- Vad, S.; Szanto, A.; Tarr, T.; Vegh, J.; Naggy, G.; Zeher, M. Immunological disorders in reproductive failure. J. Reprod. Immunol. 2014, 101–102, 40–60. [Google Scholar] [CrossRef]
- Neimark, A.I.; Neimark, B.A.; Davydov, A.V.; Saldan, I.; Nozdrachev, N. Rehabilitation of patients with male infertility after varicocelectomy. Eff. Pharmacother. 2018, 9, 8–12. (In Russian) [Google Scholar]
- Evdokimov, V.V.; Zakharikov, S.V.; Kastrikin Yu, V. Varicocele in children and adolescents. Treat. Prev. 2017, 21, 53–56. (In Russian) [Google Scholar]
- Will, M.A.; Swain, J.; Fode, M.; Sonksen, J.; Christman, G.M.; Ohl, D. The great debate: Varicocele treatment and impact on fertility. Fertil. Steril. 2011, 95, 841–852. [Google Scholar] [CrossRef]
- Komarova, S.; Tsap, N.A. Ways to reduce the risk of reproductive loss in children with varicocele. Med. Sci. Educ. Urals 2017, 1, 98–101. (In Russian) [Google Scholar]
- Neimark, A.I.; Popov, I.S.; Gazamatov, A.V. Features of the microcirculation of the prostate and gonads in young men suffering from isolated varicocele and varicocele in combination with pelvic congestion. Andrology 2013, 2, 56–60. (In Russian) [Google Scholar]
- Efremov, E.A.; Shekhovtsov, S.; Butov, A.O.; Kastrikin, V.; Kozdoba, A.S.; Garaev, T.I. The effect of varicocele on hormones and the reproductive system in men. Exp. Clin. Urol. 2019, 1, 102–106. (In Russian) [Google Scholar]
- Ramalingam, M.; Kini, S.; Mahmood, T. Male fertility and infertility. Obstet. Gynaecol. Reprod. Med. 2014, 24, 326–332. [Google Scholar] [CrossRef]
- Komarova, S. Problems and prospects of andrological care for children in predicting the reproductive status of a megacity. Ural Med. J. Pediatr. Urol. 2013, 9, 10–13. (In Russian) [Google Scholar]
- Madar, J.; Urbánek, V.; Chaloupková, A.; Nouza, K.; Kinský, R. Role of sperm antibodies and cellular autoimmunity to sperm in the pathogenesis of male infertility. Ceska Gynekol. 2002, 67, 3–7. [Google Scholar] [PubMed]
- Hassanin, A.M.; Ahmed, H.H.; Kaddah, A.N. A Global View of the Pathophysiology of Varicocele. Andrology 2018, 6, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Bonfig, R.; Wilbert, D.M.; Strohmaier, W.L.; Engelmann, U.H. Risk factors for antisperm antibodies in infertile men. Am. J. Reprod. Immunol. 1994, 31, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, B.R.; Witkin, S.S.; Goldstein, M. Correlation of sperm-bound immunoglobulins with impaired semen analysis in infertile men with varicoceles. Fertil. Steril. 1989, 52, 469–473. [Google Scholar] [CrossRef]
- Knudson, G.; Ross, L.; Stuhldreher, D.; Houlihan, D.; Bruns, E.; Prins, G. Prevalence of sperm bound antibodies in infertile men with varicocele: The effect of varicocele ligation on antibody levels and semen response. J. Urol. 1994, 151, 1260–1262. [Google Scholar] [CrossRef]
- Ulcova-Gallova, Z.; Gruberova, J.; Vrzalova, J.; Bibkova, K.; Peknicova, J.; Micanova, Z.; Topolcan, O. Sperm antibodies, intra-acrosomal sperm proteins, and cytokines in semen in men from infertile couples. Am. J. Reprod. Immunol. 2009, 61, 236–245. [Google Scholar] [CrossRef]
- Ferrer, M.S.; Miller, L.M.J. Equine sperm-bound antisperm antibodies are associated with poor semen quality. Theriogenology 2018, 15, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Kanevskaya, T.A.; Yatsyk, S.P.; Bezlepkina, O.B. Hormonal status and markers of autoimmune disorders of spermatogenesis in adolescents who underwent surgical treatment for varicocele. Pediatr. Pharmacol. 2010, 7, 92–94. (In Russian) [Google Scholar]
- Oshinsky, G.S.; Rodriguez, M.V.; Mellinger, B.C. Varicocele-related infertility is not associated with increased sperm-bound antibody. J. Urol. 1993, 150, 871–873. [Google Scholar] [CrossRef]
- Veräjänkorva, E.; Laato, M.; Pöllänen, P. Analysis of 508 infertile male patients in south-western Finland in 1980-2000: Hormonal status and factors predisposing to immunological infertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 111, 173–178. [Google Scholar] [CrossRef]
- Yatsyk, S.P.; Kanevskaya, T.A.; Abramov, K.S.; Sharkov, S.M.; Fomin, D.K. Reproductive health of children and adolescents who have undergone surgical correction in connection with andrological pathology. Pediatr. Pharmacol. 2009, 6, 15–22. (In Russian) [Google Scholar]
- Jensen, C.F.S.; Khan, O.; Nagras, Z.G.; Sønksen, J.; Fode, M.; Østergren, P.B.; Shah, T.; Ohl, D.A.; Collaborative, C. Male infertility problems of patients with strict sperm morphology between 5-14% may be missed with the current WHO guidelines. Scand. J. Urol. 2018, 5–6, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Berger, G.K.; Smith-Harrison, L.I.; Sandlow, J.I. Sperm agglutination: Prevalence and contributory factors. Andrologia 2019, 51, e13254. [Google Scholar] [CrossRef]
- Djaladat, H.; Mehrsai, A.; Rezazade, M.; Djaladat, Y.; Pourmand, G. Varicocele and antisperm antibody: Fact or fiction? South Med. J. 2006, 99, 44–47. [Google Scholar] [CrossRef]
- Wigby, S.; Suarez, S.S.; Lazzaro, B.P.; Pizzari, T.; Wolfner, M.F. Sperm success and immunity. Curr. Top Dev. Biol. 2019, 135, 287–313. [Google Scholar] [CrossRef]
- Eggert-Kruse, W.; Weltin, M.; Strowitzki, T. Are chlamydial lipopolysaccharide-directed antibodies in seminal plasma or serum clinically significant during investigation of male infertility? Urology 2011, 77, 1101–1106. [Google Scholar] [CrossRef]
- Garolla, A.; Pizzol, D.; Bertoldo, A.; De Toni, L.; Barzon, L.; Foresta, C. Association, prevalence, andclearance of human papillomavirusand antisperm antibodies in infected semen samples from infertile patients. Fertil. Steril. 2013, 99, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Thaper, D.; Prabha, V. Molecular mimicry: An explanation for autoimmune diseases and infertility. Scand. J. Immunol. 2018, 88, e12697. [Google Scholar] [CrossRef]
- Merezhkovsky, K.S. The Theory of Two Plasmas as a Basis Symbiogenesis, a New Doctrine of the Origin of Organisms; Imperial Univ. Publishing House: Kazan, Russia, 1909; p. 102. [Google Scholar]
- Kanduc, D. The role of proteomics in defining autoimmunity. Expert Rev. Proteom. 2021, 18, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Pashnina, I.A.; Krivolapova, I.M.; Fedotkina, T.V.; Ryabkova, V.A.; Chereshneva, M.V.; Churilov, L.P.; Chereshnev, V.A. Antinuclear Autoantibodies in Health: Autoimmunity Is Not a Synonym of Autoimmune Disease. Antibodies 2021, 10, 9. [Google Scholar] [CrossRef]
- Ahuja, A.K.; Cheema, R.S. Homology between cattle bull sperm and bacterial antigenic proteins viz a viz possible role in immunological infertility. Reprod. Domest. Anim. 2018, 53, 1530–1538. [Google Scholar] [CrossRef]
- Zupin, L.; Pascolo, L.; Zito, G.; Ricci, G.; Crovella, S. SARS-CoV-2 and the next generations: Which impact on reproductive tissues? J. Assist. Reprod. Genet. 2020, 37, 2399–2403. [Google Scholar] [CrossRef]
- Haghpanah, A.; Masjedi, F.; Alborzi, S.; Hosseinpour, A.; Dehghani, A.; Malekmakan, L.; Roozbeh, J. Potential mechanisms of SARS-CoV-2 action on male gonadal function and fertility: Current status and future prospects. Andrologia 2021, 53, e13883. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qi, L.; Chi, X.; Yang, J.; Wei, X.; Gong, E.; Peh, S.; Gu, J. Orchitis: A complication of severe acute respiratory syndrome (SARS). Biol. Reprod. 2006, 74, 410–416. [Google Scholar] [CrossRef]
- Gagliardi, L.; Bertacca, C.; Centenari, C.; Merusi, I.; Parolo, E.; Ragazzo, V.; Tarabella, V. Orchiepididymitis in a boy with COVID-19. Pediatr. Infect. Dis. J. 2020, 39, e200–e202. [Google Scholar] [CrossRef] [PubMed]
- Shetty, J.; Klotz, K.L.; Wolkowicz, M.J.; Flickinger, C.J.; Herr, J.C. Radial spoke protein 44 (human meichroacidin) is an axonemal alloantigen of sperm and cilia. Gene 2007, 396, 93–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krassas, G.E.; Markou, K.B. The impact of thyroid diseases starting from birth on reproductive function. Hormones 2019, 18, 365–381. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Vita, R.; Condorelli, R.A.; Mongioì, L.M.; Presti, S.; Benvenga, S.; Calogero, A.E. Impact of thyroid disease on testicular function. Endocrine 2017, 58, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Churilov, L.P.; Stroev, Y.I.; Serdyuk, I.Y.; Kaminova-Mudzhikova, O.M.; Belyaeva, I.V.; Gvozdetsky, A.N.; Nitsa, N.A.; Mikhailova, L.R. Autoimmune thyroiditis: Centennial jubilee of a social disease and its comorbidity. Pathophysiology 2014, 21, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Linquette, M.; Fossati, P. Hyperprolactinismes et anti-prolactiniques. Sem. Hop. 1977, 53, 1431-8. [Google Scholar] [PubMed]
- Borba, V.V.; Zandman-Goddard, G.; Shoenfeld, Y. Prolactin and autoimmunity: The hormone as an inflammatory cytokine. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101324. [Google Scholar] [CrossRef]
- Krassas, G.E.; Poppe, K.; Glinoer, D. Thyroid function and human reproductive health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar] [CrossRef]
- Gubbi, S.; Hannah-Shmouni, F.; Verbalis, J.G.; Koch, C.A. Hypophysitis: An update on the novel forms, diagnosis and management of disorders of pituitary inflammation. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101371. [Google Scholar] [CrossRef] [PubMed]
- Iukhta, A.I.; Stroev, I.; Churilov, L.P. Reproductiveness in men with Hashimoto’s thyroiditis. Health Bas. Hum. Potential Probl. Ways Solve Them. 2020, 15, 314–322. (In Russian) [Google Scholar]
- Iukhta, A.I. On the pathogenesis of male infertility in Hashimoto’s thyroiditis. In Proceedings of the Actual Problems of Biomedicine—2021: Proceedings of the XXVII All-Russia’s Conference of Young Scientists with International Participation, St. Petersburg, Russia, 25–26 March 2021; I.P. Pavlov First SPbSMU Publisher: Saint Petersburg, Russia, 2021; pp. 134–136. (In Russian). [Google Scholar]
- Churilov, L.P.; Stroev, I.; Mudzhikova, O.M. Ageing, thyroid and autoallergy: New insight into pathogenesis and treatment. Wien. Klin. Wochenschr. 2009, 121, 70–71. Available online: https://link.springer.com/content/pdf/10.1007/s00508-009-1195-6.pdf (accessed on 12 September 2021).
- Mihara, S.; Suzuki, N.; Wakisaka, S.; Suzuki, S.; Sekita, N.; Yamamoto, S.; Saito, N.; Hoshino, T.; Sakane, T. Effects of thyroid hormones on apoptotic cell death of human lymphocytes. J. Clin. Endocrinol. Metab. 1999, 84, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Dasu, N.; Khalid, Y.; Panuganti, S.; Daly, S. Amiodarone induced epididymo-orchitis. Urol. Case Rep. 2019, 26, 100929. [Google Scholar] [CrossRef] [PubMed]
- Safarian, G.K.; Niauri, D.A.; Borodina, Y.S.; Dzhemlikhanova, L.K.; Gzgzyan, A.M. Gender characteristics of autoimmune hypogonadism. Vestn. St. Petersburg Univ. 2020, 15, 9–23. [Google Scholar] [CrossRef]
- Fichorova, R.; Nakov, L.; Baleva, M.; Nikolov, K.; Gegova, I. Sperm, nuclear, phospholipid, and red blood cell antibodies and isotype RF in infertile couples and patients with autoimmune rheumatic diseases. Am. J. Reprod. Immunol. 1996, 36, 309–316. [Google Scholar] [CrossRef]
- Silva, C.A.; Cocuzza, M.; Borba, E.F.; Bonfá, E. Cutting-edge issues in autoimmune orchitis. Clin. Rev. Allergy Immunol. 2012, 42, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Shibahara, H.; Koriyama, J.; Hirano, Y.; Okazaki, H.; Minota, S.; Suzuki, M. Incidence of antisperm antibodies in males with systemic autoimmune diseases. Am. J. Reprod. Immunol. 2009, 61, 183–189. [Google Scholar] [CrossRef]
- Sikka, S.C.; Hellstrom, W.J.G. Tests for antisperm antibodies. Infertil. Male 2019, 603–612. [Google Scholar] [CrossRef]
- Ferguson, A.C. Detection of antisperm antibodies in equine semen using the immunobead test. Anim. Reprod. Sci. 2010, 121S, 151–152. [Google Scholar] [CrossRef]
- WHO. Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO Press: Geneva, Switzerland, 2021; p. 292. [Google Scholar]
- Shibahara, H.; Wakimoto, Y.; Fukui, A.; Hasegawa, A. Anti-sperm antibodies and reproductive failures. Am. J. Reprod. Immunol. 2021, 85, e13337. [Google Scholar] [CrossRef]
- Barbonetti, A.; Castellini, C.; D’Andrea, S.; Cordeschi, G.; Santucci, R. Prevalence of anti-sperm antibodies and relationship of degree of sperm auto-immunization to semen parameters and post-coital test outcome: A retrospective analysis of over 10 000 men. Hum. Reprod. 2019, 34, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Barbonetti, A.; Castellini, C.; D’Andrea, S.; Minaldi, E.; Totaro, M. Relationship between natural and intrauterine insemination-assisted live births and the degree of sperm autoimmunization. Hum. Reprod. 2020, 35, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chereshnev, V.A.; Pichugova, S.V.; Beikin, Y.B.; Chereshneva, M.V.; Iukhta, A.I.; Stroev, Y.I.; Churilov, L.P. Pathogenesis of Autoimmune Male Infertility: Juxtacrine, Paracrine, and Endocrine Dysregulation. Pathophysiology 2021, 28, 471-488. https://doi.org/10.3390/pathophysiology28040030
Chereshnev VA, Pichugova SV, Beikin YB, Chereshneva MV, Iukhta AI, Stroev YI, Churilov LP. Pathogenesis of Autoimmune Male Infertility: Juxtacrine, Paracrine, and Endocrine Dysregulation. Pathophysiology. 2021; 28(4):471-488. https://doi.org/10.3390/pathophysiology28040030
Chicago/Turabian StyleChereshnev, Valeriy A., Svetlana V. Pichugova, Yakov B. Beikin, Margarita V. Chereshneva, Angelina I. Iukhta, Yuri I. Stroev, and Leonid P. Churilov. 2021. "Pathogenesis of Autoimmune Male Infertility: Juxtacrine, Paracrine, and Endocrine Dysregulation" Pathophysiology 28, no. 4: 471-488. https://doi.org/10.3390/pathophysiology28040030
APA StyleChereshnev, V. A., Pichugova, S. V., Beikin, Y. B., Chereshneva, M. V., Iukhta, A. I., Stroev, Y. I., & Churilov, L. P. (2021). Pathogenesis of Autoimmune Male Infertility: Juxtacrine, Paracrine, and Endocrine Dysregulation. Pathophysiology, 28(4), 471-488. https://doi.org/10.3390/pathophysiology28040030






