Preeclampsia Status Controls Interleukin-6 and Soluble IL-6 Receptor Release from Neutrophils and Endothelial Cells: Relevance to Increased Inflammatory Responses
Abstract
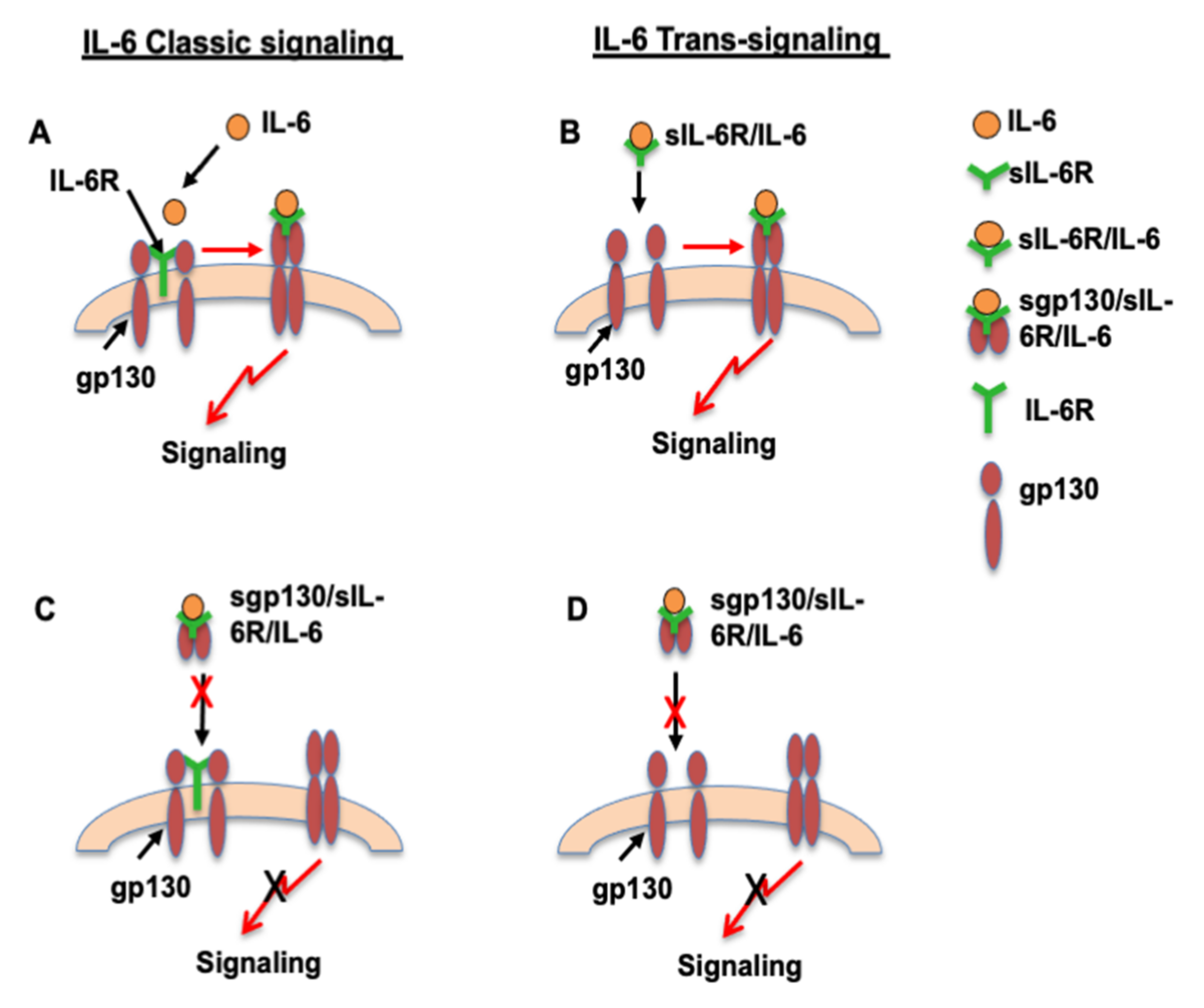
1. Introduction
2. Materials and Methods
2.1. Study Samples
2.2. HUVEC Isolation and Culture
2.3. Neutrophil Isolation and Culture
2.4. Preparation of Placental Conditioned Medium
2.5. Measurement of IL-6, sIL-6R, and sgp130
2.6. Statistical Analysis
3. Results
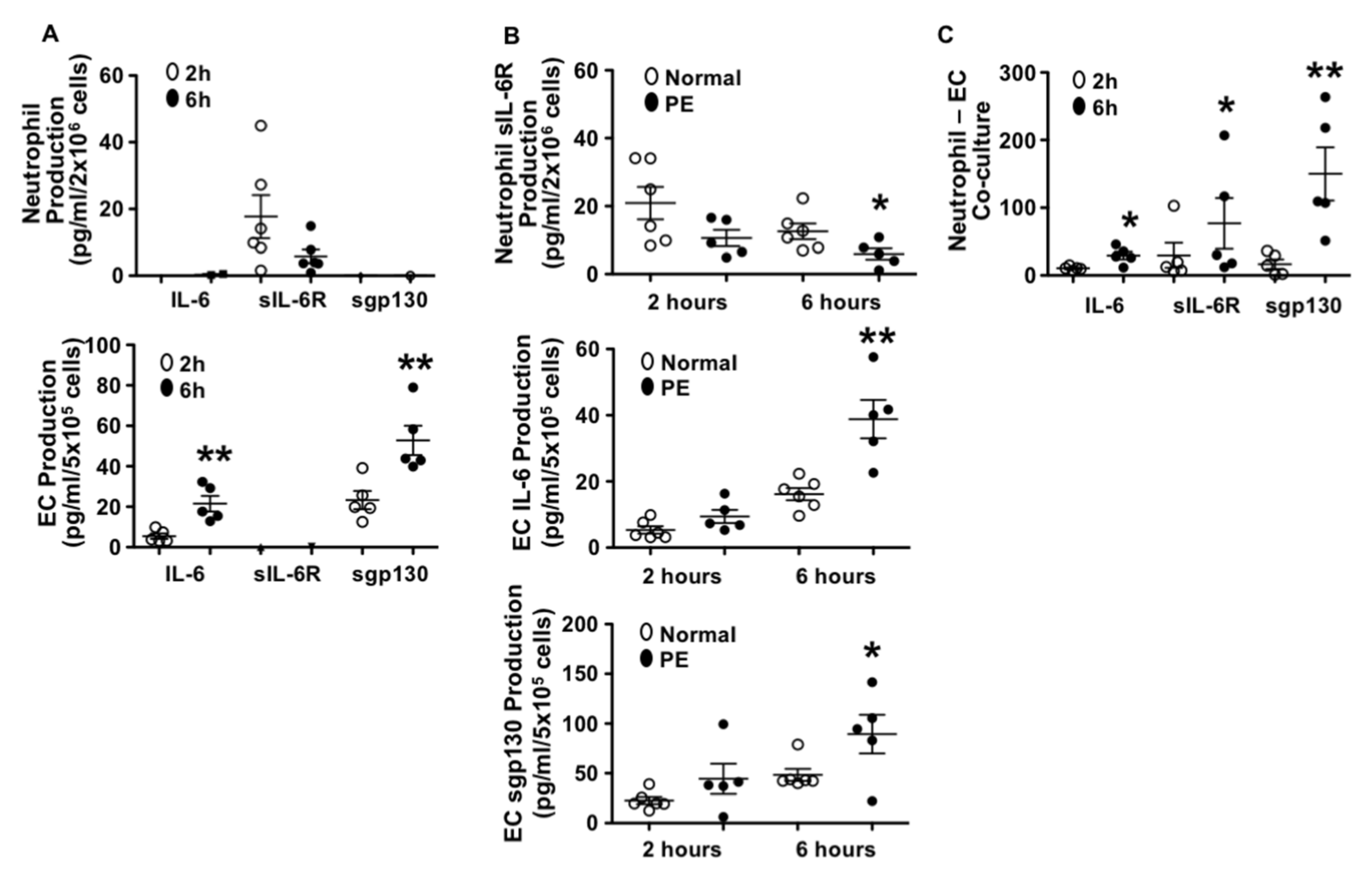
3.1. Different Patterns in IL-6, sIL-6R, and sgp130 Production by Endothelial Cells and Neutrophils from Normal Pregnant Women
3.2. Neutrophils Produced Less sIL-6R and Endothelial Cells Produced More IL-6 and sgp130 from Preeclamptic than Those from Normal Pregnancies
3.3. Production of IL-6, sIL-6R, and sgp130 in Neutrophil–Endothelial Co-Culture
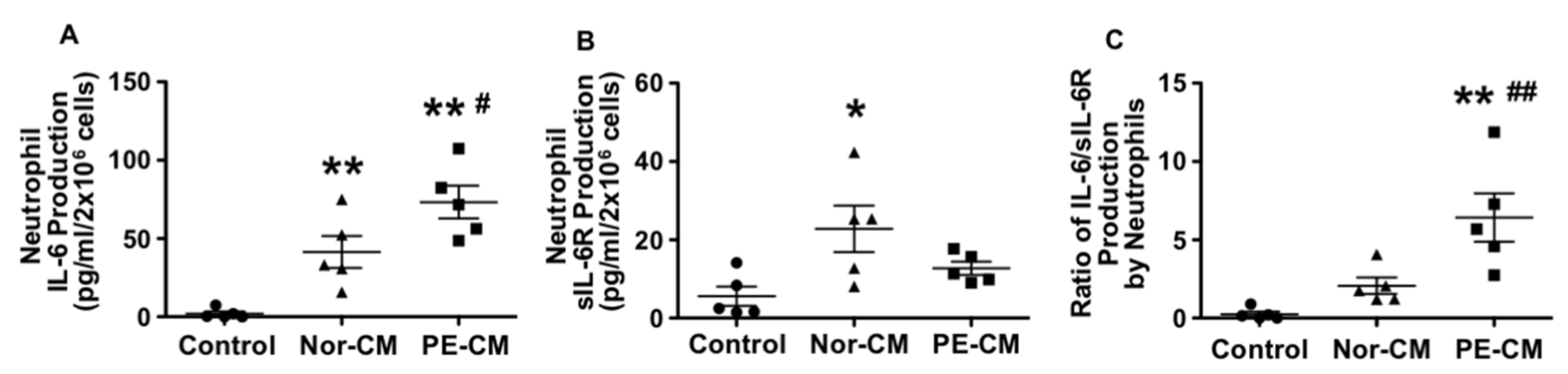
3.4. Effects of Placenta on Neutrophil Production of IL-6, sIL-6R, and sgp130
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Redman, C.W.G.; Sacks, G.P.; Sargent, I.L. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am. J. Obstet. Gynecol. 1999, 180, 499–506. [Google Scholar] [CrossRef]
- Vince, G.S.; Starkey, P.M.; Austgulen, R.; Kwiatkowski, D.; Redman, C.W.G. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br. J. Obstet. Gynaecol. 1995, 102, 20–25. [Google Scholar] [CrossRef]
- Conrad, K.P.; Benyo, D.F. Placental cytokines and the pathogenesis of preeclampsia. Am. J. Reprod. Immunol. 1997, 37, 240–249. [Google Scholar] [CrossRef]
- Lewis, D.F.; Canzoneri, B.J.; Wang, Y. Maternal circulating TNFα levels are highly correlated with IL-10 levels, but not IL-6 and IL-8 levels, in women with preeclampsia. Am. J. Reprod. Immunol. 2006, 62, 269–274. [Google Scholar] [CrossRef]
- Heyl, W.; Handt, S.; Reister, F.; Gehlen, J.; Schroder, W.; Mittermayer, C.; Rath, W. Elevated soluble adhesion molecules in women with pre-eclampsia. Do cytokines like tumour necrosis factor-alpha and interleukin-1beta cause endothelial activation. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 86, 35–41. [Google Scholar] [CrossRef]
- Austgulen, R.; Lien, E.; Vince, G.; Redman, C.W. Increased maternal plasma levels of soluble adhesion molecules (ICAM-1, VCAM-1, E-selectin) in preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997, 71, 53–58. [Google Scholar] [CrossRef]
- Greer, I.A.; Haddad, N.G.; Dawes, J.; Johnstone, F.D.; Calder, A.A. Neutrophil activation in pregnancy-induced hypertension. Br. J. Obstet. Gynaecol. 1989, 96, 978–982. [Google Scholar] [CrossRef]
- Holthe, M.R.; Staff, A.C.; Berge, L.N.; Lyberg, T. Leukocyte adhesion molecules and reactive oxygen species in preeclampsia. Obstet. Gynecol. 2004, 103, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Hurst, S.M.; Wilkinson, T.S.; McLoughlin, R.M.; Jones, S.; Horiuchi, S.; Yamamoto, N.; Rose-John, S.; Fuller, G.M.; Topley, N.; Jones, S.A. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 2001, 14, 705–714. [Google Scholar] [CrossRef]
- Jones, S.A. Directing transition from innate to acquired immunity: Defining a role for IL-6. J. Immunol. 2005, 175, 3463–3468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lewis, D.F.; Gu, Y.; Zhao, S.; Groome, L.J. Elevated maternal soluble gp130 and IL-6 levels and reduced gp130 and SOCS-3 expressions in women with preeclampsia. Hypertension 2011, 57, 336–342. [Google Scholar] [CrossRef]
- Krebs, D.L.; Hilton, D.J. SOCS proteins: Negative regulators of cytokine signaling. Stem Cells 2001, 19, 378–387. [Google Scholar] [CrossRef]
- Alexander, W.S.; Starr, R.; Metcalf, D.; Nicholson, S.E.; Farley, A.; Elefanty, A.G.; Brysha, M.; Kile, B.T.; Richardson, R.; Baca, M.; et al. Suppressors of cytokine signaling (SOCS): Negative regulators of signal transduction. J. Leukoc. Biol. 1999, 66, 588–592. [Google Scholar] [CrossRef]
- Croker, B.A.; Krebs, D.L.; Zhang, J.G.; Wormald, S.; Willson, T.A.; Stanley, E.G.; Robb, L.; Greenhalgh, C.J.; Förster, I.; Clausen, B.E.; et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat. Immunol. 2003, 4, 540–545. [Google Scholar] [CrossRef]
- Wang, Y.; Adair, C.D.; Coe, L.; Weeks, J.W.; Lewis, D.F.; Alexander, J.S. Activation of endothelial cells in preeclampsia: Increased neutrophil-endothelial adhesion correlates with up-regulation of adhesion molecule P-selectin in human umbilical vein endothelial cells isolated from preeclampsia. J. Soc. Gynecol. Investig. 1998, 5, 237–243. [Google Scholar]
- Markert, M.; Andrews, P.C.; Babior, B.M. Measurement of O2- production by human neutrophils. The preparation and assay of NADPH oxidase-containing particles from human neutrophils. Methods Enzymol. 1984, 105, 358–365. [Google Scholar]
- Wang, Y.; Adair, C.D.; Weeks, J.W.; Lewis, D.F.; Alexander, J.S. Increased neutrophil-endothelial adhesion induced by placental factors is mediated by platelet-activating factor in preeclampsia. J. Soc. Gynecol. Investig. 1999, 6, 136–141. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Y.; Lucas, M.J. Expression of thrombin receptors in endothelial cells and neutrophils from normal and preeclamptic pregnancies. J. Clin. Endocrinol. Metab. 2002, 87, 3728–3734. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Gu, Y.; Philibert, L.; Lucas, M.J. Neutrophil activation induced by placental factors in normal and pre-eclamptic pregnancies in vitro. Placenta 2001, 22, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.; Boswell, F.; Greer, I.A. The neutrophil and preeclampsia. Sem. Reprod. Endocrinol. 1998, 16, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.; Graham, D.; Beilin, L.J.; Ritchie, J.; Baker, R.; Walters, B.N.; Michael, C.A. Neutrophil CD 11B expression and neutrophil activation in pre-eclampsia. Clin. Sci. 1997, 92, 37–44. [Google Scholar] [CrossRef]
- Jostock, T.; Müllberg, J.; Ozbek, S.; Atreya, R.; Blinn, G.; Voltz, N.; Fischer, M.; Neurath, M.F.; Rose-John, S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur. J. Biochem. 2001, 268, 160–167. [Google Scholar] [CrossRef]
- Rose-John, S. IL-6 trans-signaling via the soluble IL-6 receptor: Importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef]
- IL6R Genetics Consortium Emerging Risk Factors Collaboration; Sarwar, N.; Butterworth, A.S.; Freitag, D.F.; Gregson, J.; Willeit, P. Interleukin-6 receptor pathways in coronary heart disease: A collaborative meta-analysis of 82 studies. Lancet 2012, 379, 1205–1213. [Google Scholar]
- Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium; Hingorani, A.D.; Casas, J.P. The interleukin-6 receptor as a target for prevention of coronary heart disease: A mendelian randomisation analysis. Lancet 2012, 379, 1214–1224. [Google Scholar]
- Yudkin, J.S.; Kumari, M.; Humphries, S.E.; Mohamed-Ali, V. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis 2000, 148, 209–214. [Google Scholar] [CrossRef]
- Luc, G.; Bard, J.M.; Juhan-Vague, I.; Ferrieres, J.; Evans, A.; Amouyel, P.; Arveiler, D.; Fruchart, J.C.; Ducimetiere, P.; PRIME Study Group. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: The PRIME Study. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Pauleau, A.L.; Parganas, E.; Takahashi, Y.; Mages, J.; Ihle, J.N.; Rutschman, R.; Murray, P.J. SOCS3 regulates the plasticity of gp130 signaling. Nat. Immunol. 2003, 4, 546–550. [Google Scholar] [CrossRef]
- Gaillard, J.; Pugnière, M.; Tresca, J.; Mani, J.; Klein, B.; Brochier, J. Interleukin-6 receptor signaling. II. Bio-availability of interleukin-6 in serum. Eur. Cytokine Netw. 1999, 10, 337–344. [Google Scholar] [PubMed]
- Modur, V.; Li, Y.; Zimmerman, G.A.; Prescott, S.M.; McIntyre, T.M. Retrograde inflammatory signaling from neutrophils to endothelial cells by soluble interleukin-6 receptor alpha. J. Clin. Investig. 1997, 100, 2752–2756. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, Y.; Zhang, Y.; Lewis, D.F. Evidence of endothelial dysfunction in preeclampsia: Decreased endothelial nitric oxide synthase expression is associated with increased cell permeability in endothelial cells from preeclampsia. Am. J. Obstet. Gynecol. 2004, 190, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zou, Q.Y.; Li, H.; Wang, R.F.; Liu, A.X.; Magness, R.R.; Zheng, J. Preeclampsia Downregulates MicroRNAs in Fetal Endothelial Cells: Roles of miR-29a/c-3p in Endothelial Function. J. Clin. Endocrinol. Metab. 2017, 102, 3470–3479. [Google Scholar] [CrossRef]
- Mellembakken, J.R.; Aukrust, P.; Olafsen, M.K.; Ueland, T.; Hestdal, K.; Videm, V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension 2002, 39, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Taga, T.; Saito, M.; Suematsu, S.; Kumanogoh, A.; Tanaka, T.; Fujiwara, H.; Hirata, M.; Yamagami, T.; Nakahata, T.; et al. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc. Natl. Acad. Sci. USA 1996, 93, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.J. Cytokines and eicosanoids in rheumatic diseases. Ann. Rheum. Dis. 1990, 49, 207–210. [Google Scholar] [CrossRef] [PubMed]
| Variable | Normal (n = 13) | Preeclampsia (n = 5) | p Value |
|---|---|---|---|
| Maternal Age (years) | 23 ± 5 | 24 ± 4 | 0.6822 |
| Racial Status | |||
| White | 1 | 2 | ND |
| Black | 12 | 2 | ND |
| BMI | 30 ± 6 | 40 ± 7 | 0.0088 |
| Blood Pressure | |||
| Systolic | 119 ± 13 | 179 ± 11 | <0.0001 |
| Diastolic | 75 ± 9 | 109 ± 3 | <0.0101 |
| Primigravida | 10 | 3 | ND |
| Gestational Age (weeks+days) | |||
| at blood draw | 32+5 ± 4+5 | 29+5 ± 2+1 | 0.2401 |
| at delivery | 39+5 ± 1+0 | 33+0 ± 3+1 | 0.0023 |
| Delivery Mode | |||
| Vaginal | 12 | 1 | ND |
| C-section | 1 | 4 | ND |
| Variable | Normal (n = 10) | Preeclampsia (n = 11) | p Value |
|---|---|---|---|
| Maternal Age (years) | 27 ± 7 | 23 ± 5 | 0.1818 |
| Racial Status | |||
| White | 2 | 1 | ND |
| Black | 7 | 10 | ND |
| Other | 1 | 0 | ND |
| BMI | 31 ± 8 | 35 ± 9 | 0.285 |
| Blood Pressure (mmHg) | |||
| Systolic | 114 ± 11 | 165 ± 12 | <0.0001 |
| Diastolic | 70 ± 11 | 101 ± 8 | <0.0001 |
| Primigravida | 5 | 6 | ND |
| Gestational Age (weeks+days) | 39+0 ± 1+1 | 35+0 ± 3+5 | 0.0052 |
| Delivery Mode | |||
| Vaginal | 6 | 5 | ND |
| C-section | 4 | 6 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Gu, Y.; Alexander, J.S.; Lewis, D.F. Preeclampsia Status Controls Interleukin-6 and Soluble IL-6 Receptor Release from Neutrophils and Endothelial Cells: Relevance to Increased Inflammatory Responses. Pathophysiology 2021, 28, 202-211. https://doi.org/10.3390/pathophysiology28020013
Wang Y, Gu Y, Alexander JS, Lewis DF. Preeclampsia Status Controls Interleukin-6 and Soluble IL-6 Receptor Release from Neutrophils and Endothelial Cells: Relevance to Increased Inflammatory Responses. Pathophysiology. 2021; 28(2):202-211. https://doi.org/10.3390/pathophysiology28020013
Chicago/Turabian StyleWang, Yuping, Yang Gu, J. Steven Alexander, and David F. Lewis. 2021. "Preeclampsia Status Controls Interleukin-6 and Soluble IL-6 Receptor Release from Neutrophils and Endothelial Cells: Relevance to Increased Inflammatory Responses" Pathophysiology 28, no. 2: 202-211. https://doi.org/10.3390/pathophysiology28020013
APA StyleWang, Y., Gu, Y., Alexander, J. S., & Lewis, D. F. (2021). Preeclampsia Status Controls Interleukin-6 and Soluble IL-6 Receptor Release from Neutrophils and Endothelial Cells: Relevance to Increased Inflammatory Responses. Pathophysiology, 28(2), 202-211. https://doi.org/10.3390/pathophysiology28020013







