Mathematical Modeling of Ion Quantum Tunneling Reveals Novel Properties of Voltage-Gated Channels and Quantum Aspects of Their Pathophysiology in Excitability-Related Disorders
Abstract
1. Introduction
2. The Mathematical Model
2.1. The Conductance of the Voltage-Gated Channels According to the Laws of Thermodynamics
2.2. The Conductance of the Voltage-Gated Channels According to Quantum Mechanics
3. Results
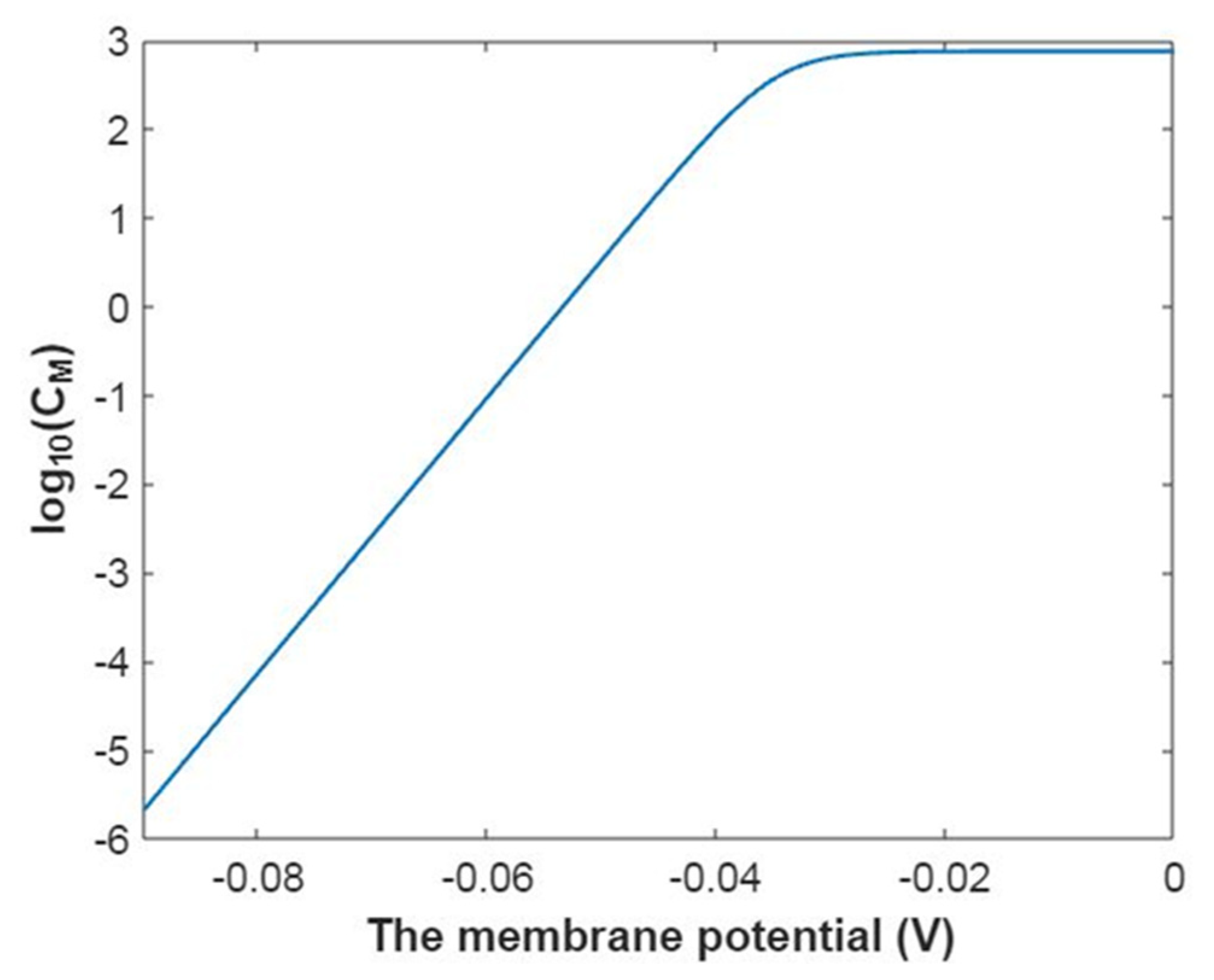
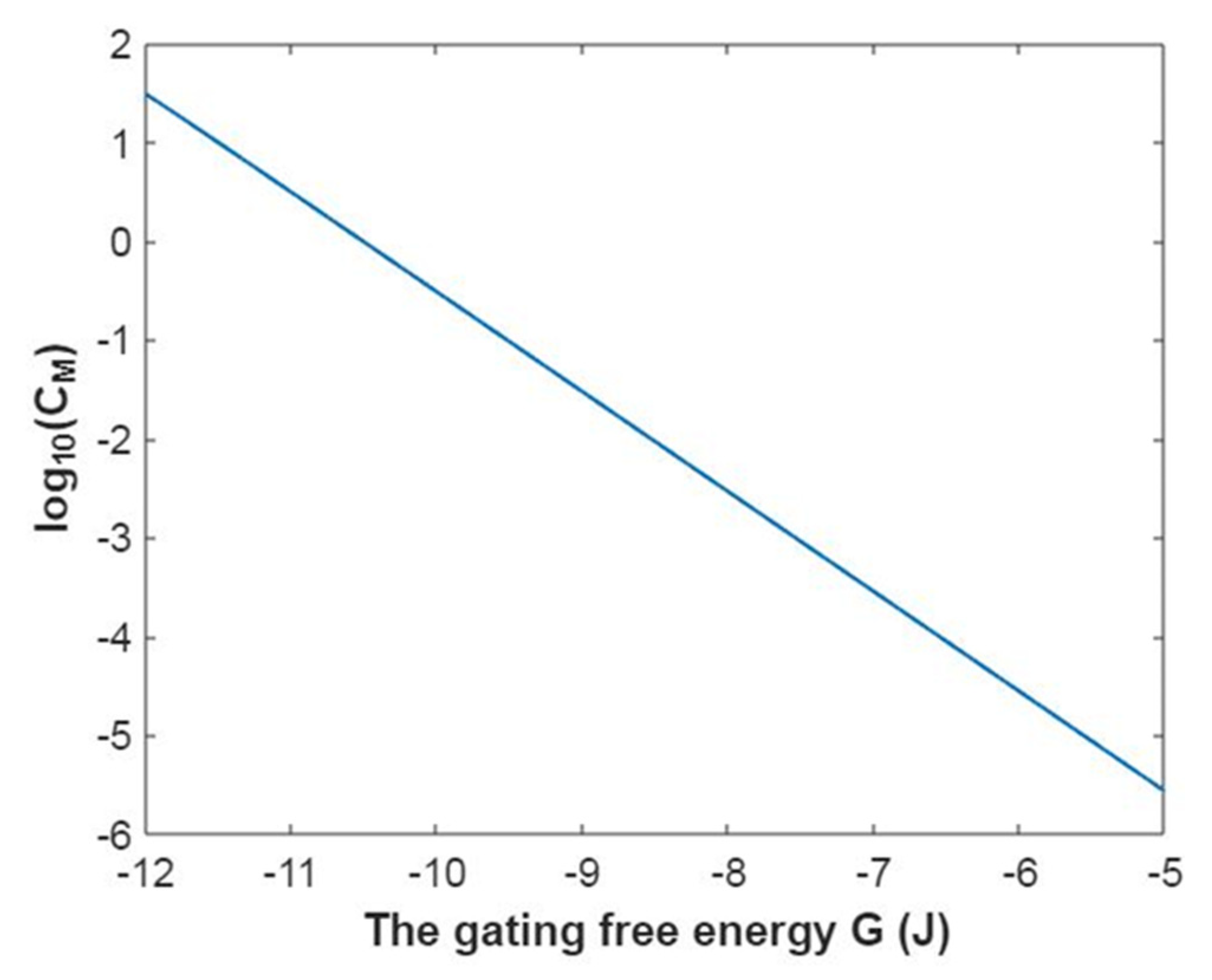
3.1. The Conductance of the Voltage-Gated Sodium Channels According to the Boltzmann Distribution
3.2. The Conductance of the Voltage-Gated Sodium Channels According to the Quantum Mechanics
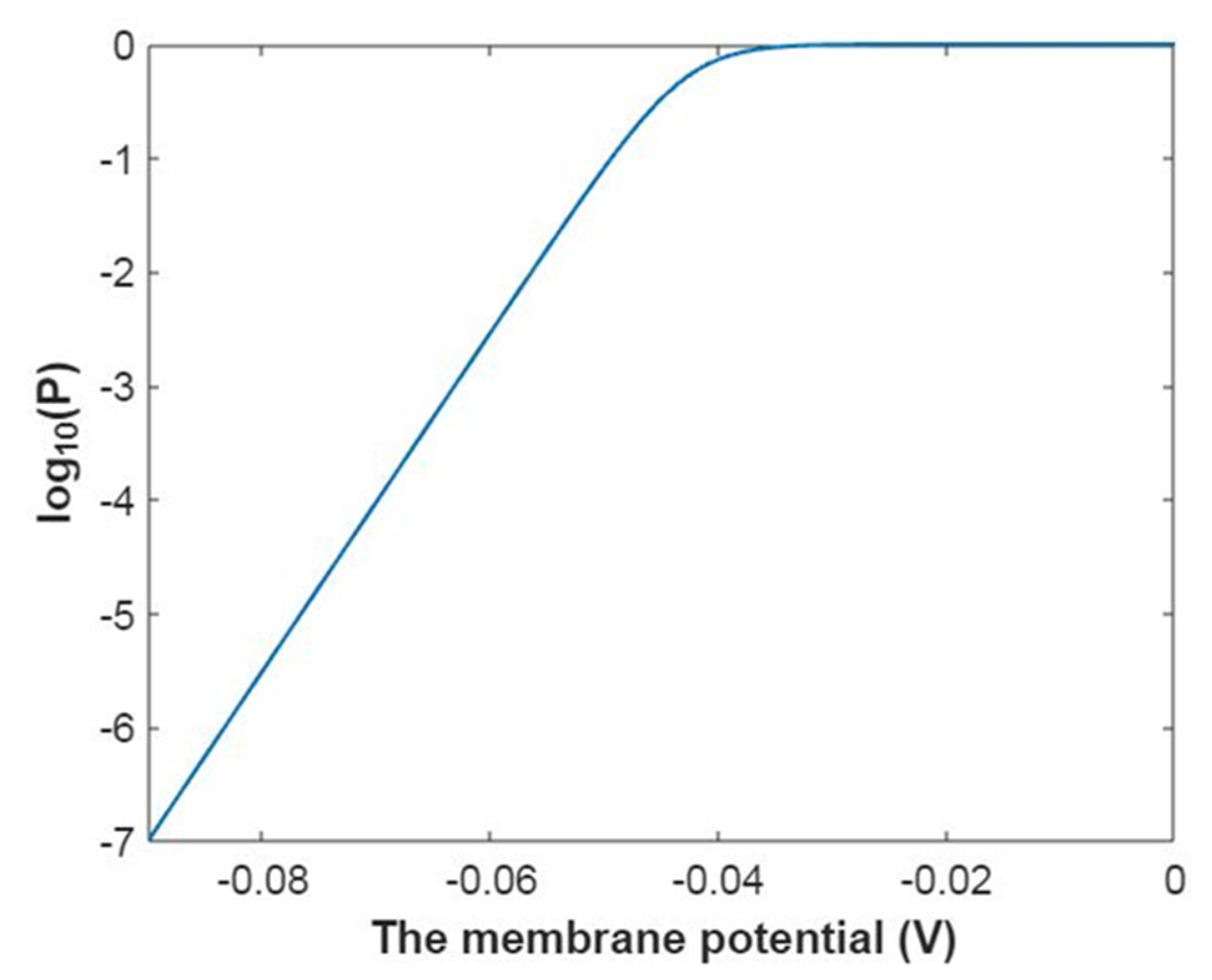
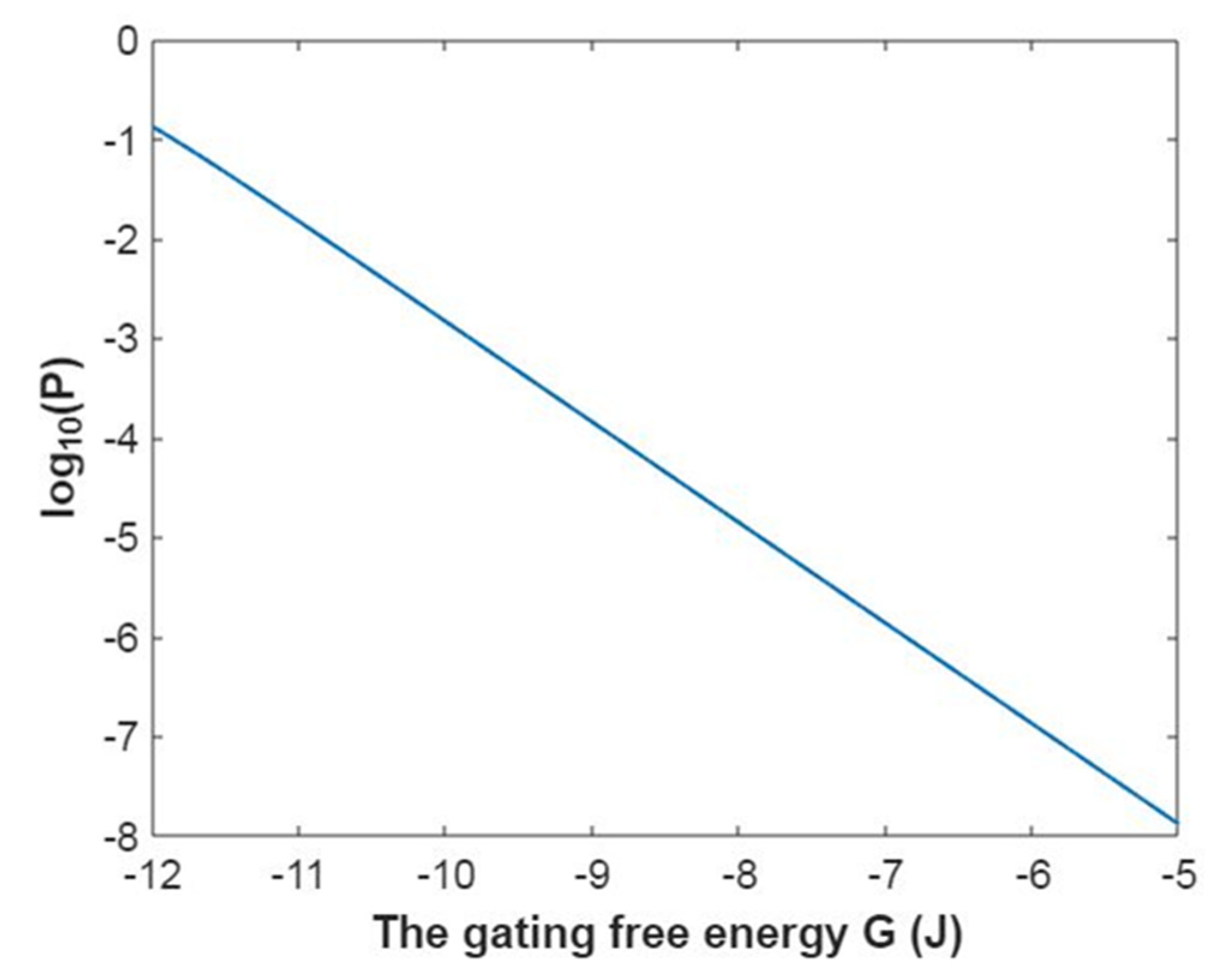
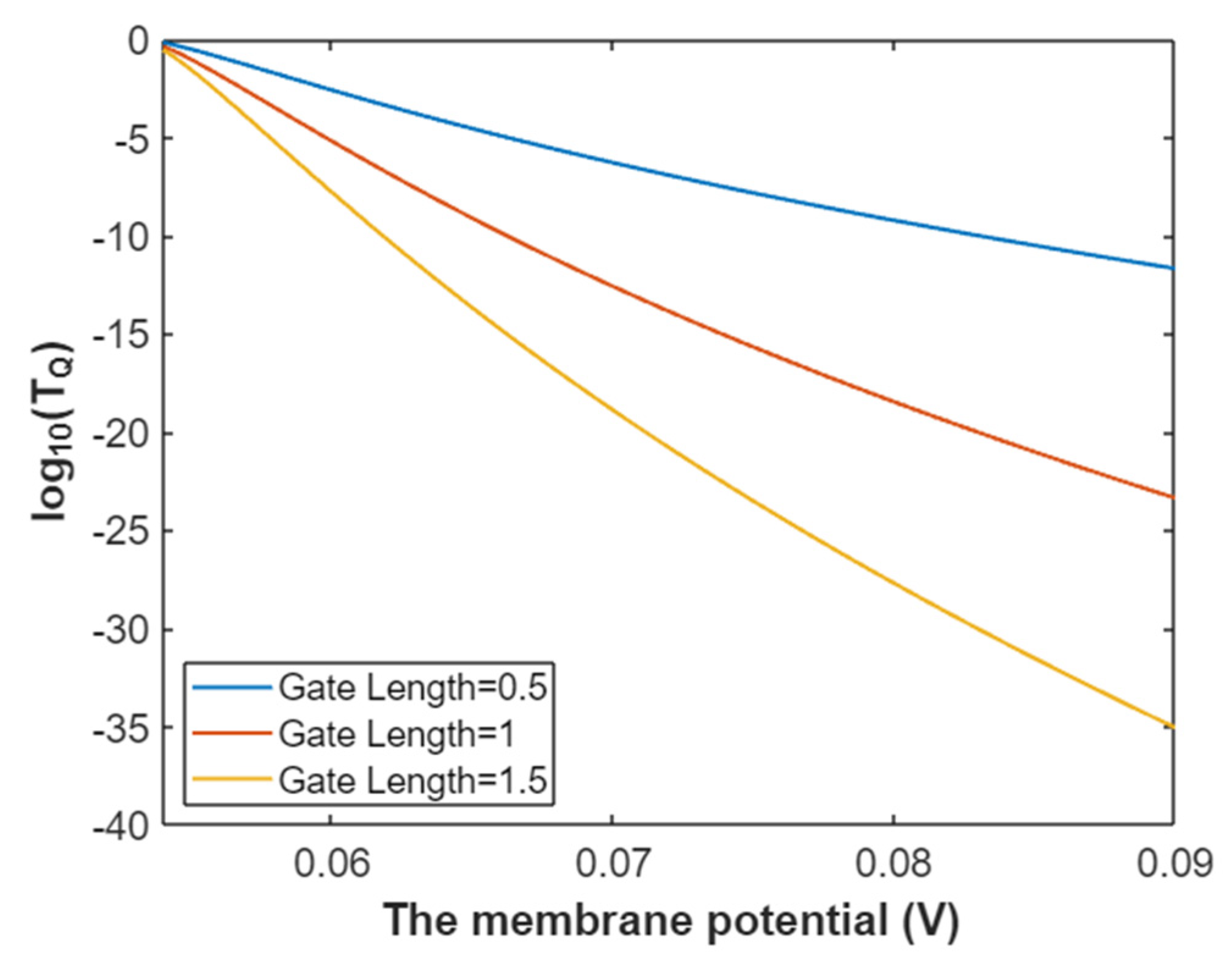
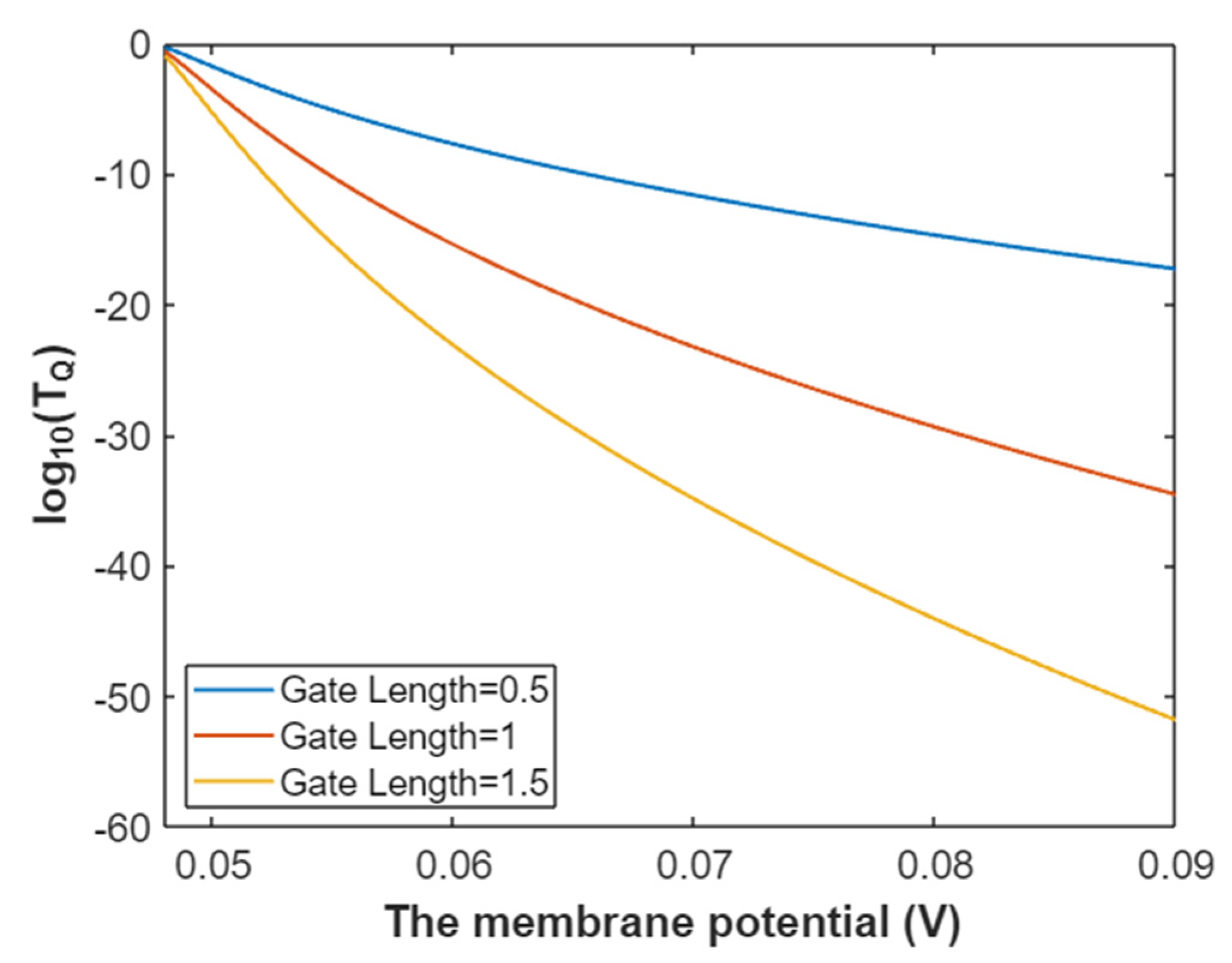
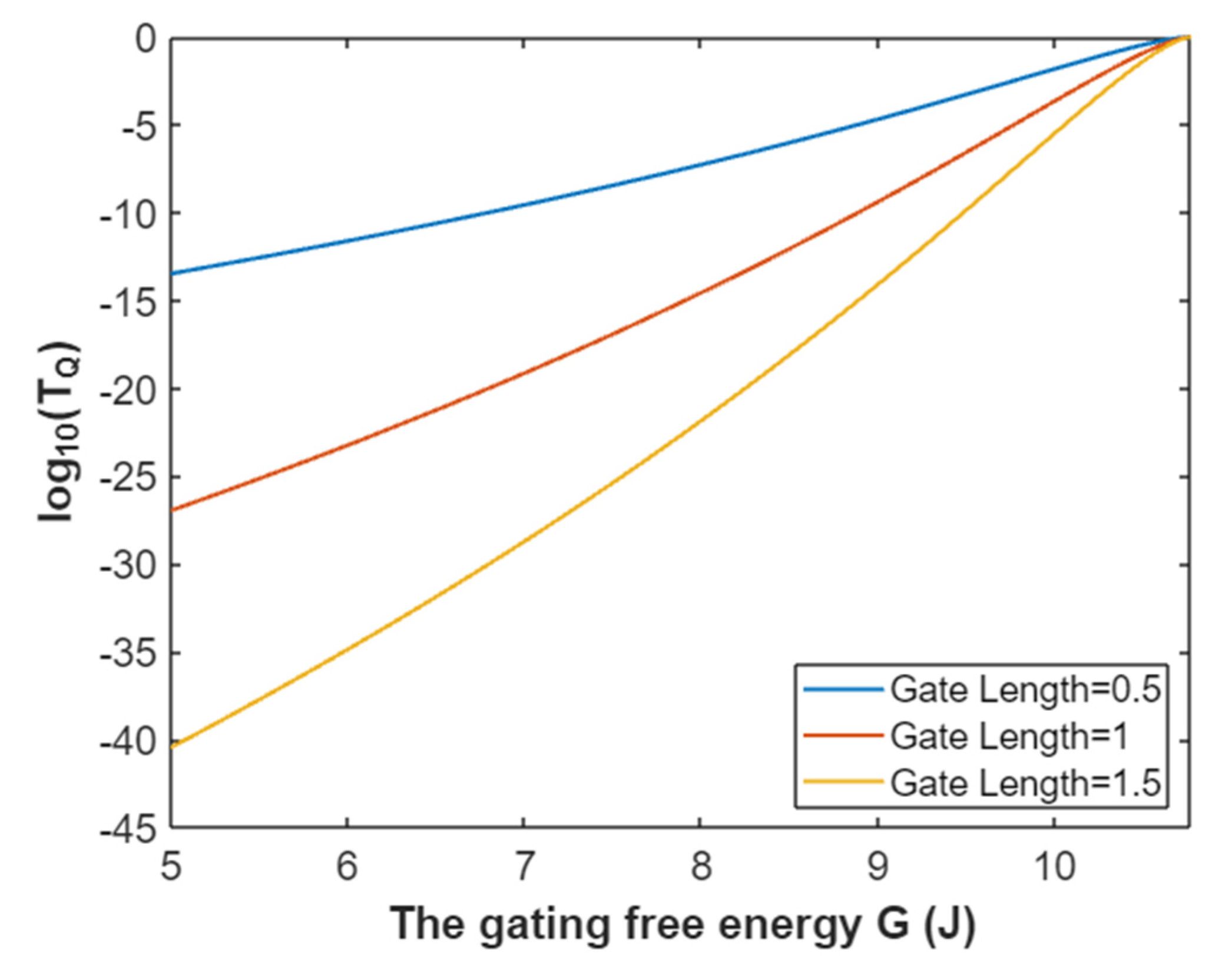
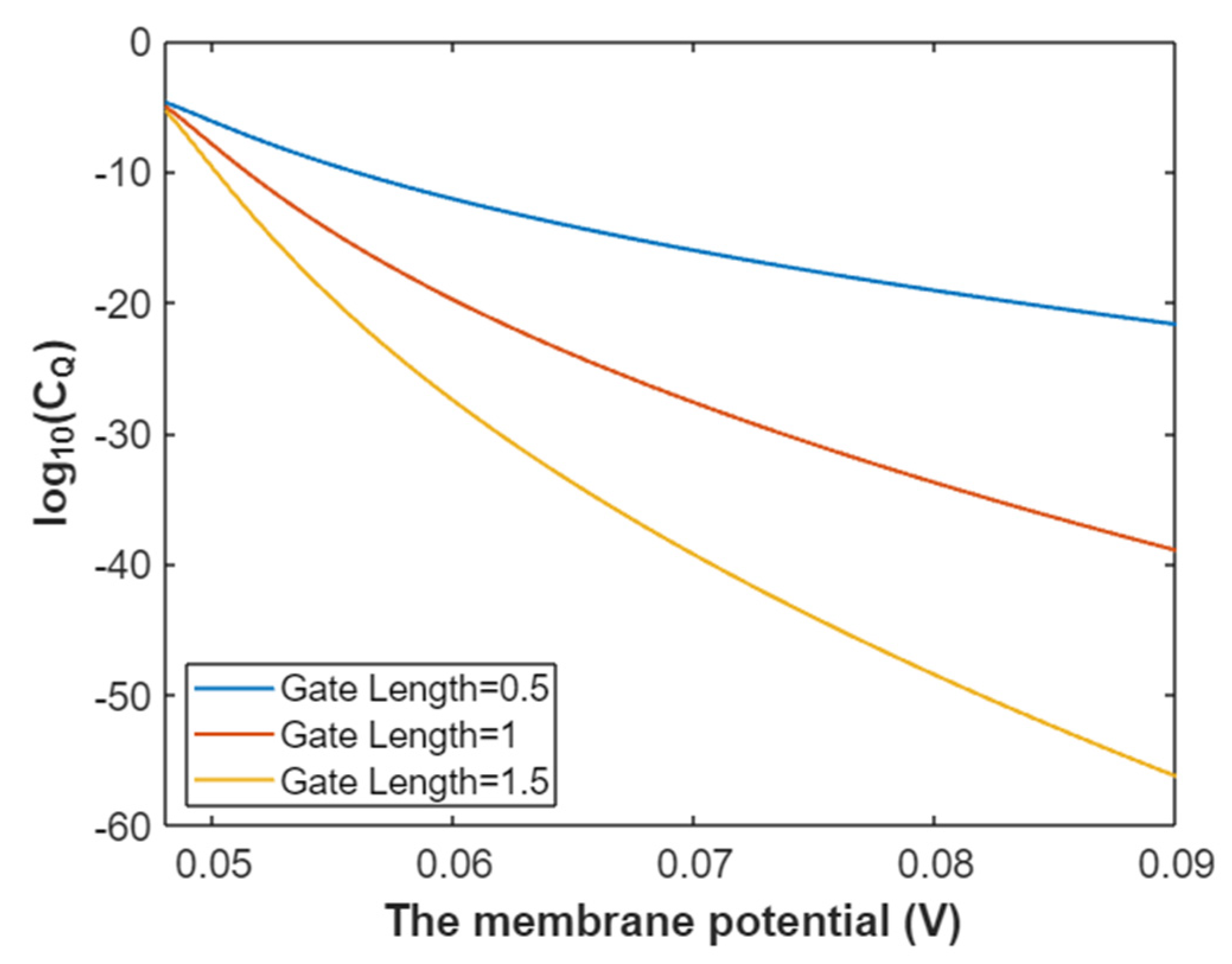
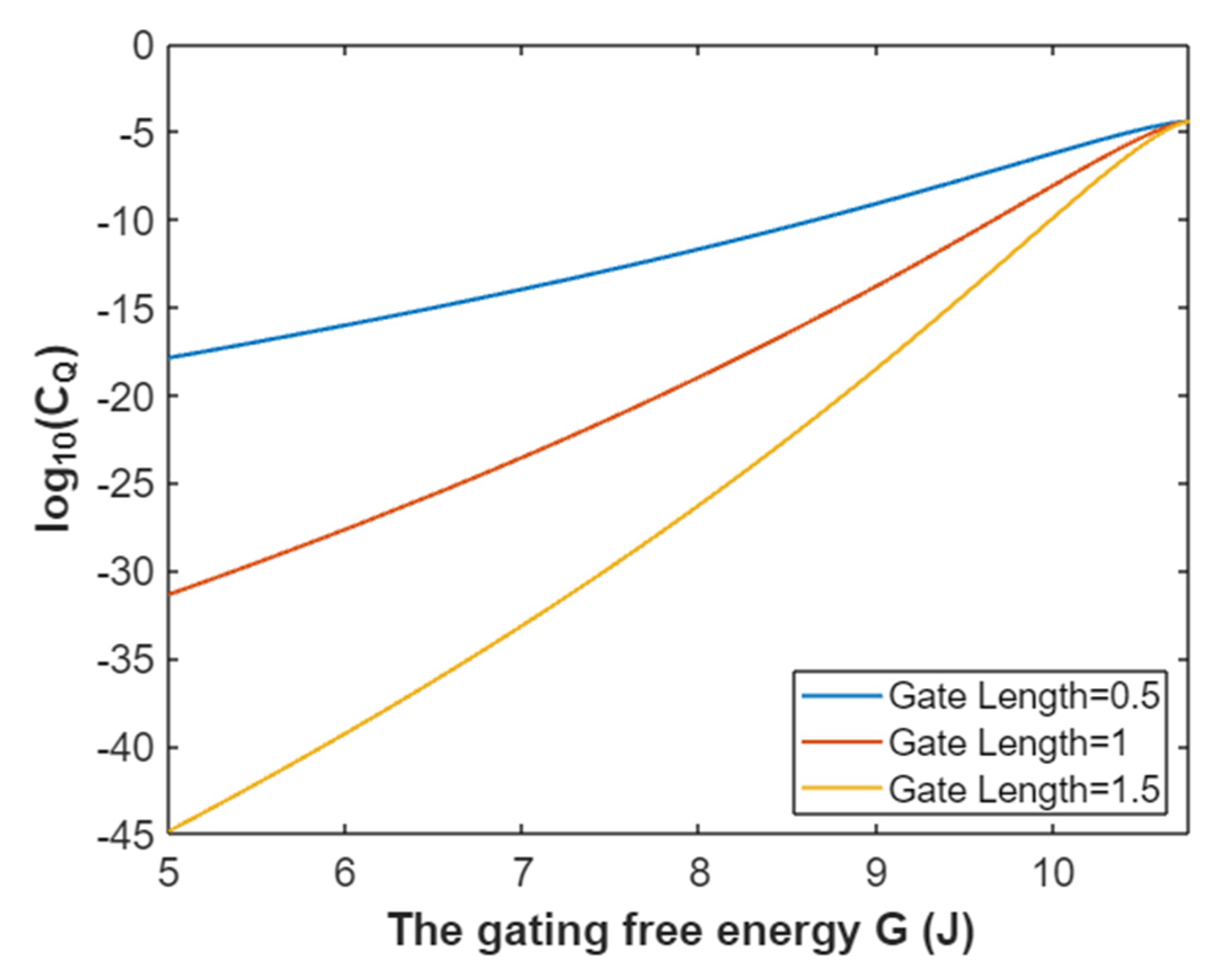
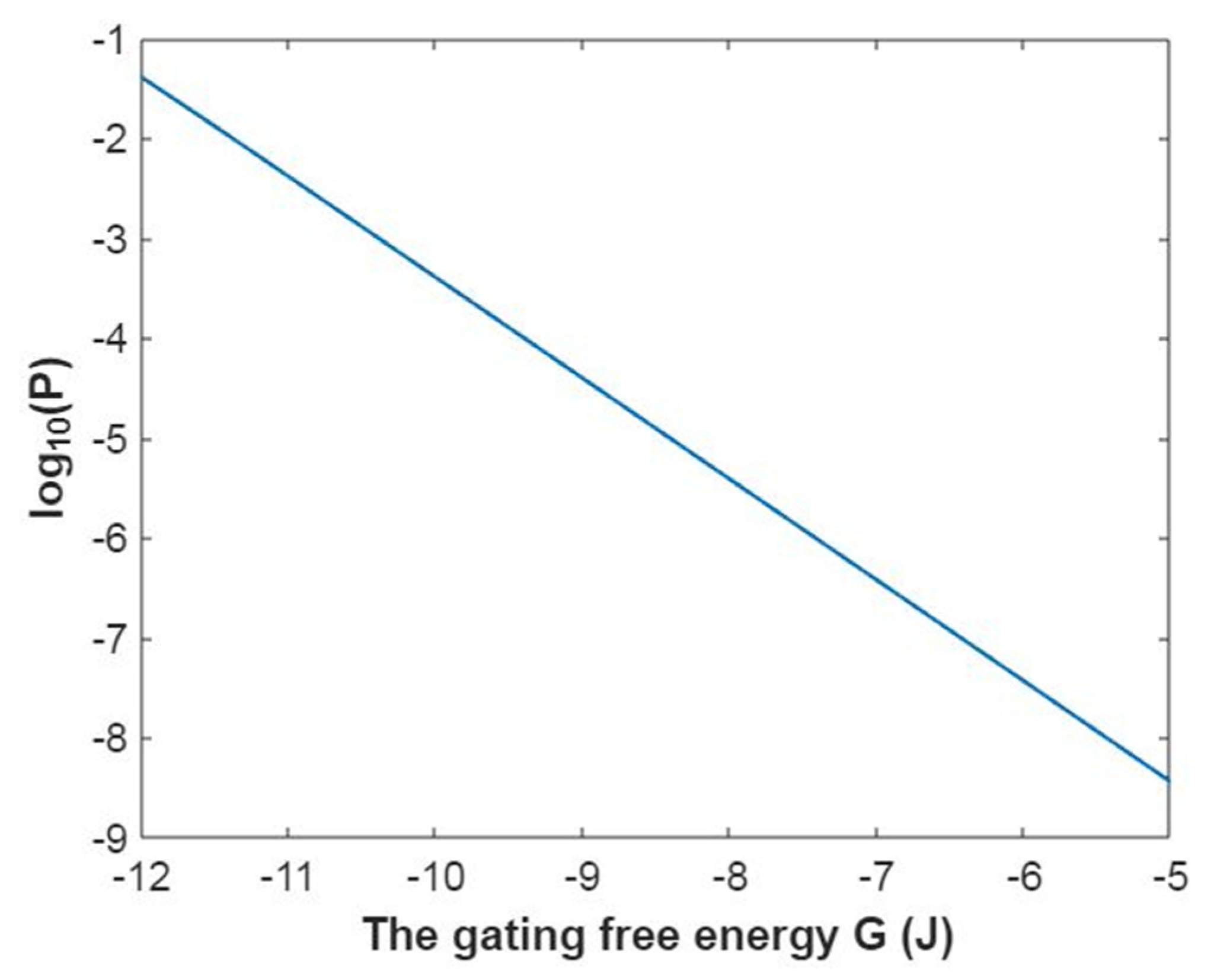
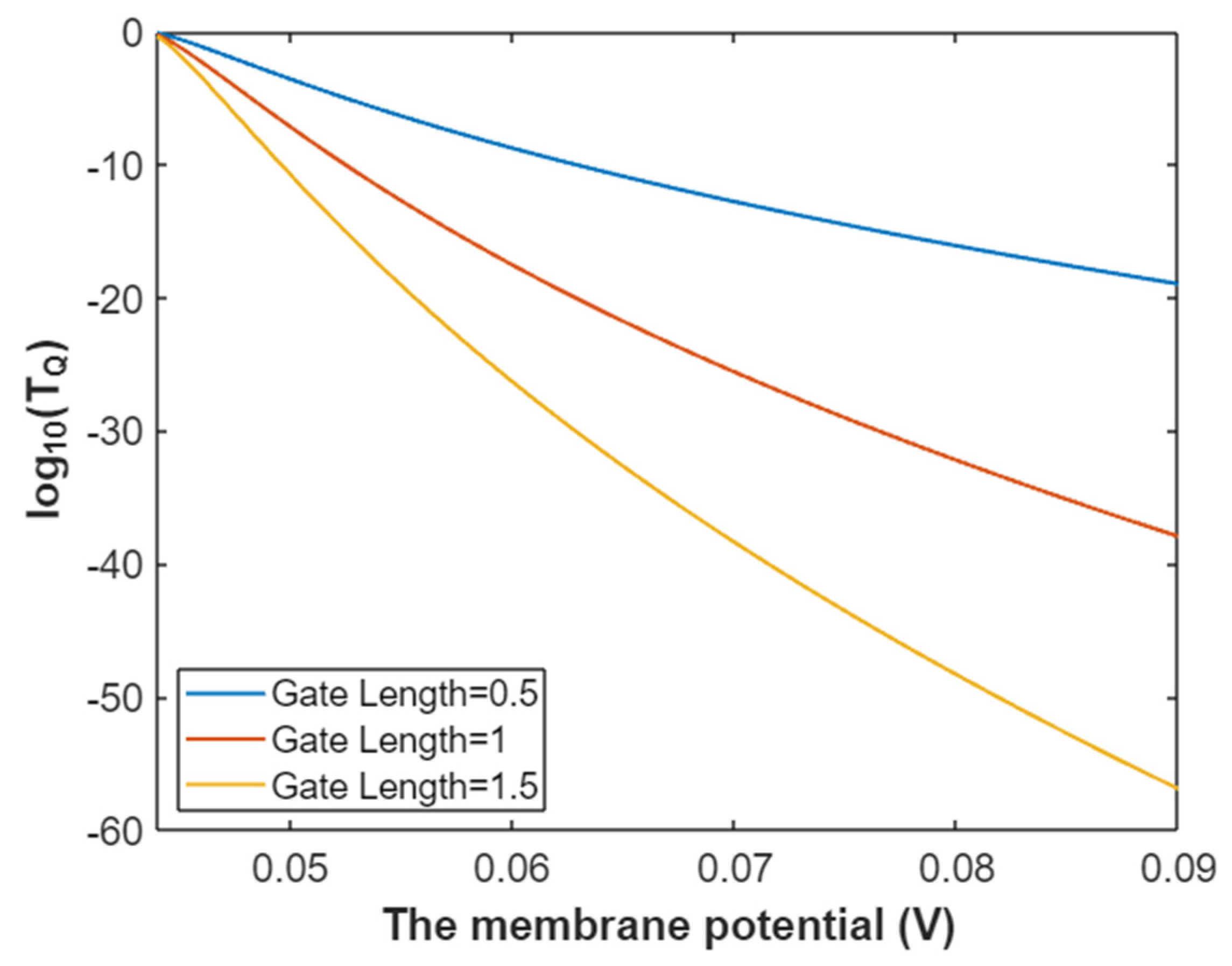
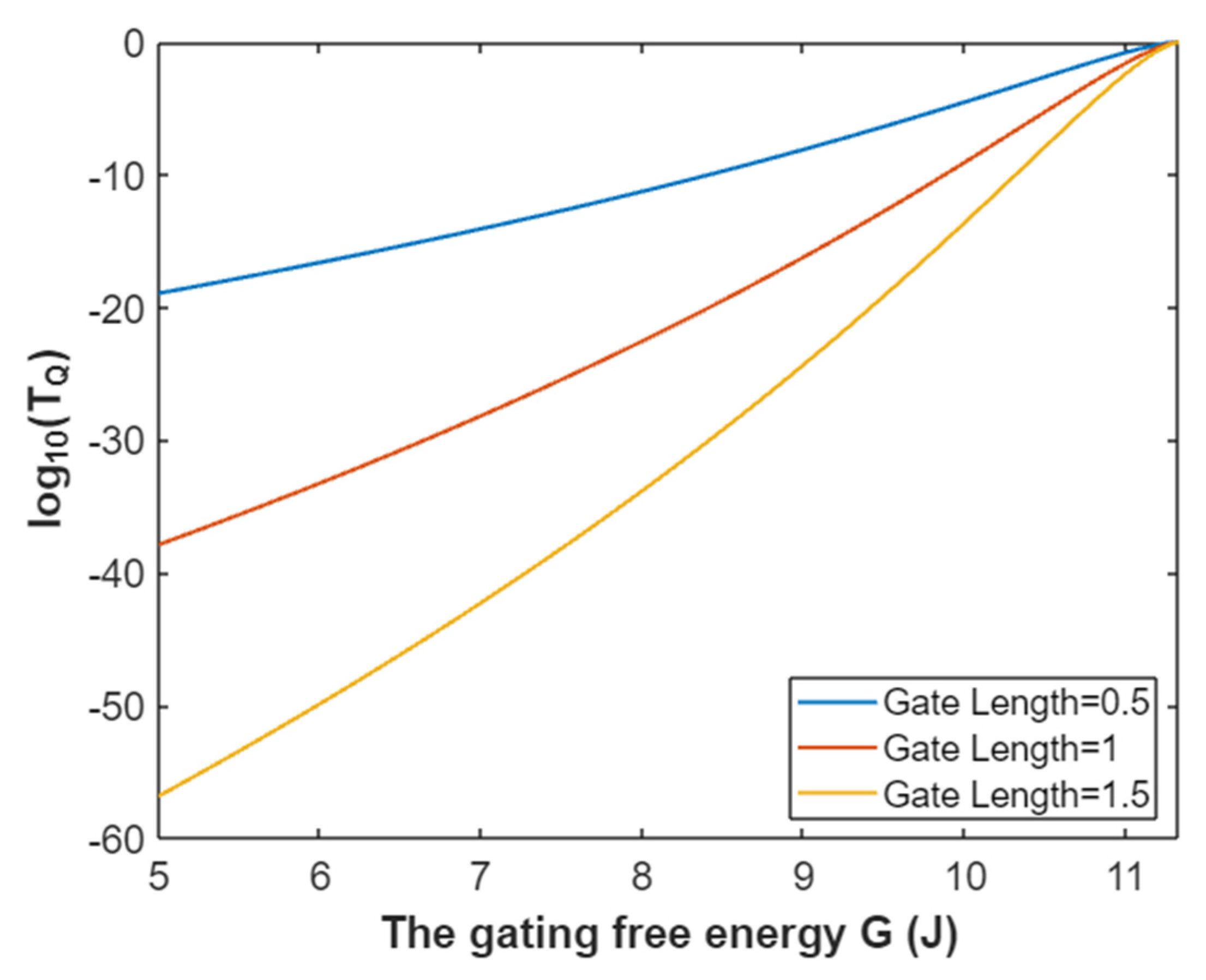
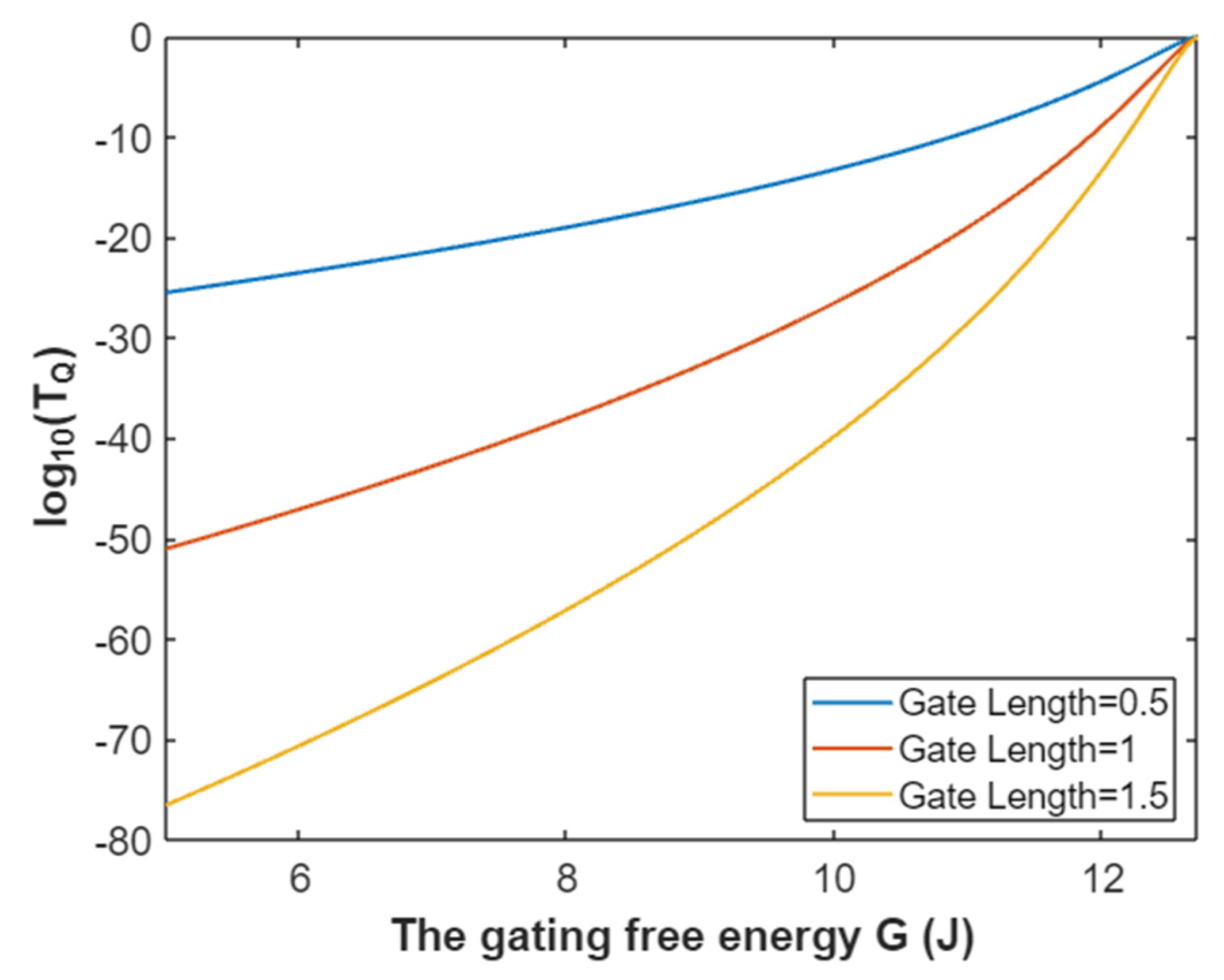
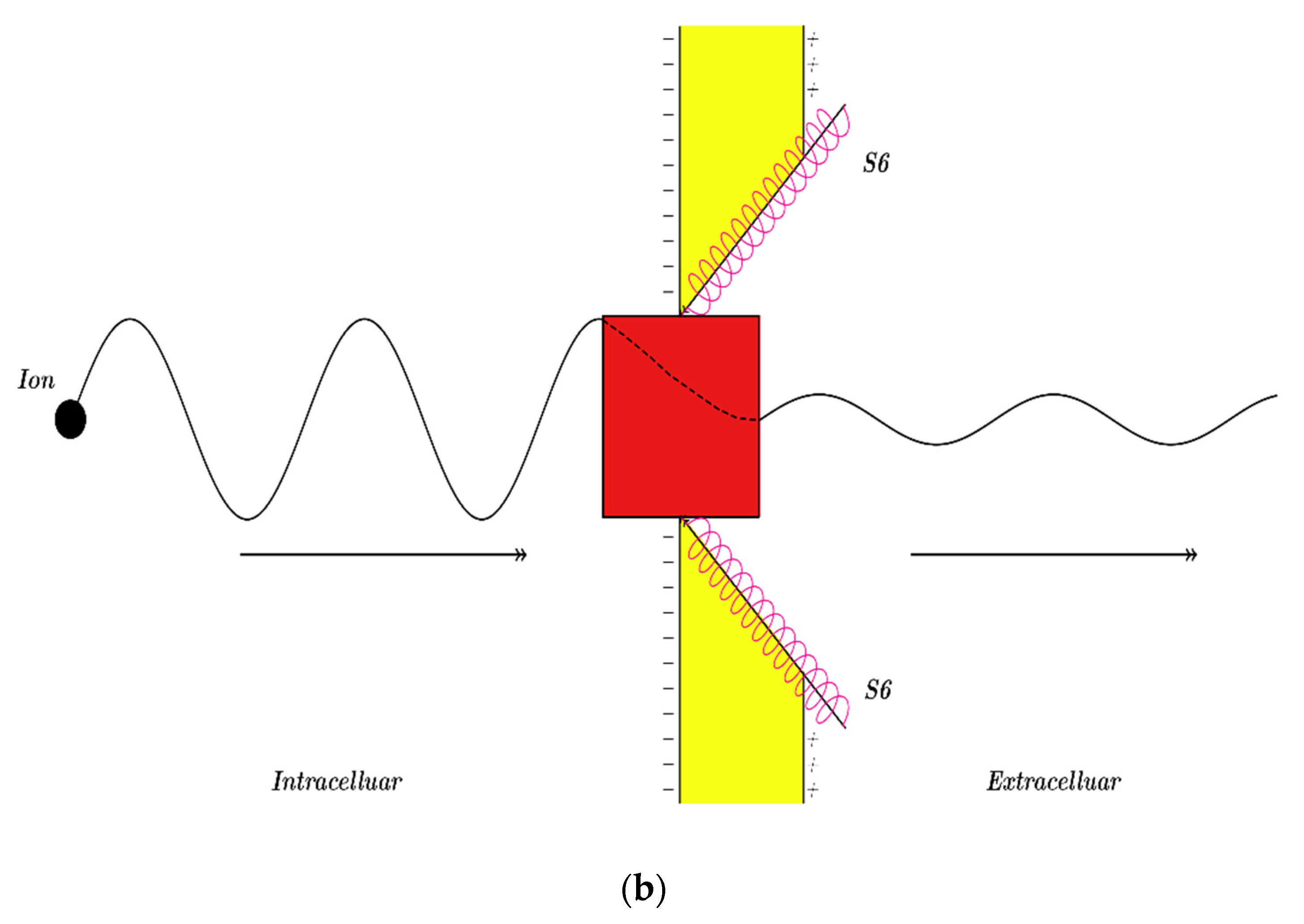
3.2.1. The Tunneling Probability of Sodium Ions through the Closed Intracellular Hydrophobic Gate
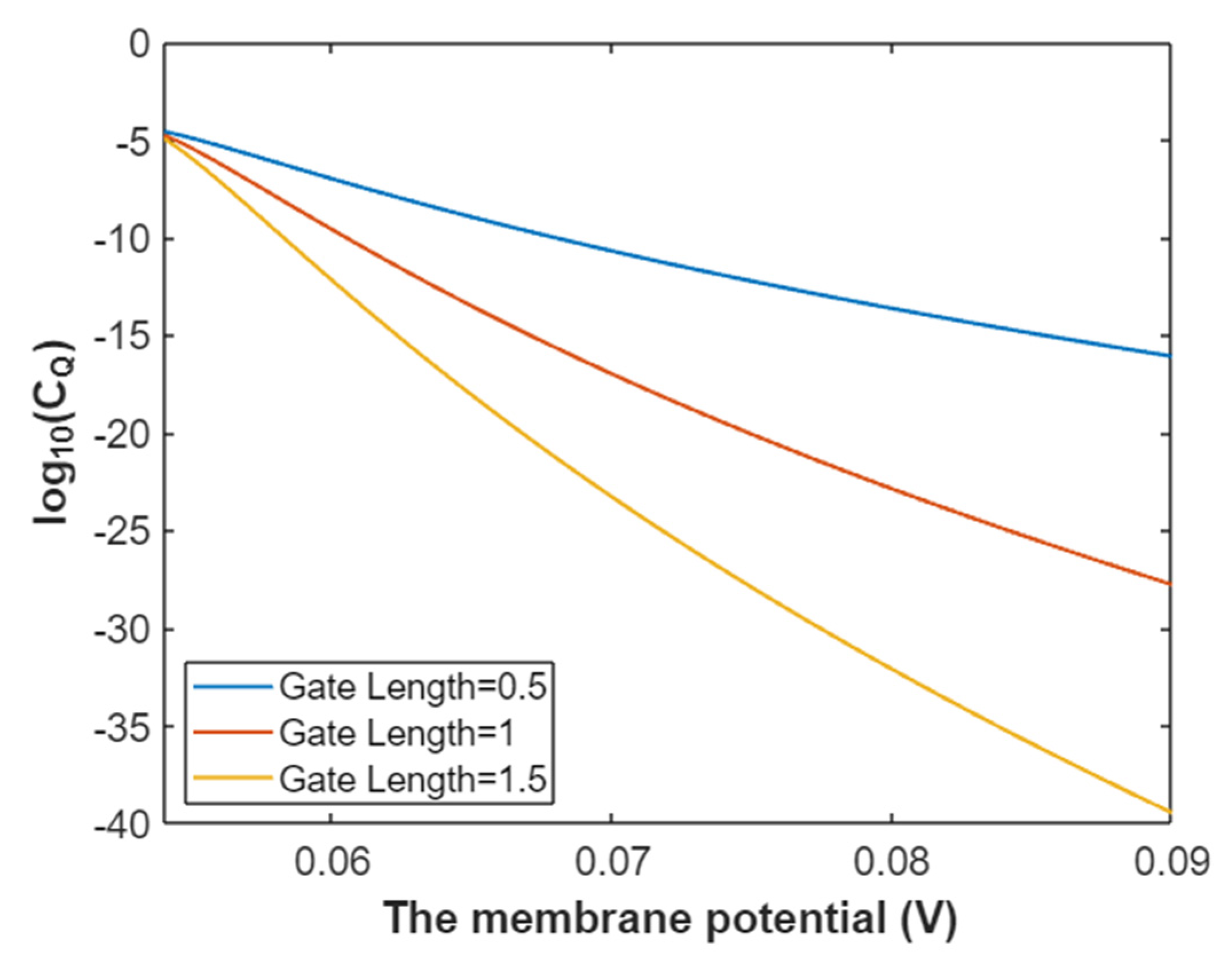
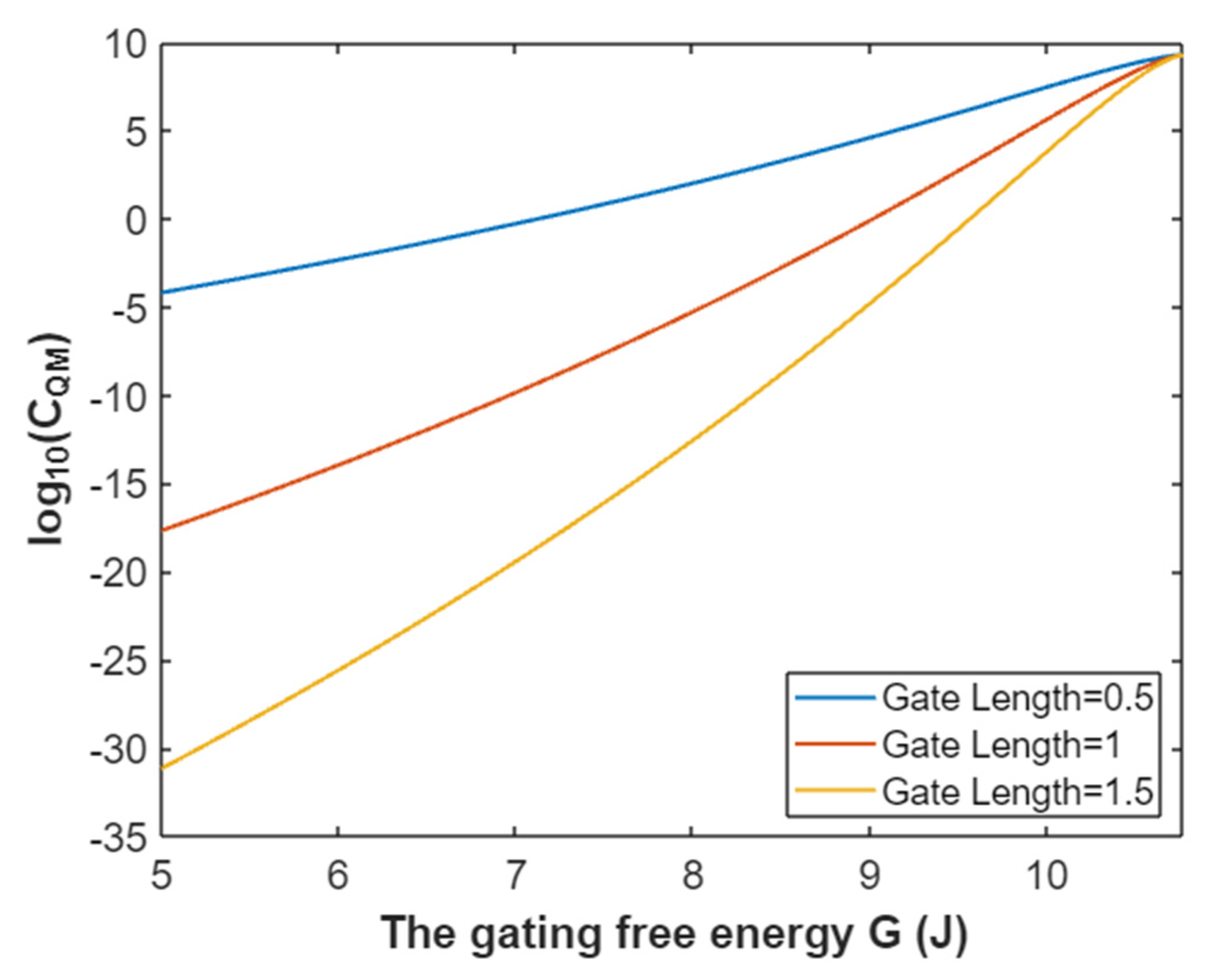
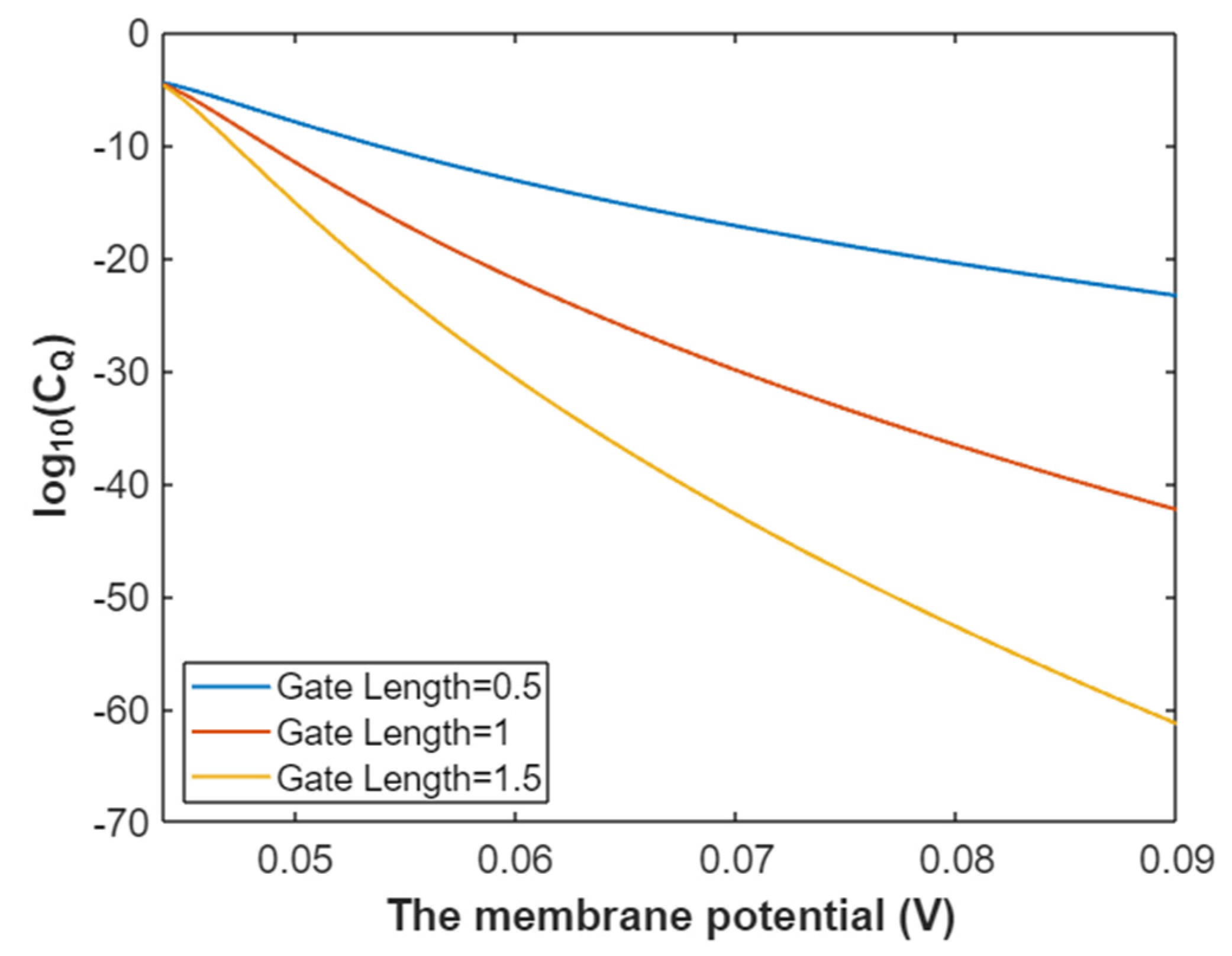
3.2.2. The Quantum Conductance of Single Voltage-Gated Sodium Channel
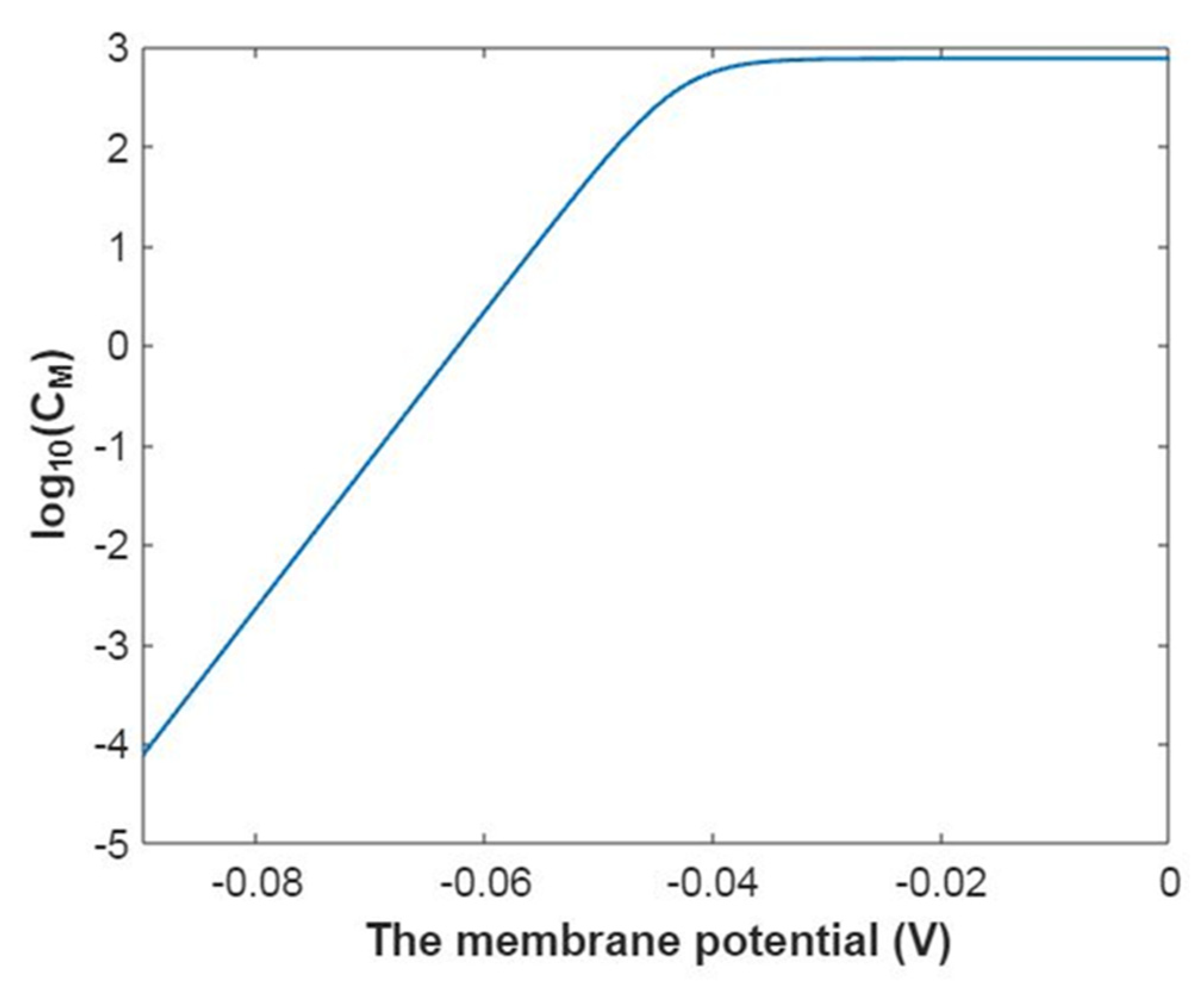
3.2.3. The Quantum Membrane Conductance of Sodium Ions
3.3. The Conductance of the Voltage-Gated Potassium Channels According to the Boltzmann Distribution
3.4. The Conductance of the Voltage-Gated Potassium Channels According to the Quantum Mechanics
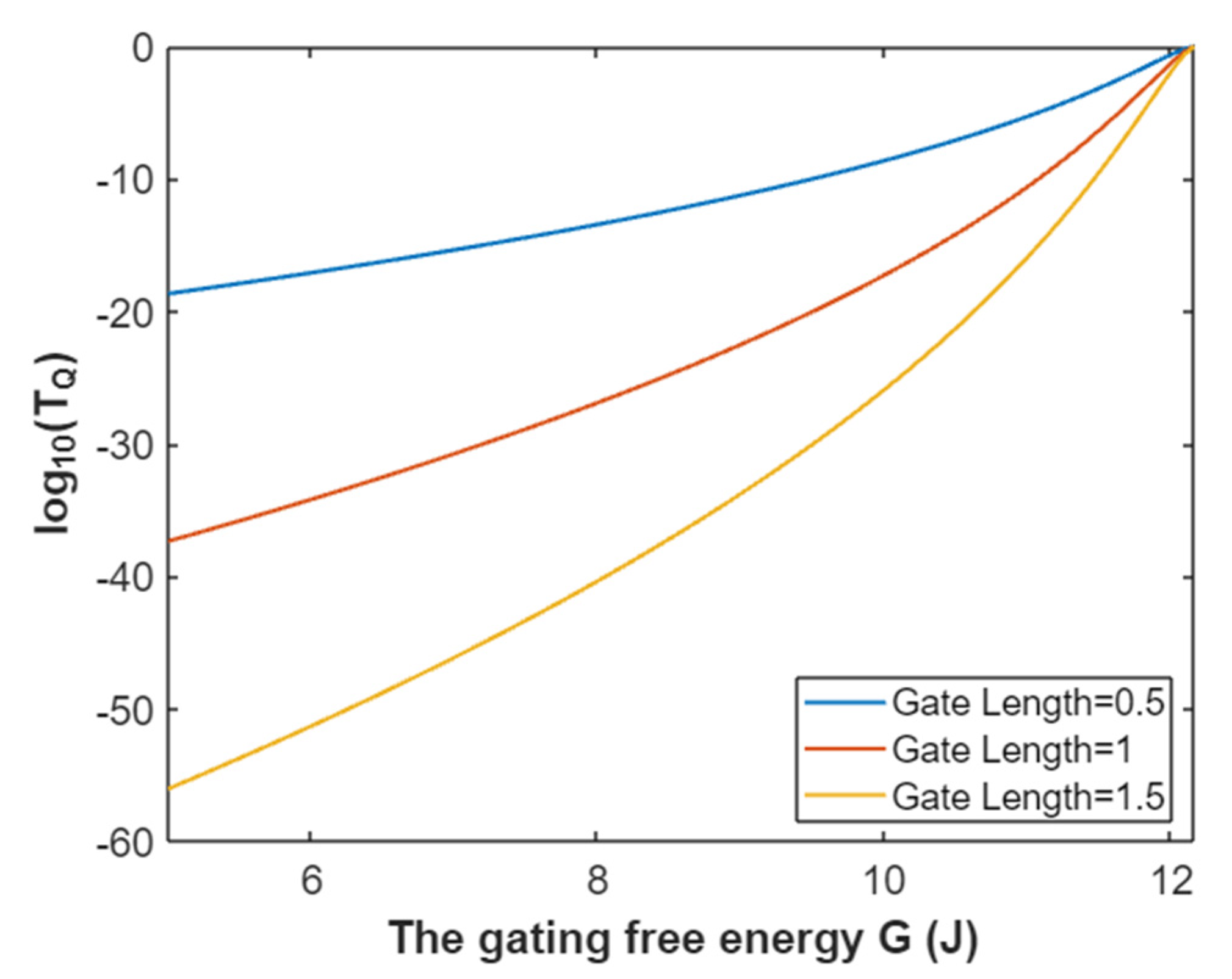
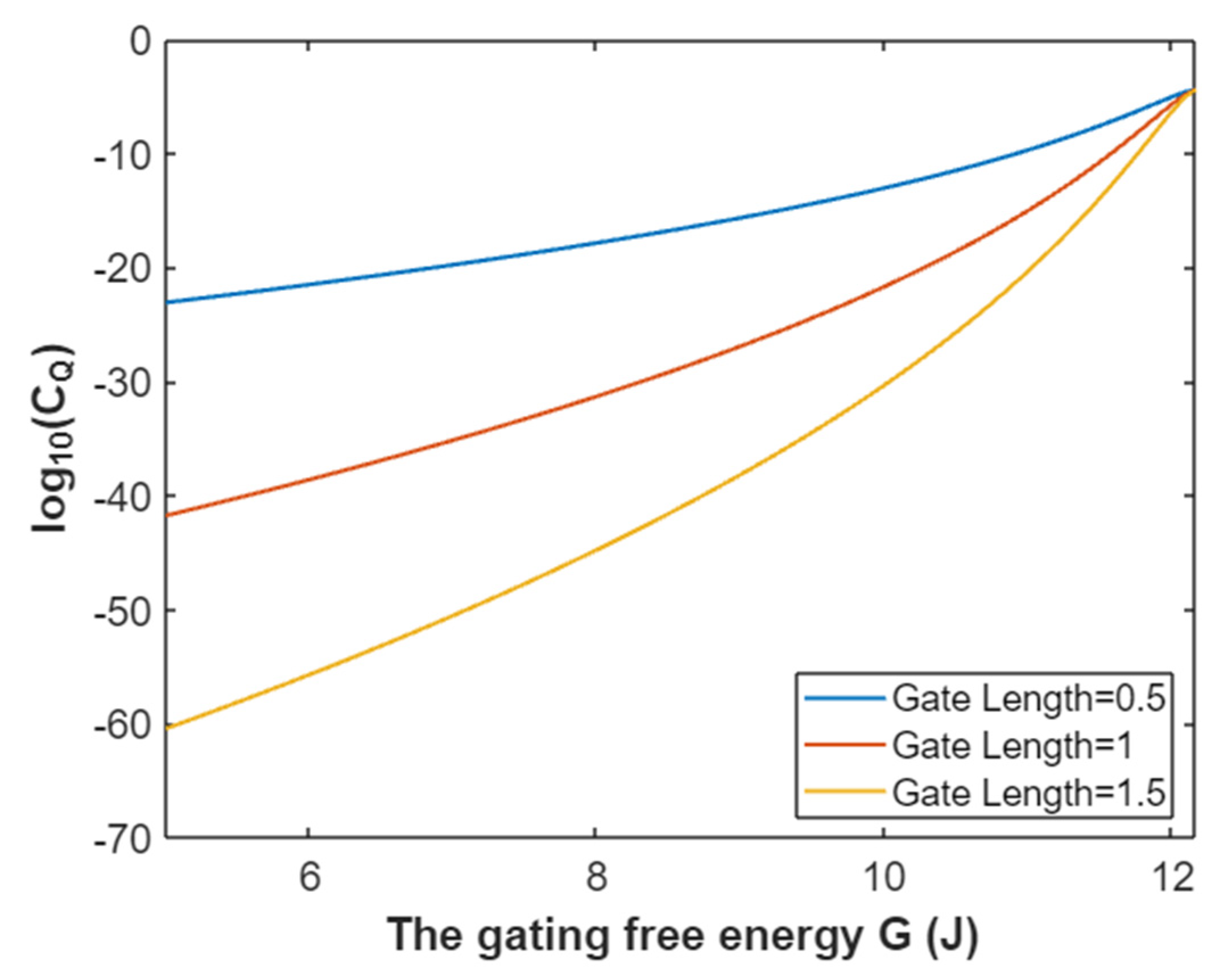
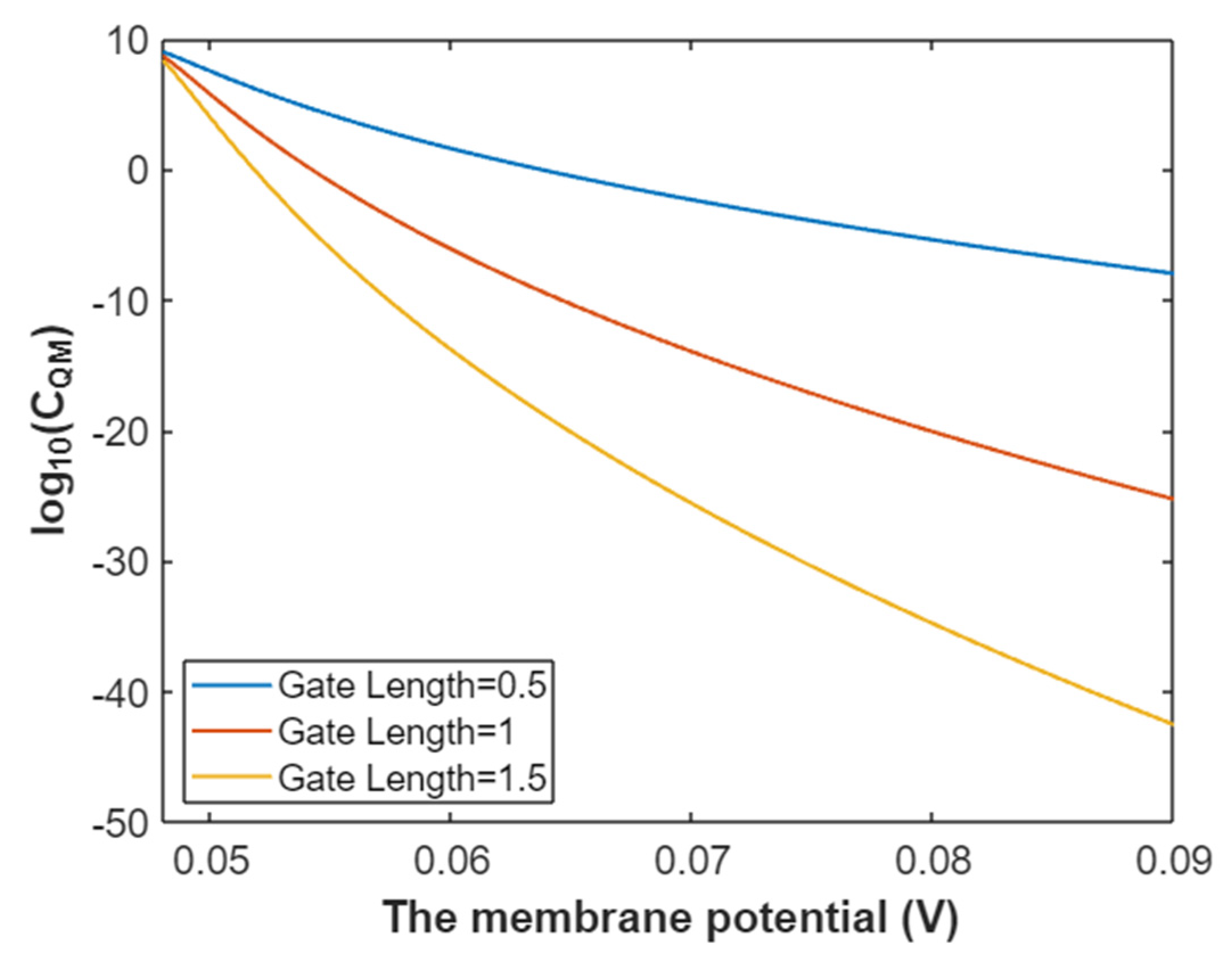
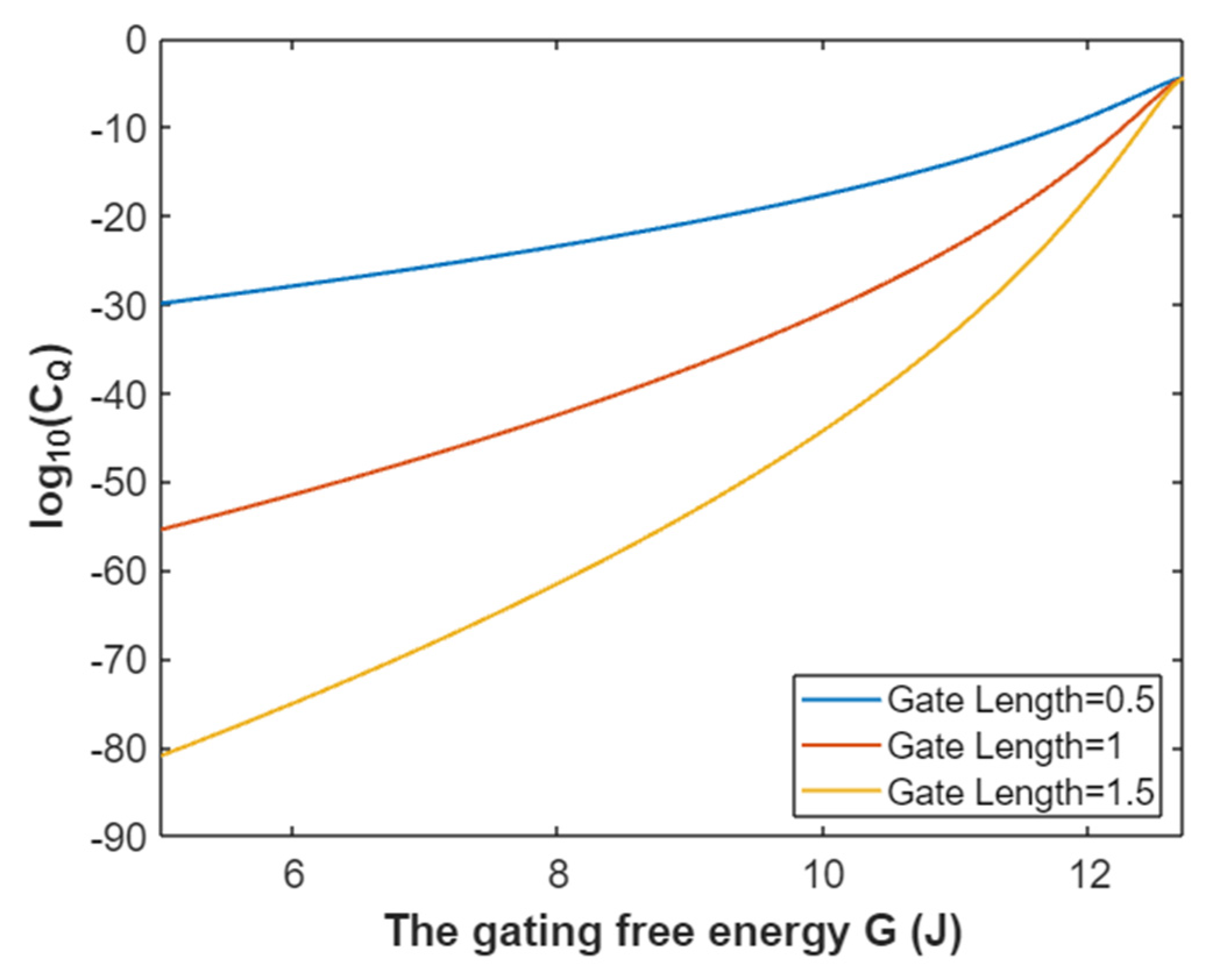
3.4.1. The Tunneling Probability of Potassium Ions through the Intracellular Hydrophobic Gate
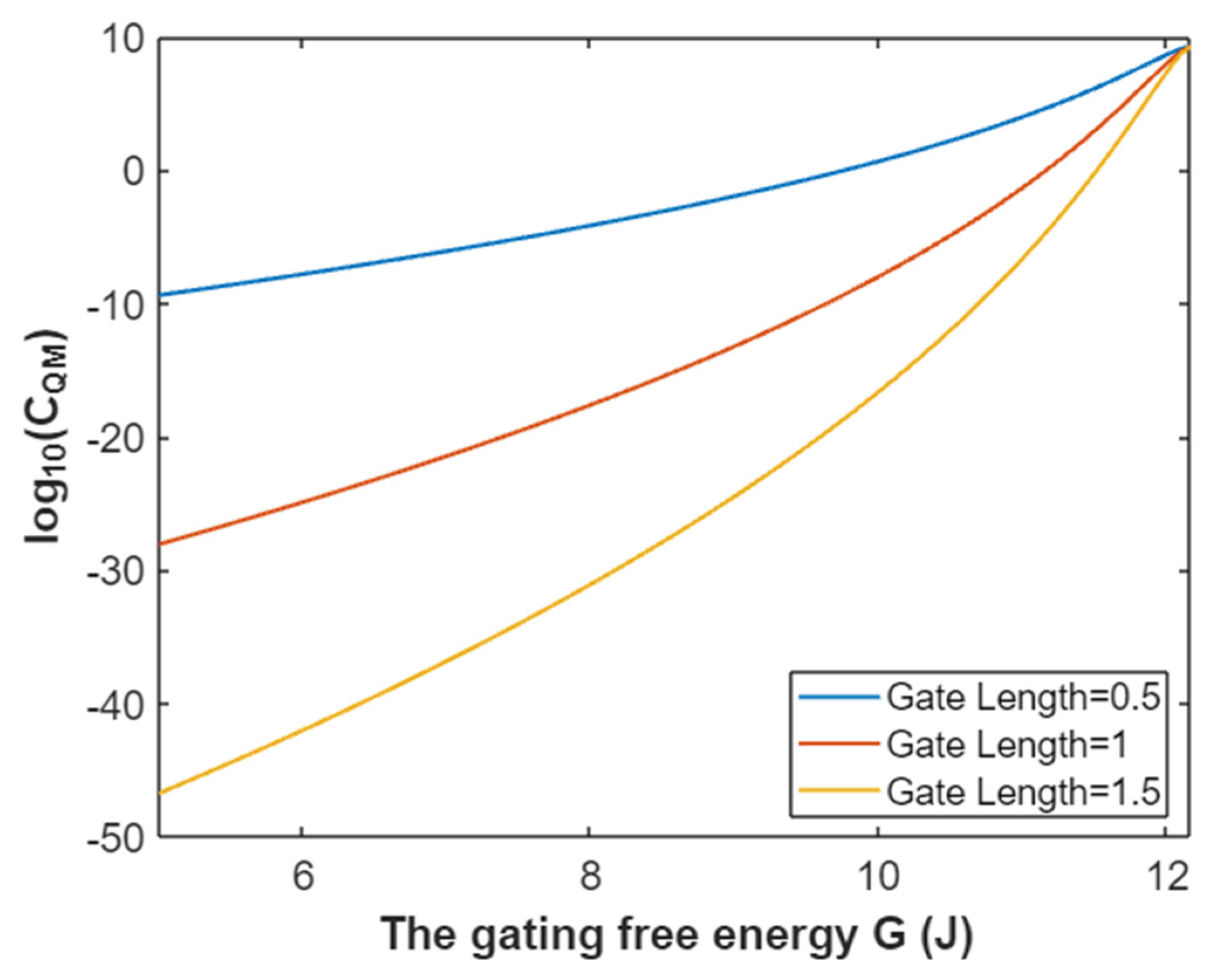
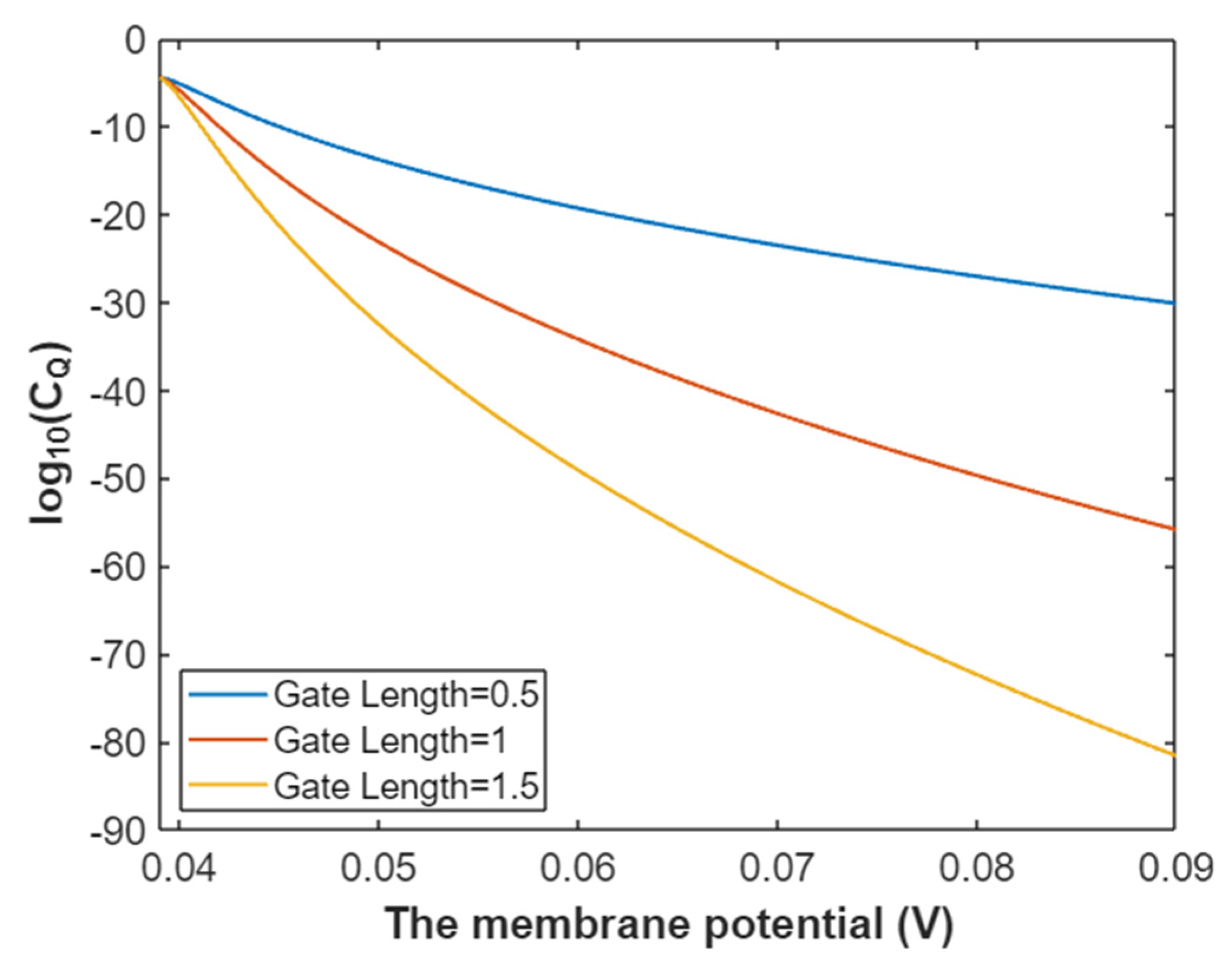
3.4.2. The Quantum Conductance of Single Voltage-Gated Potassium Channel
3.4.3. The Quantum Membrane Conductance of Potassium Ions
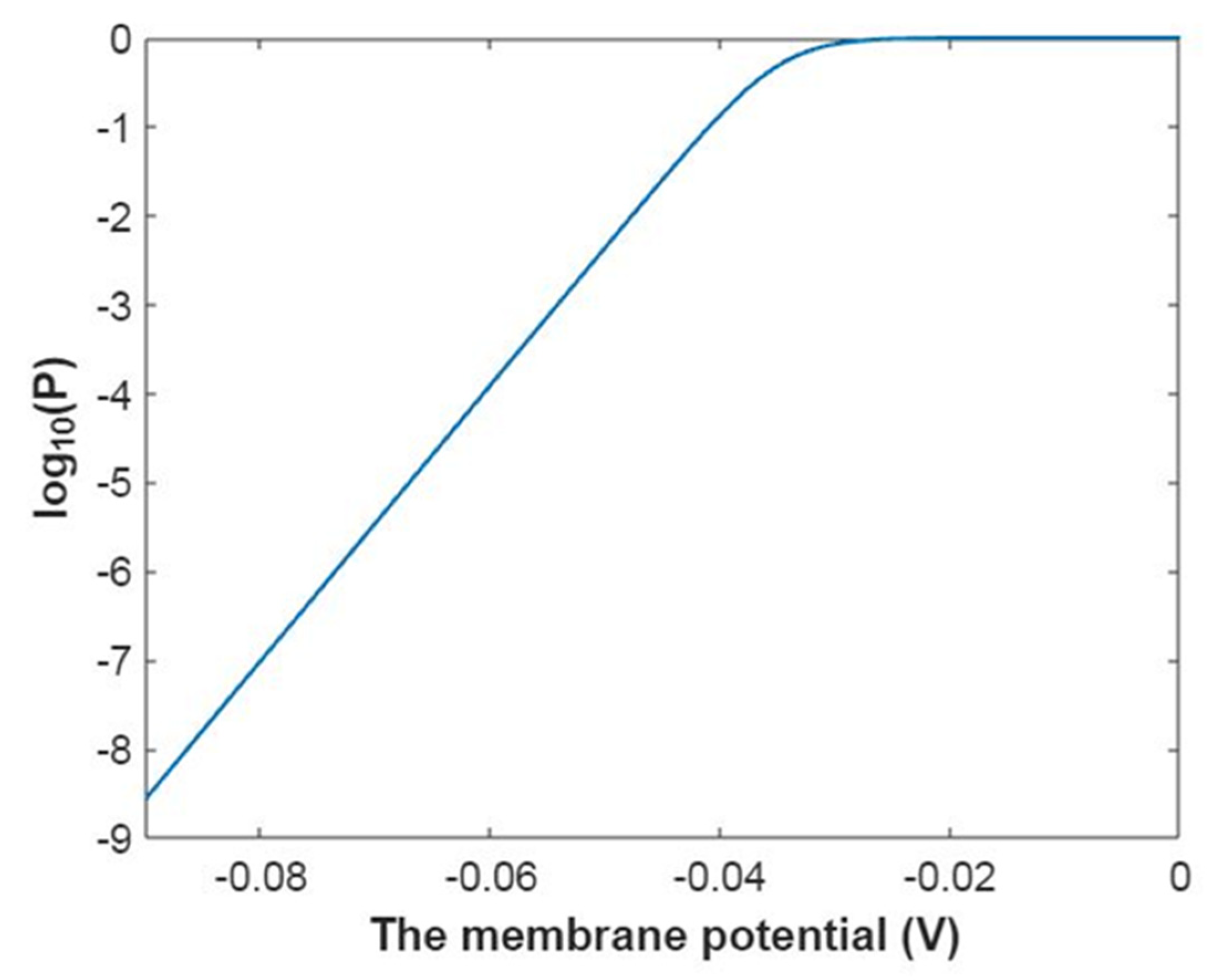
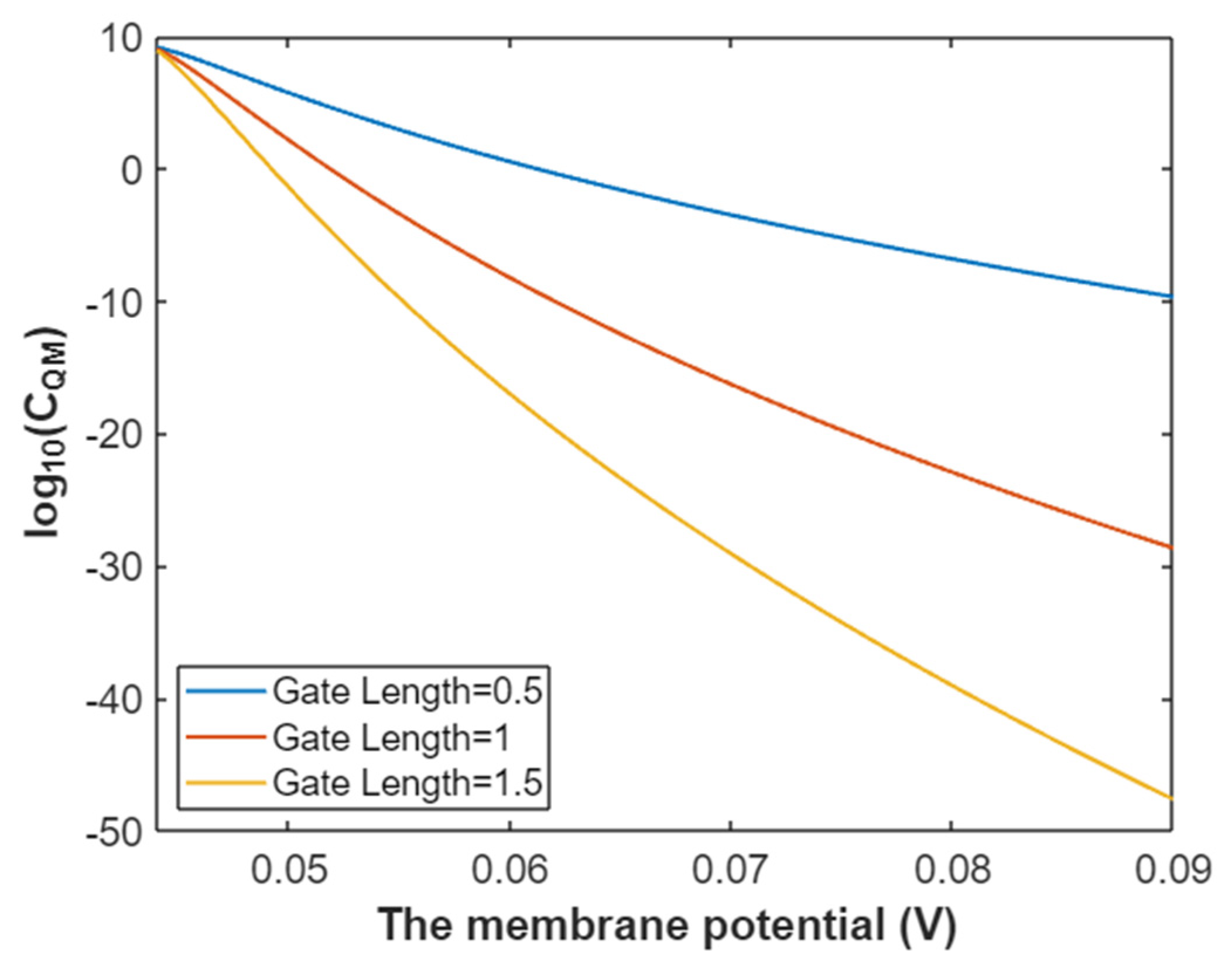
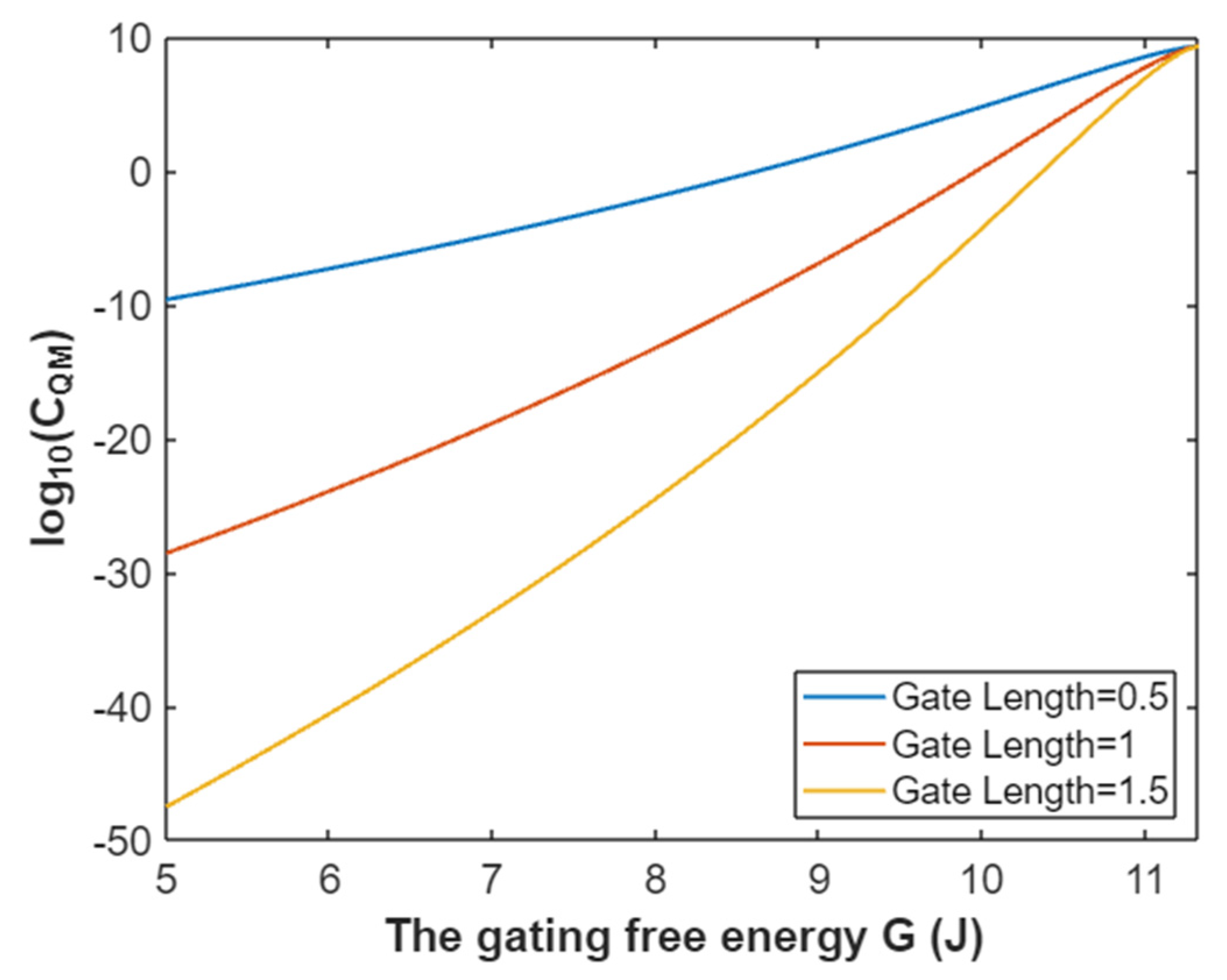
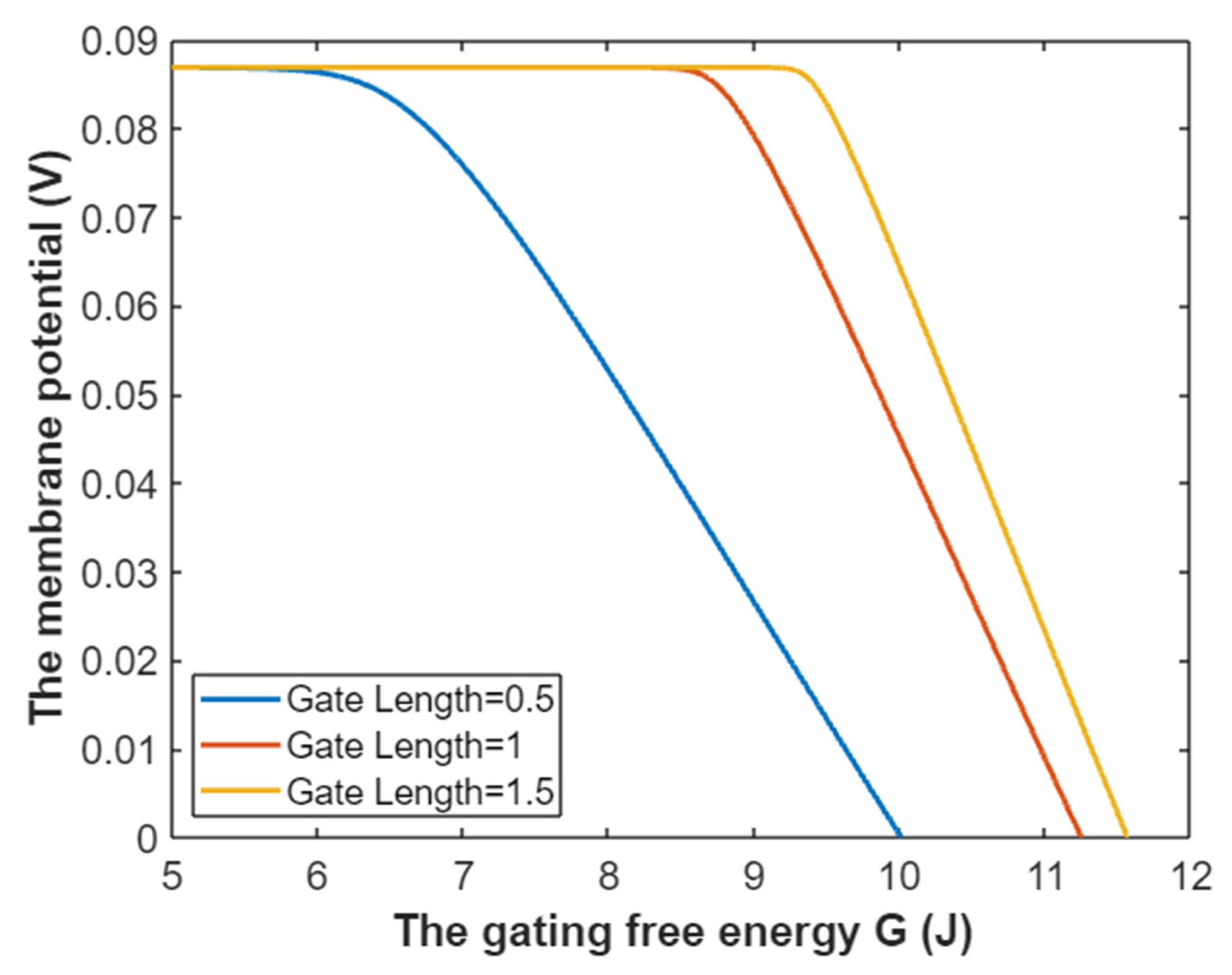
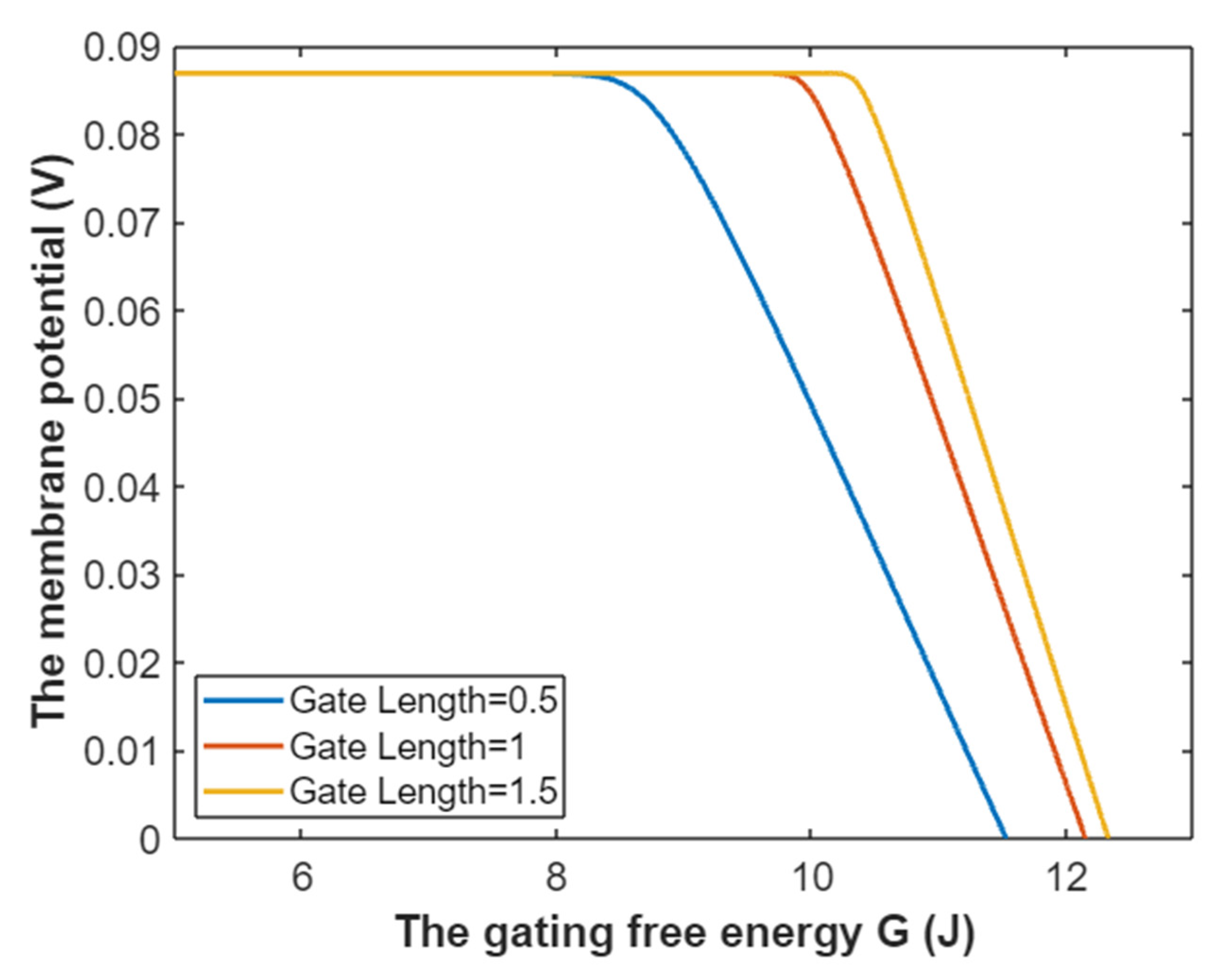
3.5. The Influence of Quantum Tunneling of Ions on the Resting Membrane Potential
4. Discussion
- Because voltage-gated channels and ions are a part of the biological system, it is expected that they could operate far from the thermal equilibrium, which indicates that ions may cool down through the hydrophobic gate even at higher body temperatures. This ‘cooling down’ can sustain the quantum coherence and make the quantum effects more apparent [17,74,75,76,77].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertil, H. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Lerche, H.; Shah, M.; Beck, H.; Noebels, J.; Johnston, D.; Vincent, A. Ion channels in genetic and acquired forms of epilepsy. J. Physiol. 2013, 591, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.D.; Geha, P.; Waxman, S.G. Sodium channels in pain disorders: Pathophysiology and prospects for treatment. Pain 2017, 158, S97–S107. [Google Scholar] [CrossRef]
- Dehghani-Samani, A.; Madreseh-Ghahfarokhi, S.; Dehghani-Samani, A. Mutations of voltage-gated ionic channels and risk of severe cardiac arrhythmias. Acta Cardiol. Sin. 2019, 35, 99–110. [Google Scholar] [PubMed]
- Tejada, J.; Costa, K.M.; Bertti, P.; Garcia-Cairasco, N. The epilepsies: Complex challenges needing complex solutions. Epilepsy Behav. 2013, 26, 212–228. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.S.; Kayani, K.; Whyte-Oshodi, D.; Whyte-Oshodi, A.; Nachiappan, N.; Gnanarajah, S.; Mohammed, R. Voltage gated sodium channels as therapeutic targets for chronic pain. J. Pain Res. 2019, 12, 2709–2722. [Google Scholar] [CrossRef]
- Ragab, A.A.; Sitorus, G.D.; Brundel, B.B.; de Groot, N.M. The Genetic Puzzle of Familial Atrial Fibrillation. Front. Cardiovasc. Med. 2020, 7, 14. [Google Scholar] [CrossRef]
- Privitera, M. Current challenges in the management of epilepsy. Am. J. Manag. Care 2011, 17 (Suppl. 7), S195–S203. [Google Scholar]
- Domenichiello, A.F.; Ramsden, C.E. The silent epidemic of chronic pain in older adults. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 93, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.A. Pain management–the challenge. Ochsner J. 2003, 5, 15–21. [Google Scholar]
- Nattel, S.; Andrade, J.; Macle, L.; Rivard, L.; Dyrda, K.; Mondesert, B.; Khairy, P. New directions in cardiac arrhythmia management: Present challenges and future solutions. Can. J. Cardiol. 2014, 30, S420–S430. [Google Scholar] [CrossRef] [PubMed]
- Serway, R.A.; Moses, C.J.; Moyer, C.A. Modern Physics; Cengage Learning: Boston, MA, USA, 2004. [Google Scholar]
- Lambert, N.; Chen, Y.N.; Cheng, Y.C.; Li, C.M.; Chen, G.Y.; Nori, F. Quantum biology. Nat. Phys. 2013, 9, 10–18. [Google Scholar] [CrossRef]
- Ball, P. Physics of life: The dawn of quantum biology. Nature 2011, 474, 272–274. [Google Scholar] [CrossRef]
- Kim, Y.; Bertagna, F.; D’Souza, E.M.; Heyes, D.J.; Johannissen, L.O.; Nery, E.T.; Pantelias, A.; Sanchez-Pedreño Jimenez, A.; Slocombe, L.; Spencer, M.G.; et al. Quantum Biology: An Update and Perspective. Quantum Rep. 2021, 3, 6. [Google Scholar] [CrossRef]
- Kong, J.; Jiménez-Martínez, R.; Troullinou, C.; Lucivero, V.G.; Tóth, G.; Mitchell, M.W. Measurement-induced, spatially-extended entanglement in a hot, strongly-interacting atomic system. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Arndt, M.; Juffmann, T.; Vedral, V. Quantum physics meets biology. HFSP J. 2009, 3, 386–400. [Google Scholar] [CrossRef]
- Oelstrom, K.; Goldschen-Ohm, M.P.; Holmgren, M.; Chanda, B. Evolutionarily conserved intracellular gate of voltage-dependent sodium channels. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Labro, A.J.; Snyders, D.J. Being flexible: The voltage-controllable activation gate of Kv channels. Front. Pharmacol. 2012, 3, 168. [Google Scholar] [CrossRef]
- Ingber, L.; Pappalepore, M.; Stesiak, R.R. Electroencephalographic field influence on calcium momentum waves. J. Theor. Biol. 2014, 343, 138–153. [Google Scholar] [CrossRef]
- Ingber, L. Quantum calcium-ion interactions with EEG. Sci 2019, 1, 20. [Google Scholar] [CrossRef]
- Summhammer, J.; Sulyok, G.; Bernroider, G. Quantum Mechanical Coherence of K+ Ion Wave Packets Increases Conduction in the KcsA Ion Channel. Appl. Sci. 2020, 10, 4250. [Google Scholar] [CrossRef]
- Koch, H.J. Quantum biology: Unit membrane reduces entropy due to wave particle duality. NeuroQuantology 2017, 15. [Google Scholar] [CrossRef]
- Juffmann, T.; Milic, A.; Müllneritsch, M.; Asenbaum, P.; Tsukernik, A.; Tüxen, J.; Mayor, M.; Cheshnovsky, O.; Arndt, M. Real-time single-molecule imaging of quantum interference. Nat. Nanotechnol. 2012, 7, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Gerlich, S.; Eibenberger, S.; Tomandl, M.; Nimmrichter, S.; Hornberger, K.; Fagan, P.J.; Tüxen, J.; Mayor, M.; Arndt, M. Quantum interference of large organic molecules. Nat. Commun. 2011, 2, 263. [Google Scholar] [CrossRef] [PubMed]
- Eibenberger, S.; Gerlich, S.; Arndt, M.; Mayor, M.; Tüxen, J. Matter–wave interference of particles selected from a molecular library with masses exceeding 10000 amu. Phys. Chem. Chem. Phys. 2013, 15, 14696–14700. [Google Scholar] [CrossRef]
- Vaziri, A.; Plenio, M.B. Quantum coherence in ion channels: Resonances, transport and verification. New J. Phys. 2010, 12, 085001. [Google Scholar] [CrossRef]
- Ganim, Z.; Tokmakoff, A.; Vaziri, A. Vibrational excitons in ionophores: Experimental probes for quantum coherence-assisted ion transport and selectivity in ion channels. New J. Phys. 2011, 13, 113030. [Google Scholar] [CrossRef]
- Salari, V.; Naeij, H.; Shafiee, A. Quantum interference and selectivity through biological ion channels. Sci. Rep. 2017, 7, 41625. [Google Scholar] [CrossRef] [PubMed]
- Aryal, P.; Sansom, M.S.; Tucker, S.J. Hydrophobic gating in ion channels. J. Mol. Biol. 2015, 427, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Qaswal, A.B. Quantum tunneling of ions through the closed voltage-gated channels of the biological membrane: A mathematical model and implications. Quantum Rep. 2019, 1, 19. [Google Scholar] [CrossRef]
- Qaswal, A.B. Quantum Electrochemical Equilibrium: Quantum Version of the Goldman–Hodgkin–Katz Equation. Quantum Rep. 2020, 2, 17. [Google Scholar] [CrossRef]
- Zhang, X.C.; Yang, H.; Liu, Z.; Sun, F. Thermodynamics of voltage-gated ion channels. Biophys. Rep. 2018, 4, 300–319. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Chanda, B. Estimating the voltage-dependent free energy change of ion channels using the median voltage for activation. J. Physiol. 2012, 139, 3–17. [Google Scholar] [CrossRef]
- Chen, F.; Hihath, J.; Huang, Z.; Li, X.; Tao, N.J. Measurement of single-molecule conductance. Annu. Rev. Phys. Chem. 2007, 58, 535–564. [Google Scholar] [CrossRef]
- Gonzalez, C.; Contreras, G.F.; Peyser, A.; Larsson, P.; Neely, A.; Latorre, R. Voltage sensor of ion channels and enzymes. Biophys. Rev. 2012, 4, 1–5. [Google Scholar] [CrossRef]
- Feigenspan, A.; Dedek, K.; Weiler, R.; Thanos, S.; Schlich, K. Expression and biophysical characterization of voltage-gated sodium channels in axons and growth cones of the regenerating optic nerve. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1789–1799. [Google Scholar] [CrossRef]
- Hu, W.; Tian, C.; Li, T.; Yang, M.; Hou, H.; Shu, Y. Distinct contributions of Na v 1.6 and Na v 1.2 in action potential initiation and backpropagation. Nat. Neurosci. 2009, 12, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Qaswal, A.B. Magnesium Ions Depolarize the Neuronal Membrane via Quantum Tunneling through the Closed Channels. Quantum Rep. 2020, 2, 5. [Google Scholar] [CrossRef]
- Qaswal, A.B. A Theoretical Study to Explain the Referred Pain Phenomenon and Its Characteristics via Quantum Tunneling of Potassium Ions through the Channels of Neuronal Membrane. NeuroQuantology 2019, 17. [Google Scholar] [CrossRef]
- Richards, L.A.; Schäfer, A.I.; Richards, B.S.; Corry, B. The importance of dehydration in determining ion transport in narrow pores. Small 2012, 8, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Ishida, I.G.; Rangel-Yescas, G.E.; Carrasco-Zanini, J.; Islas, L.D. Voltage-dependent gating and gating charge measurements in the Kv1. 2 potassium channel. J. Physiol. 2015, 145, 345–358. [Google Scholar]
- Islas, L.D. Functional diversity of potassium channel voltage-sensing domains. Channels 2016, 10, 202–213. [Google Scholar] [CrossRef]
- Issa, Z.F.; Miller, J.M.; Zipes, D.P. Molecular mechanisms of cardiac electrical activity. In Clinical Arrhythmology and Electrophysiology: A Companion to Braunwald’s Heart Disease, 2nd ed.; WB Saunders: Philadelphia, PA, USA, 2012; pp. 1–9. [Google Scholar]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology e-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Payandeh, J.; El-Din, T.M.; Scheuer, T.; Zheng, N.; Catterall, W.A. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature 2012, 486, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Cuello, L.G.; Jogini, V.; Cortes, D.M.; Perozo, E. Structural mechanism of C-type inactivation in K+ channels. Nature 2010, 466, 203–208. [Google Scholar] [CrossRef]
- Bagnéris, C.; Naylor, C.E.; McCusker, E.C.; Wallace, B.A. Structural model of the open–closed–inactivated cycle of prokaryotic voltage-gated sodium channels. J. Physiol. 2015, 145, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Ion channel voltage sensors: Structure, function, and pathophysiology. Neuron 2010, 67, 915–928. [Google Scholar] [CrossRef]
- Soler-Llavina, G.J.; Holmgren, M.; Swartz, K.J. Defining the conductance of the closed state in a voltage-gated K+ channel. Neuron 2003, 38, 61–67. [Google Scholar] [CrossRef]
- Banh, R.; Cherny, V.V.; Morgan, D.; Musset, B.; Thomas, S.; Kulleperuma, K.; Smith, S.M.; Pomès, R.; DeCoursey, T.E. Hydrophobic gasket mutation produces gating pore currents in closed human voltage-gated proton channels. Proc. Natl. Acad. Sci. USA 2019, 116, 18951–18961. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, M.; Eldstrom, J.; Murray, C.I.; Thompson, E.; Fedida, D. IKs ion-channel pore conductance can result from individual voltage sensor movements. Proc. Natl. Acad. Sci. USA 2019, 116, 7879–7888. [Google Scholar] [CrossRef]
- Moradi, N.; Scholkmann, F.; Salari, V. A study of quantum mechanical probabilities in the classical Hodgkin–Huxley model. J. Integr. Neurosci. 2015, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bernroider, G.; Summhammer, J. Can quantum entanglement between ion transition states effect action potential initiation? Cognit. Comput. 2012, 4, 29–37. [Google Scholar] [CrossRef]
- Yifrach, O.; MacKinnon, R. Energetics of pore opening in a voltage-gated K+ channel. Cell 2002, 111, 231–239. [Google Scholar] [CrossRef]
- Huang, J.; Estacion, M.R.; Zhao, P.; Dib-Hajj, F.; Schulman, B.; Abicht, A.; Kurth, I.; Brockmann, K.; Waxman, S.; Dib-Hajj, S.D. A novel gain-of-function Nav1. 9 mutation in a child with episodic pain. Front. Neurosci. 2019, 13, 918. [Google Scholar] [CrossRef]
- Chamberlin, A.; Qiu, F.; Rebolledo, S.; Wang, Y.; Noskov, S.Y.; Larsson, H.P. Hydrophobic plug functions as a gate in voltage-gated proton channels. Proc. Natl. Acad. Sci. USA 2014, 111, E273–E282. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.E. Voltage-gated channel mechanosensitivity: Fact or friction? Front. Physiol. 2011, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Reeves, D.; Ursell, T.; Sens, P.; Kondev, J.; Phillips, R. Membrane mechanics as a probe of ion-channel gating mechanisms. Phys. Rev. E 2008, 78, 041901. [Google Scholar] [CrossRef]
- George, A.L., Jr. Inherited channelopathies associated with epilepsy. Epilepsy Curr. 2004, 4, 65–70. [Google Scholar] [CrossRef]
- Behere, S.P.; Weindling, S.N. Inherited arrhythmias: The cardiac channelopathies. Ann. Pediatr. Cardiol. 2015, 8, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Cregg, R.; Momin, A.; Rugiero, F.; Wood, J.N.; Zhao, J. Pain channelopathies. J. Physiol. 2010, 588, 1897–1904. [Google Scholar] [CrossRef]
- Silverman, I.E.; Restrepo, L.; Mathews, G.C. Poststroke seizures. Arch. Neurol. 2002, 59, 195–201. [Google Scholar] [CrossRef]
- Ghuran, A.V.; Camm, A.J. Ischaemic heart disease presenting as arrhythmias. Br. Med. Bull. 2001, 59, 193–210. [Google Scholar] [CrossRef]
- Bhar-Amato, J.; Davies, W.; Agarwal, S. Ventricular arrhythmia after acute myocardial infarction: ‘The Perfect Storm’. Arrhythmia Electrophysiol. Rev. 2017, 6, 134–139. [Google Scholar] [CrossRef]
- Franz, M.R.; Cima, R.; Wang, D.; Profitt, D.; Kurz, R. Electrophysiological effects of myocardial stretch and mechanical determinants of stretch-activated arrhythmias. Circulation 1992, 86, 968–978. [Google Scholar] [CrossRef]
- Bigger, J.T., Jr. Why patients with congestive heart failure die: Arrhythmias and sudden cardiac death. Circulation 1987, 75, IV28–IV35. [Google Scholar]
- Horstkotte, D. Arrhythmias in the natural history of mitral stenosis. Acta Cardiol. 1992, 47, 105–113. [Google Scholar]
- Scardigli, M.; Cannazzaro, S.; Coppini, R.; Crocini, C.; Yan, P.; Loew, L.M.; Sartiani, L.; Cerbai, E.; Pavone, F.S.; Sacconi, L.; et al. Arrhythmia susceptibility in a rat model of acute atrial dilation. Prog. Biophys. Mol. Biol. 2020, 154, 21–29. [Google Scholar] [CrossRef]
- Niday, Z.; Tzingounis, A.V. Potassium channel gain of function in epilepsy: An unresolved paradox. Neuroscientist 2018, 24, 368–380. [Google Scholar] [CrossRef]
- Stafstrom, C.E. Persistent sodium current and its role in epilepsy. Epilepsy Curr. 2007, 7, 15–22. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Wang, C.; Ortega, E.; Wang, X.; Xie, Y.F.; Shen, J.; Gao, C.; Van der Bruggen, B. Electric field-based ionic control of selective separation layers. J. Mater. Chem. A 2020, 8, 4244–4251. [Google Scholar] [CrossRef]
- Salari, V.; Tuszynski, J.; Rahnama, M.; Bernroider, G. Plausibility of quantum coherent states in biological systems. J. Phys. Conf. Ser. 2011, 306, 012075. [Google Scholar] [CrossRef]
- Hagan, S.; Hameroff, S.R.; Tuszyński, J.A. Quantum computation in brain microtubules: Decoherence and biological feasibility. Phys. Rev. E 2002, 65, 061901. [Google Scholar] [CrossRef]
- Lidar, D.A.; Chuang, I.L.; Whaley, K.B. Decoherence-free subspaces for quantum computation. Phys. Rev. Lett. 1998, 81, 2594. [Google Scholar] [CrossRef]
- Matsuno, K. Cell motility as an entangled quantum coherence. BioSystems 1999, 51, 15–19. [Google Scholar] [CrossRef]
- Cai, J.; Popescu, S.; Briegel, H.J. Dynamic entanglement in oscillating molecules and potential biological implications. Phys. Rev. E 2010, 82, 021921. [Google Scholar] [CrossRef]
- Jedlicka, P. Revisiting the quantum brain hypothesis: Toward quantum (neuro) biology? Front. Mol. Neurosci. 2017, 10, 366. [Google Scholar] [CrossRef]
- Jedlicka, P. Quantum stochasticity and (the end of) neurodeterminism. Quantum Phys. Meets Philos. Mind 2014, 183–197. [Google Scholar] [CrossRef]
- Anastassiou, C.A.; Koch, C. Ephaptic coupling to endogenous electric field activity: Why bother? Curr. Opin. Neurobiol. 2015, 31, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, F.; McCormick, D.A. Endogenous electric fields may guide neocortical network activity. Neuron 2010, 67, 129–143. [Google Scholar] [CrossRef]
- McFadden, J.; Al-Khalili, J. Life on the Edge: The Coming of Age of Quantum Biology; Crown Publishing Group: New York, NY, USA, 26 July 2016. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qaswal, A.B.; Ababneh, O.; Khreesha, L.; Al-Ani, A.; Suleihat, A.; Abbad, M. Mathematical Modeling of Ion Quantum Tunneling Reveals Novel Properties of Voltage-Gated Channels and Quantum Aspects of Their Pathophysiology in Excitability-Related Disorders. Pathophysiology 2021, 28, 116-154. https://doi.org/10.3390/pathophysiology28010010
Qaswal AB, Ababneh O, Khreesha L, Al-Ani A, Suleihat A, Abbad M. Mathematical Modeling of Ion Quantum Tunneling Reveals Novel Properties of Voltage-Gated Channels and Quantum Aspects of Their Pathophysiology in Excitability-Related Disorders. Pathophysiology. 2021; 28(1):116-154. https://doi.org/10.3390/pathophysiology28010010
Chicago/Turabian StyleQaswal, Abdallah Barjas, Omar Ababneh, Lubna Khreesha, Abdallah Al-Ani, Ahmad Suleihat, and Mutaz Abbad. 2021. "Mathematical Modeling of Ion Quantum Tunneling Reveals Novel Properties of Voltage-Gated Channels and Quantum Aspects of Their Pathophysiology in Excitability-Related Disorders" Pathophysiology 28, no. 1: 116-154. https://doi.org/10.3390/pathophysiology28010010
APA StyleQaswal, A. B., Ababneh, O., Khreesha, L., Al-Ani, A., Suleihat, A., & Abbad, M. (2021). Mathematical Modeling of Ion Quantum Tunneling Reveals Novel Properties of Voltage-Gated Channels and Quantum Aspects of Their Pathophysiology in Excitability-Related Disorders. Pathophysiology, 28(1), 116-154. https://doi.org/10.3390/pathophysiology28010010






