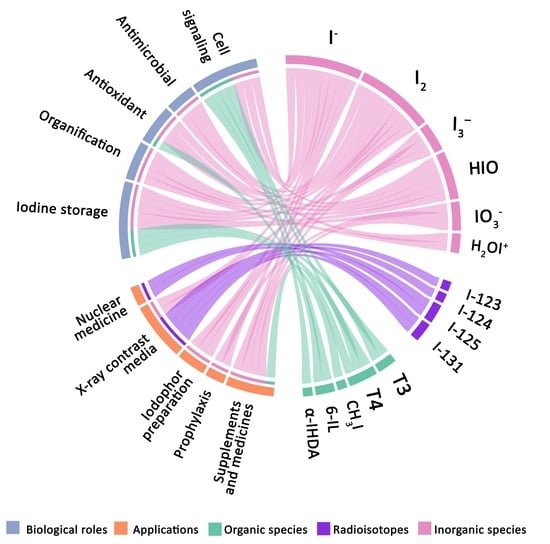
Implications and Practical Applications of the Chemical Speciation of Iodine in the Biological Context
Abstract
:1. Introduction
2. History and Chemical Characteristics
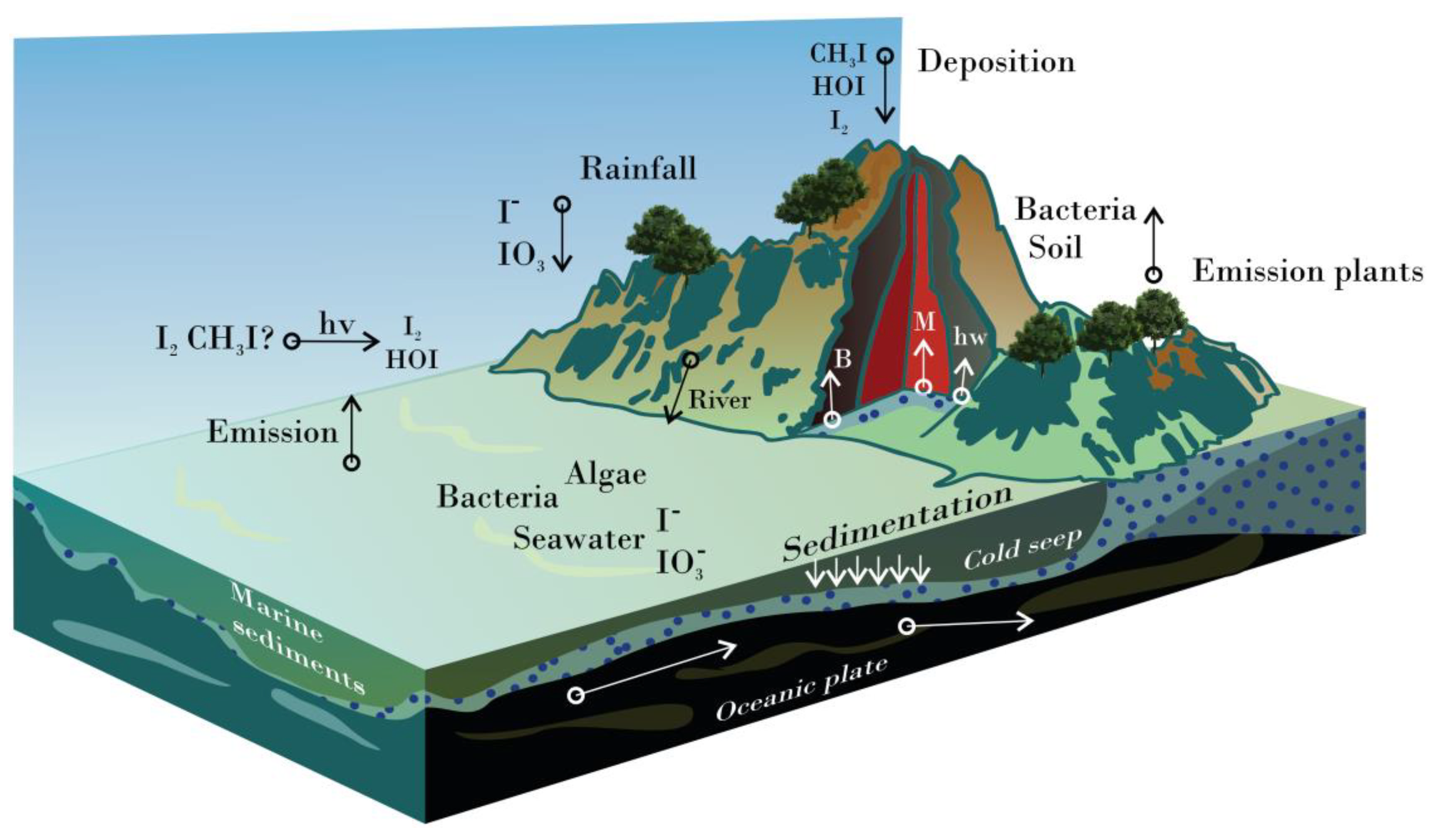
3. Distribution of Iodine
4. Relevant Iodine Species
4.1. Main Inorganic Forms
4.2. Representative Organic Species
5. Biological Roles of Iodine
5.1. Antimicrobial Action
5.2. Nutrition and Human Health
5.3. Thyroid Hormones
5.3.1. TH Synthesis and Metabolism
5.3.2. Actions of THs
5.3.3. THs out of Vertebrates
5.4. Antioxidant Capacity
5.4.1. Markers of Oxidative Stress in Mammals
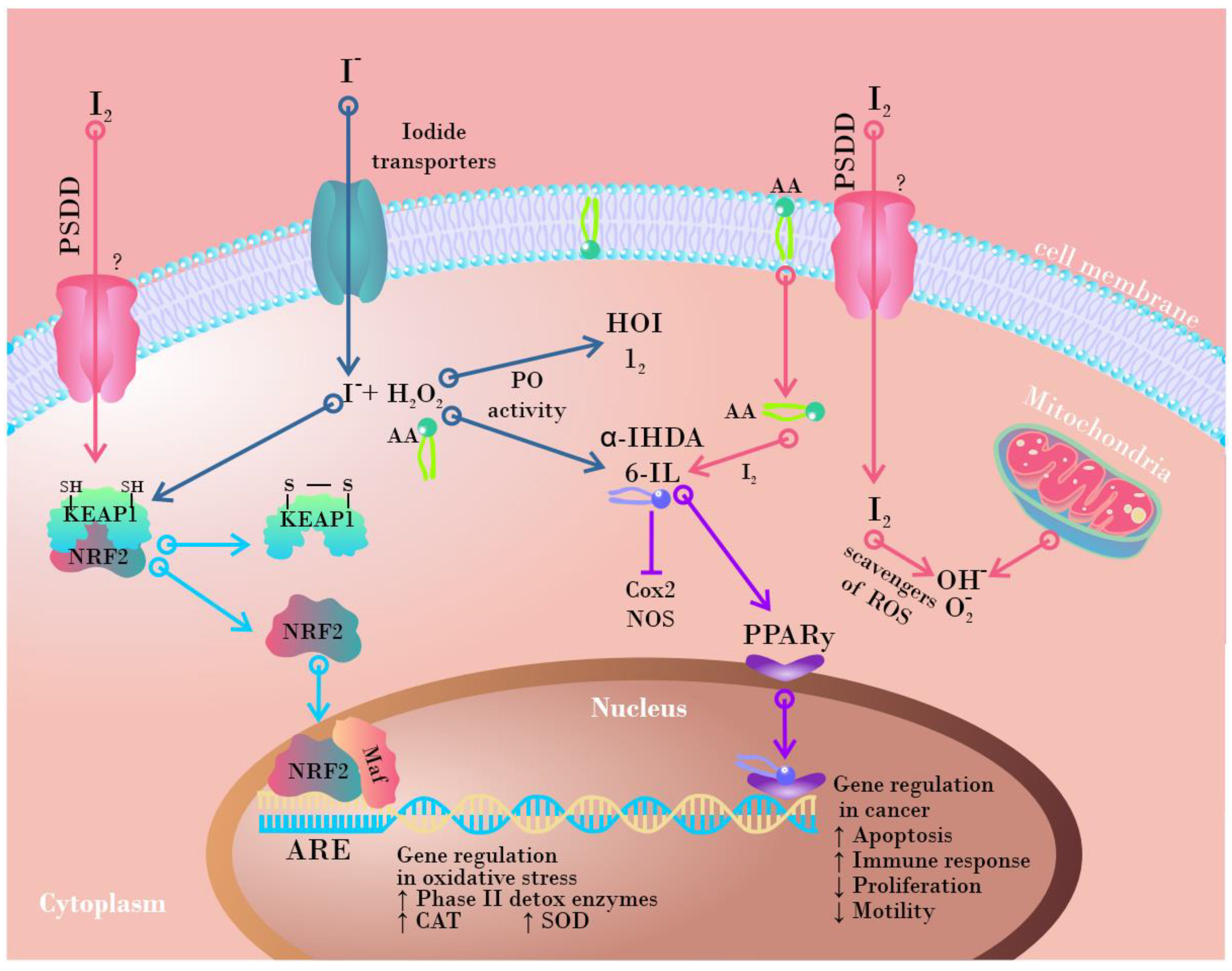
5.4.2. Regulation of Antioxidant Pathways
5.5. Immunomodulation
5.6. Anticarcinogenic Effect
5.6.1. Effects on Cells and Tissues
5.6.2. Animal Models and Humans
5.6.3. Additional Considerations
6. Iodine in the Industry
7. Applications in the Health Sciences
7.1. Disinfection, Asepsis, and Wound Care
7.2. Supplementation
7.3. Contrast Agents
7.4. Applications in Nuclear Medicine
7.5. Prophylaxis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Venturi, S.; Donati, F.M.; Venturi, A.; Venturi, M. Environmental Iodine Deficiency: A Challenge to the Evolution of Terrestrial Life? Thyroid 2000, 10, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Gottardi, W. Iodine as Disinfectant. In Iodine Chemistry and Applications; Kaiho, T., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; Volume 9781118466, pp. 375–410. [Google Scholar]
- Crockford, S.J. Evolutionary Roots of Iodine and Thyroid Hormones in Cellcell Signaling. Integr. Comp. Biol. 2009, 49, 155–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Küpper, F.C.; Feiters, M.C.; Olofsson, B.; Kaiho, T.; Yanagida, S.; Zimmermann, M.B.; Carpenter, L.J.; Luther, G.W.; Lu, Z.; Jonsson, M.; et al. Commemorating Two Centuries of Iodine Research: An Interdisciplinary Overview of Current Research. Angew. Chemie Int. Ed. 2011, 50, 11598–11620. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem Element Summary for AtomicNumber 53, Iodine. Available online: https://pubchem.ncbi.nlm.nih.gov/element/Iodine (accessed on 24 April 2022).
- Meija, J.; Coplen, T.B.; Berglund, M.; Brand, W.A.; De Bièvre, P.; Gröning, M.; Holden, N.E.; Irrgeher, J.; Loss, R.D.; Walczyk, T.; et al. Isotopic Compositions of the Elements 2013 (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 293–306. [Google Scholar] [CrossRef] [Green Version]
- Edwards, R.R. Iodine-129: Its Occurrence in Nature and Its Utility as a Tracer. Science 1962, 137, 851–853. [Google Scholar] [CrossRef]
- Kaiho, T. Inorganic Iodides. In Iodine Chemistry and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; Volume 9781118466, pp. 55–73. [Google Scholar]
- Muramatsu, Y.; Yoshida, S.; Fehn, U.; Amachi, S.; Ohmomo, Y. Studies with Natural and Anthropogenic Iodine Isotopes: Iodine Distribution and Cycling in the Global Environment. J. Environ. Radioact. 2004, 74, 221–232. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, A.; Romarís-Hortas, V.; Bermejo-Barrera, P. A Review on Iodine Speciation for Environmental, Biological and Nutrition Fields. J. Anal. At. Spectrom. 2011, 26, 2107. [Google Scholar] [CrossRef]
- Amachi, S. Microbial Contribution to Global Iodine Cycling: Volatilization, Accumulation, Reduction, Oxidation, and Sorption of Iodine. Microbes Environ. 2008, 23, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Kocher, D.C. A Dynamic Model of the Global Iodine Cycle and Estimation of Dose to the World Population from Releases of Iodine-129 to the Environment. Environ. Int. 1981, 5, 15–31. [Google Scholar] [CrossRef]
- Elmore, D.; Gove, H.E.; Ferraro, R.; Kilius, L.R.; Lee, H.W.; Chang, K.H.; Beukens, R.P.; Litherland, A.E.; Russo, C.J.; Purser, K.H.; et al. Determination of 129I Using Tandem Accelerator Mass Spectrometry. Nature 1980, 286, 138–140. [Google Scholar] [CrossRef]
- Brown, C.F.; Geiszler, K.N.; Lindberg, M.J. Analysis of 129I in Groundwater Samples: Direct and Quantitative Results below the Drinking Water Standard. Appl. Geochemistry 2007, 22, 648–655. [Google Scholar] [CrossRef]
- Crivello, J.V. Diaryliodonium Salt Photoacid Generators. In Iodine Chemistry and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 457–478. [Google Scholar]
- Carpenter, L.J. Atmospheric Chemistry of Iodine. In Iodine Chemistry and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 591–601. [Google Scholar]
- Cox, R.A.; Bloss, W.J.; Jones, R.L.; Rowley, D.M. OIO and the Atmospheric Cycle of Iodine. Geophys. Res. Lett. 1999, 26, 1857–1860. [Google Scholar] [CrossRef]
- Chameides, W.L.; Davis, D.D. Iodine: Its Possible Role in Tropospheric Photochemistry. J. Geophys. Res. Ocean. 1980, 85, 7383–7398. [Google Scholar] [CrossRef]
- Raso, A.R.W.; Custard, K.D.; May, N.W.; Tanner, D.; Newburn, M.K.; Walker, L.; Moore, R.J.; Huey, L.G.; Alexander, L.; Shepson, P.B.; et al. Active Molecular Iodine Photochemistry in the Arctic. Proc. Natl. Acad. Sci. USA 2017, 114, 10053–10058. [Google Scholar] [CrossRef] [Green Version]
- Seidell, A. Solubilities of Inorganic and Organic Compounds. A Compilation of Quantitative Solubility Data from the Periodical Literature. J. Am. Med. Assoc. 1928, 91, 1131. [Google Scholar]
- Lewis, R.A. Hawley’s Condensed Chemical Dictionary, 15th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Yuita, K. Dynamics of Iodine, Bromine, and Chlorine in Soil: II. Chemical Forms of Iodine in Soil Solutions. Soil Sci. Plant Nutr. 1992, 38, 281–287. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Guo, W.; Xie, X.; Zhang, L. Factors Controlling Spatial Variation of Iodine Species in Groundwater of the Datong Basin, Northern China. Procedia Earth Planet. Sci. 2013, 7, 483–486. [Google Scholar] [CrossRef] [Green Version]
- Gottardi, W. Iodine and Disinfection: Theoretical Study on Mode of Action, Efficiency, Stability, and Analytical Aspects in the Aqueous System. Arch. Pharm. 1999, 332, 151–157. [Google Scholar] [CrossRef]
- Gottardi, W. Potentiometrische Bestimmung Der Gleichgewichtskonzentrationen an Freiem Und Komplex Gebundenem Iod in W~iflrigen Liisungen von Polyvinylpyrrolidon-Iod (PVP-Iod) Polyvinylpyrrolidon-Iod (PVP-Iod). Fresenius’ Zeitschrift für Anal. Chemie 1983, 314, 582–585. [Google Scholar] [CrossRef]
- Bowen, H.J.M. Environmental Chemistry of the Elements; Academic Press: London, UK, 1979. [Google Scholar]
- Rackur, H. New Aspects of Mechanism of Action of Povidone-Iodine. J. Hosp. Infect. 1985, 6, 13–23. [Google Scholar] [CrossRef]
- Bafort, F.; Parisi, O.; Perraudin, J.-P.; Jijakli, M.H. Mode of Action of Lactoperoxidase as Related to Its Antimicrobial Activity: A Review. Enzyme Res. 2014, 2014, 517164. [Google Scholar] [CrossRef]
- Cooper, R.A. Iodine Revisited. Int. Wound J. 2007, 4, 124–137. [Google Scholar] [CrossRef]
- Punyani, S.; Narayana, P.; Singh, H.; Vasudevan, P. Iodine Based Water Disinfection: A Review. J. Sci. Ind. Res. 2006, 65, 116–120. [Google Scholar]
- Information National Center for Biotechnology PubChem Compound Summary for CID 23665710, Potassium Iodate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Potassium-iodate (accessed on 24 April 2022).
- Dembitsky, V.M. Biogenic Iodine and Iodine-Containing Metabolites. Nat. Prod. Commun. 2006, 1, 1934578X0600100. [Google Scholar] [CrossRef] [Green Version]
- Edmonds, J.S.; Morita, M. The Determination of Iodine Species in Environmental and Biological Samples (Technical Report). Pure Appl. Chem. 1998, 70, 1567–1584. [Google Scholar] [CrossRef]
- Cakmak, I.; Prom-u-thai, C.; Guilherme, L.R.G.; Rashid, A.; Hora, K.H.; Yazici, A.; Savasli, E.; Kalayci, M.; Tutus, Y.; Phuphong, P.; et al. Iodine Biofortification of Wheat, Rice and Maize through Fertilizer Strategy. Plant Soil 2017, 418, 319–335. [Google Scholar] [CrossRef]
- Kiferle, C.; Gonzali, S.; Holwerda, H.T.; Ibaceta, R.R.; Perata, P. Tomato Fruits: A Good Target for Iodine Biofortification. Front. Plant Sci. 2013, 4, 205. [Google Scholar] [CrossRef] [Green Version]
- Smoleń, S.; Kowalska, I.; Halka, M.; Ledwożyw-Smoleń, I.; Grzanka, M.; Skoczylas, Ł.; Czernicka, M.; Pitala, J. Selected Aspects of Iodate and Iodosalicylate Metabolism in Lettuce Including the Activity of Vanadium Dependent Haloperoxidases as Affected by Exogenous Vanadium. Agronomy 2019, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Kiferle, C.; Martinelli, M.; Salzano, A.M.; Gonzali, S.; Beltrami, S.; Salvadori, P.A.; Hora, K.; Holwerda, H.T.; Scaloni, A.; Perata, P. Evidences for a Nutritional Role of Iodine in Plants. Front. Plant Sci. 2021, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Koukkou, E.G.; Roupas, N.D.; Markou, K.B. Effect of Excess Iodine Intake on Thyroid on Human Health. Minerva Med. 2017, 108, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Zicker, S.; Schoenherr, B. Focus on Nutrition: The Role of Iodine in Nutrition and Metabolism. Compend. Contin. Educ. Vet. 2012, 34, E1-4. [Google Scholar] [PubMed]
- Hulbert, A.J. Thyroid Hormones and Their Effects: A New Perspective. Biol. Rev. 2000, 75, 519–631. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J. Thyroid Hormones and Derivatives: Endogenous Thyroid Hormones and Their Targets. In Thyroid Hormone Nuclear Receptor; Springer: Berlin, Germany, 2018; Volume 1897, pp. 85–104. [Google Scholar]
- Citterio, C.E.; Targovnik, H.M.; Arvan, P. The Role of Thyroglobulin in Thyroid Hormonogenesis. Nat. Rev. Endocrinol. 2019, 15, 323–338. [Google Scholar] [CrossRef]
- Dedieu, A.; Gaillard, J.-C.; Pourcher, T.; Darrouzet, E.; Armengaud, J. Revisiting Iodination Sites in Thyroglobulin with an Organ-Oriented Shotgun Strategy. J. Biol. Chem. 2011, 286, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Fong, P. Thyroid Iodide Efflux: A Team Effort? J. Physiol. 2011, 589, 5929–5939. [Google Scholar] [CrossRef]
- Pereira, A.; Braekman, J.C.; Dumont, J.E.; Boeynaems, J.M. Identification of a Major Iodolipid from the Horse Thyroid Gland as 2-Iodohexadecanal. J. Biol. Chem. 1990, 265, 17018–17025. [Google Scholar] [CrossRef]
- Aceves, C.; Anguiano, B.; Delgado, G. Is Iodine a Gatekeeper of the Integrity of the Mammary Gland? J. Mammary Gland Biol. Neoplasia 2005, 10, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Zamocky, M.; Jakopitsch, C.; Furtmüller, P.G.; Dunand, C.; Obinger, C. The Peroxidase-Cyclooxygenase Superfamily: Reconstructed Evolution of Critical Enzymes of the Innate Immune System. Proteins Struct. Funct. Bioinforma. 2008, 72, 589–605. [Google Scholar] [CrossRef]
- Chino, Y.; Saito, M.; Yamasu, K.; Suyemitsu, T.; Ishihara, K. Formation of the Adult Rudiment of Sea Urchins Is Influenced by Thyroid Hormones. Dev. Biol. 1994, 161, 1–11. [Google Scholar] [CrossRef]
- Salvatore, G.; Covelli, I.; Roche, J. La Fixation Des Hormones Thyroidiennes Par Escherichia Coli et Son Mécanisme. Gen. Comp. Endocrinol. 1963, 3, 15–25. [Google Scholar] [CrossRef]
- Gräsbeck, R.; Lamberg, B.-A.; Björkstén, F. The Formation of Thyroxine Metabolites by Escherichia Coli. Acta Endocrinol. 1960, XXXIV, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Gkotsi, D.S.; Ludewig, H.; Sharma, S.V.; Connolly, J.A.; Dhaliwal, J.; Wang, Y.; Unsworth, W.P.; Taylor, R.J.K.; McLachlan, M.M.W.; Shanahan, S.; et al. A Marine Viral Halogenase That Iodinates Diverse Substrates. Nat. Chem. 2019, 11, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Kuhlisch, C.; Schleyer, G.; Shahaf, N.; Vincent, F.; Schatz, D.; Vardi, A. Viral Infection of Algal Blooms Leaves a Unique Metabolic Footprint on the Dissolved Organic Matter in the Ocean. Sci. Adv. 2021, 7, 1–14. [Google Scholar] [CrossRef]
- Capriotti, K.; Pelletier, K.; Barone, S.; Capriotti, J. Efficacy of Dilute Povidone-Iodine against Multi- Drug Resistant Bacterial Biofilms, Fungal Biofilms and Fungal Spores. J. Clin. Res. Dermatology 2018, 5, 1–5. [Google Scholar]
- Hoekstra, M.J.; Westgate, S.J.; Mueller, S. Povidone-Iodine Ointment Demonstrates in Vitro Efficacy against Biofilm Formation. Int. Wound J. 2017, 14, 172–179. [Google Scholar] [CrossRef]
- Chang, S.L. The Use of Active Iodine as a Water Disinfectant. J. Am. Pharm. Assoc. 1958, 47, 417–423. [Google Scholar] [CrossRef]
- HSU, Y.-C.; NOMURA, S.; KRUSÉ, C.W. Some Bactericidal and Virucidal Properties of Iodine Not Affecting Infectious RNA and DNA. Am. J. Epidemiol. 1965, 82, 317–328. [Google Scholar] [CrossRef]
- Schreier, H.; Erdos, G.; Reimer, K.; König, B.; König, W.; Fleischer, W. Molecular Effects of Povidone-Iodine on Relevant Microorganisms: An Electron-Microscopic and Biochemical Study. Dermatology 1997, 195, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, D.G. The Oxidation of Cysteine with Iodine: Formation of a Sulfinic Acid. J. Biol. Chem. 1933, 101, 35–42. [Google Scholar] [CrossRef]
- Apostolov, K. The Effects of Iodine on the Biological Activities of Myxoviruses. J. Hyg. 1980, 84, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Kohler, H.; Jenzer, H. Interaction of Lactoperoxidase with Hydrogen Peroxide. Formation of Enzyme Intermediates and Generation of Free Radicals. Free Radic. Biol. Med. 1989, 6, 323–339. [Google Scholar] [CrossRef]
- Winkler, R. Iodine—A Potential Antioxidant and the Role of Iodine/Iodide in Health and Disease. Nat. Sci. 2015, 07, 548–557. [Google Scholar] [CrossRef] [Green Version]
- Thomas, E.L.; Aune, T.M. Lactoperoxidase, Peroxide, Thiocyanate Antimicrobial System: Correlation of Sulfhydryl Oxidation with Antimicrobial Action. Infect. Immun. 1978, 20, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.L.; Aune, T.M. Cofactor Role of Iodide in Peroxidase Antimicrobial Action against Escherichia Coli. Antimicrob. Agents Chemother. 1978, 13, 1000–1005. [Google Scholar] [CrossRef] [Green Version]
- Fischer, A.J.; Lennemann, N.J.; Krishnamurthy, S.; Pócza, P.; Durairaj, L.; Launspach, J.L.; Rhein, B.A.; Wohlford-Lenane, C.; Lorentzen, D.; Bánfi, B.; et al. Enhancement of Respiratory Mucosal Antiviral Defenses by the Oxidation of Iodide. Am. J. Respir. Cell Mol. Biol. 2011, 45, 874–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohner, F.; Zimmermann, M.; Jooste, P.; Pandav, C.; Caldwell, K.; Raghavan, R.; Raiten, D.J. Biomarkers of Nutrition for Development—Iodine Review. J. Nutr. 2014, 144, 1322S–1342S. [Google Scholar] [CrossRef] [Green Version]
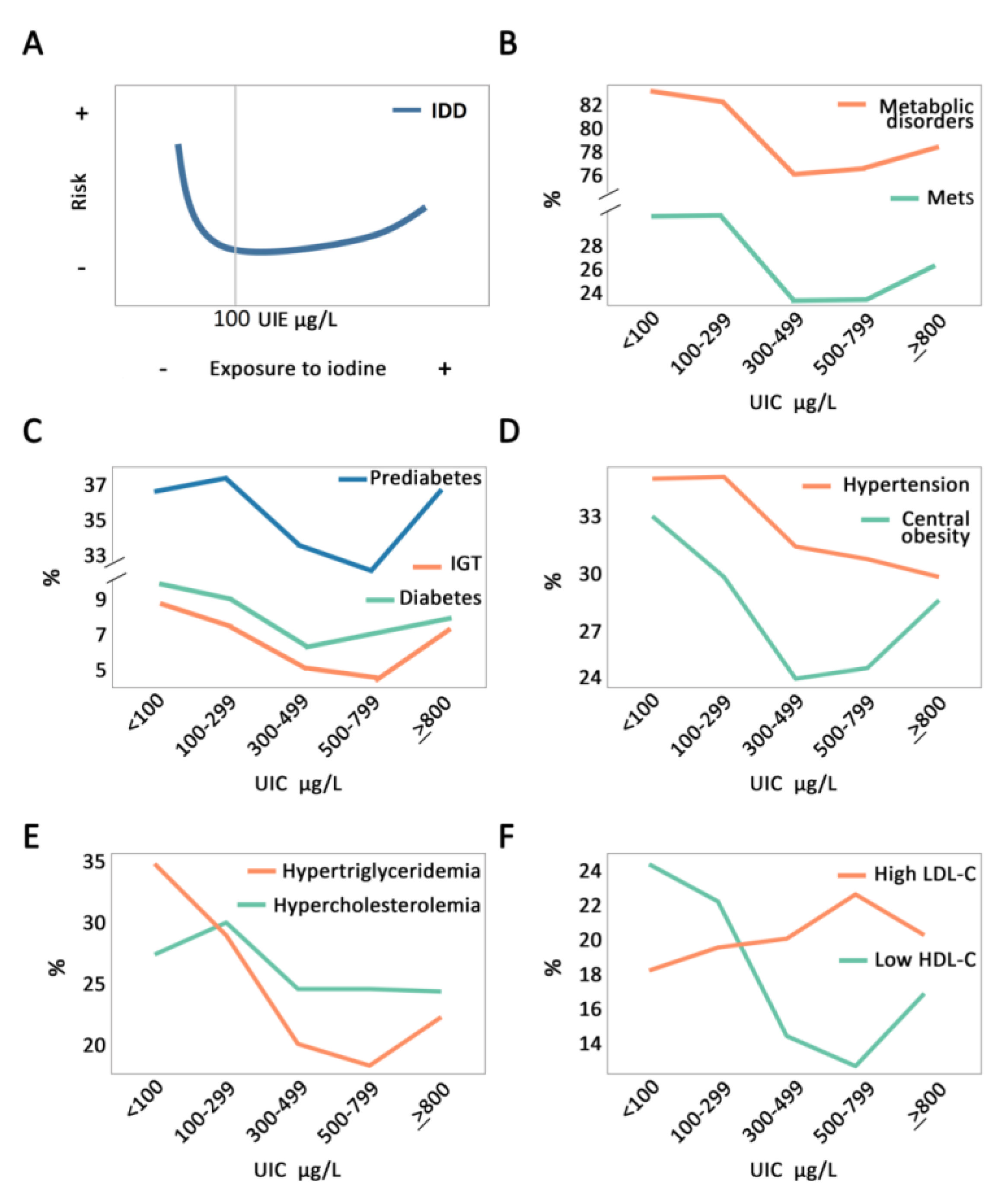
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-Deficiency Disorders. Lancet 2008, 372, 1251–1262. [Google Scholar] [CrossRef]
- Laurberg, P.; Pedersen, I.; Carlé, A.; Andersen, S.; Knudsen, N.; Ovesen, L. The U-Shaped Curve of Iodine Intake and Thyroid Disorders. In Comprehensive Handbook on Iodine: Nutritional, Endocrine and Pathological Aspects; Preedy, V.R., Burrow, G.N., Ross Watson, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 449–555. [Google Scholar]
- McCarrison, R. Observations on Endemic Cretinism In The Chitral And Gilgit Valleys.1. Lancet 1908, 172, 1275–1280. [Google Scholar] [CrossRef] [Green Version]
- Dreger, S.; Pfinder, M.; Christianson, L.; Lhachimi, S.K.; Zeeb, H. The Effects of Iodine Blocking Following Nuclear Accidents on Thyroid Cancer, Hypothyroidism, and Benign Thyroid Nodules: Design of a Systematic Review. Syst. Rev. 2015, 4, 126. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.; Zhang, Z.; Li, Y.; Teng, D.; Shi, X.; Ba, J.; Chen, B.; Du, J.; He, L.; Lai, X.; et al. U-Shaped Associations Between Urinary Iodine Concentration and the Prevalence of Metabolic Disorders: A Cross-Sectional Study. Thyroid 2020, 30, 1053–1065. [Google Scholar] [CrossRef]
- Pretell, E.A.; Delange, F.; Hostalek, U.; Corigliano, S.; Barreda, L.; Higa, A.M.; Altschuler, N.; Barragán, D.; Cevallos, J.L.; Gonzales, O.; et al. Iodine Nutrition Improves in Latin America. Thyroid 2004, 14, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Michikawa, T.; Inoue, M.; Shimazu, T.; Sawada, N.; Iwasaki, M.; Sasazuki, S.; Yamaji, T.; Tsugane, S. Seaweed Consumption and the Risk of Thyroid Cancer in Women: The Japan Public Health Center-Based Prospective Study. Eur. J. Cancer Prev. 2012, 21, 254–260. [Google Scholar] [CrossRef]
- Bogazzi, F.; Tomisti, L.; Bartalena, L.; Aghini-Lombardi, F.; Martino, E. Amiodarone and the Thyroid: A 2012 Update. J. Endocrinol. Investig. 2012, 35, 340–348. [Google Scholar]
- Rhee, C.M. Association Between Iodinated Contrast Media Exposure and Incident Hyperthyroidism and Hypothyroidism. Arch. Intern. Med. 2012, 172, 153. [Google Scholar] [CrossRef] [PubMed]
- Nobukuni, K.; Hayakawa, N.; Namba, R.; Ihara, Y.; Sato, K.; Takada, H.; Hayabara, T.; Kawahara, S. The Influence of Long-Term Treatment with Povidone-Iodine on Thyroid Function. Dermatology 1997, 195, 69–72. [Google Scholar] [CrossRef]
- Fiore, E.; Latrofa, F.; Vitti, P. Iodine, Thyroid Autoimmunity and Cancer. Eur. Thyroid J. 2015, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Teti, C.; Panciroli, M.; Nazzari, E.; Pesce, G.; Mariotti, S.; Olivieri, A.; Bagnasco, M. Iodoprophylaxis and Thyroid Autoimmunity: An Update. Immunol. Res. 2021, 69, 129–138. [Google Scholar] [CrossRef]
- Leung, A.M.; Braverman, L.E. Consequences of Excess Iodine. Nat. Rev. Endocrinol. 2014, 10, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Laurberg, P.; Andersen, S.; Pedersen, I.B.; Carlé, A. How Do We Optimize Iodine Intake to Minimize the Occurrence of Thyroid Disorders in Europe? Hot Thyroidol. 2007. [Google Scholar]
- Zava, T.T.; Zava, D.T. Assessment of Japanese Iodine Intake Based on Seaweed Consumption in Japan: A Literature-Based Analysis. Thyroid Res. 2011, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Buell, P. Changing Incidence of Breast Cancer in Japanese-American Women. JNCI J. Natl. Cancer Inst. 1973, 51, 1479–1483. [Google Scholar] [CrossRef]
- Shimizu, H.; Ross, R.; Bernstein, L.; Yatani, R.; Henderson, B.; Mack, T. Cancers of the Prostate and Breast among Japanese and White Immigrants in Los Angeles County. Br. J. Cancer 1991, 63, 963–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statista Research Department Statistics in State of Health. Available online: https://www.statista.com (accessed on 15 April 2022).
- Sharma, A.; Stan, M.N. Thyrotoxicosis: Diagnosis and Management. Mayo Clin. Proc. 2019, 94, 1048–1064. [Google Scholar] [CrossRef] [Green Version]
- Horn-Ross, P.L.; Morris, J.S.; Lee, M.; West, D.W.; Whittemore, A.S.; McDougall, I.R.; Nowels, K.; Stewart, S.L.; Spate, V.L.; Shiau, A.C.; et al. Iodine and Thyroid Cancer Risk among Women in a Multiethnic Population: The Bay Area Thyroid Cancer Study. Cancer Epidemiol Biomarkers Prev. 2001, 10, 979–985. [Google Scholar]
- Zimmermann, M.B.; Galetti, V. Iodine Intake as a Risk Factor for Thyroid Cancer: A Comprehensive Review of Animal and Human Studies. Thyroid Res. 2015, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huwiler, M.; Bürgi, U.; Kohler, H. Mechanism of Enzymatic and Non-Enzymatic Tyrosine Iodination. Inhibition by Excess Hydrogen Peroxide and/or Iodide. Eur. J. Biochem. 1985, 147, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Massart, C.; Chico-Galdo, V.; Jin, L.; De Maertelaer, V.; Decoster, C.; Dumont, J.E.; Van Sande, J. Species Specific Thyroid Signal Transduction: Conserved Physiology, Divergent Mechanisms. Mol. Cell. Endocrinol. 2010, 319, 56–62. [Google Scholar] [CrossRef]
- Ren, B.; Zhu, Y. A New Perspective on Thyroid Hormones: Crosstalk with Reproductive Hormones in Females. Int. J. Mol. Sci. 2022, 23, 2708. [Google Scholar] [CrossRef]
- Milanesi, A.; Brent, G.A. Iodine and Thyroid Hormone Synthesis, Metabolism, and Action. In Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1896, pp. 143–150. [Google Scholar]
- Filetti, S.; Bidart, J.; Arturi, F.; Caillou, B.; Russo, D.; Schlumberger, M. Sodium/Iodide Symporter: A Key Transport System in Thyroid Cancer Cell Metabolism. Eur. J. Endocrinol. 1999, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Nicola, J.P.; Basquin, C.; Portulano, C.; Reyna-Neyra, A.; Paroder, M.; Carrasco, N. The Na+/I- Symporter Mediates Active Iodide Uptake in the Intestine. Am. J. Physiol.-Cell Physiol. 2009, 296, 654–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, Y.S.; Farhana, A. Histology, Thyroid Gland. StatPearls 2019. [Google Scholar]
- Darrouzet, E.; Lindenthal, S.; Marcellin, D.; Pellequer, J.L.; Pourcher, T. The Sodium/Iodide Symporter: State of the Art of Its Molecular Characterization. Biochim. Biophys. Acta - Biomembr. 2014, 1838, 244–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silveira, J.C.; Kopp, P.A. Pendrin and Anoctamin as Mediators of Apical Iodide Efflux in Thyroid Cells. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wei, Y.; Wang, P.; Deng, Z.; Mao, J.; Zhu, L.; Chen, L.; Peng, S.; Wang, L. Excess Iodide-Induced Reactive Oxygen Species Elicit Iodide Efflux via β-Tubulin-Associated ClC-3 in Thyrocytes. Biochem. J. 2022, 479, 629–640. [Google Scholar] [CrossRef]
- De La Vieja, A.; Santisteban, P. Role of Iodide Metabolism in Physiology and Cancer. Endocr. Relat. Cancer 2018, 25, R225–R245. [Google Scholar] [CrossRef] [Green Version]
- Smyth, P.P.A. Role of Iodine in Antioxidant Defence in Thyroid and Breast Disease. BioFactors 2003, 19, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Kopp, P.A. Reduce, Recycle, Reuse—Iodotyrosine Deiodinase in Thyroid Iodide Metabolism. N. Engl. J. Med. 2008, 358, 1856–1859. [Google Scholar] [CrossRef]
- Nicoloff, J.T.; Low, J.C.; Dussault, J.H.; Fisher, D.A. Simultaneous Measurement of Thyroxine and Triiodothyronine Peripheral Turnover Kinetics in Man. J. Clin. Invest. 1972, 51, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Lima, C.; Burman, K.D. Reverse T 3 or Perverse T 3? Still Puzzling after 40 Years. Cleve. Clin. J. Med. 2018, 85, 450–455. [Google Scholar] [CrossRef] [Green Version]
- Kelly, G.S. Peripheral Metabolism of Thyroid Hormones: A Review. Altern. Med. Rev. 2000, 5, 306–333. [Google Scholar]
- DISTEFANO, J.J. Excretion, Metabolism and Enterohepatic Circulation Pathways and Their Role in Overall Thyroid Hormone Regulation in the Rat. Am. Zool. 1988, 28, 373–387. [Google Scholar] [CrossRef]
- DiStefano, J.J.; De Luze, A.; Nguyen, T.T. Binding and Degradation of 3,5,3′-Triiodothyronine and Thyroxine by Rat Intestinal Bacteria. Am. J. Physiol. -Endocrinol. Metab. 1993, 264, 6. [Google Scholar] [CrossRef]
- Rutgers, M.; Heusdens, F.A.; Bonthuis, F.; de Herder, W.W.; Hazenberg, M.P.; Visser, T.J. Enterohepatic Circulation of Triiodothyronine (T3) in Rats: Importance of the Microflora for the Liberation and Reabsorption of T3 from Biliary T3 Conjugates. Endocrinology 1989, 125, 2822–2830. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Microbiota and Thyroid Interaction in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 479–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bargiel, P.; Szczuko, M.; Stachowska, L.; Prowans, P.; Czapla, N.; Markowska, M.; Petriczko, J.; Kledzik, J.; Jędrzejczyk-Kledzik, A.; Palma, J.; et al. Microbiome Metabolites and Thyroid Dysfunction. J. Clin. Med. 2021, 10, 3609. [Google Scholar] [CrossRef] [PubMed]
- Anyetei-Anum, C.S.; Roggero, V.R.; Allison, L.A. Thyroid Hormone Receptor Localization in Target Tissues. J. Endocrinol. 2018, 237, R19–R34. [Google Scholar] [CrossRef] [Green Version]
- Visser, W.E.; Friesema, E.C.H.; Visser, T.J. Minireview: Thyroid Hormone Transporters: The Knowns and the Unknowns. Mol. Endocrinol. 2011, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, F.; Hagenbuch, B.; Stieger, B.; Klenk, U.; Folkers, G.; Meier, P.J. Identification of a Novel Human Organic Anion Transporting Polypeptide as a High Affinity Thyroxine Transporter. Mol. Endocrinol. 2002, 16, 2283–2296. [Google Scholar] [CrossRef] [Green Version]
- Bakker, O. Thyroid Hormone Receptors. In Encyclopedia of Endocrine Diseases; Elsevier: Amsterdam, The Netherlands, 2004; pp. 490–495. [Google Scholar]
- Cheng, S.-Y.; Leonard, J.L.; Davis, P.J. Molecular Aspects of Thyroid Hormone Actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef] [Green Version]
- De Vito, P.; Incerpi, S.; Pedersen, J.Z.; Luly, P.; Davis, F.B.; Davis, P.J. Thyroid Hormones as Modulators of Immune Activities at the Cellular Level. Thyroid 2011, 21, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Flamant, F.; Cheng, S.-Y.; Hollenberg, A.N.; Moeller, L.C.; Samarut, J.; Wondisford, F.E.; Yen, P.M.; Refetoff, S. Thyroid Hormone Signaling Pathways: Time for a More Precise Nomenclature. Endocrinology 2017, 158, 2052–2057. [Google Scholar] [CrossRef]
- Cioffi, F.; Giacco, A.; Goglia, F.; Silvestri, E. Bioenergetic Aspects of Mitochondrial Actions of Thyroid Hormones. Cells 2022, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Pessemesse, L.; Lepourry, L.; Bouton, K.; Levin, J.; Cabello, G.; Wrutniak-Cabello, C.; Casas, F. P28, a Truncated Form of TRα1 Regulates Mitochondrial Physiology. FEBS Lett. 2014, 588, 4037–4043. [Google Scholar] [CrossRef] [Green Version]
- Leonard, J.L.; Farwell, A.P. Thyroid Hormone-Regulated Actin Polymerization in Brain. Thyroid 2009, 7, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Medina-Gomez, G.; Hernández, A.; Calvo, R.M.; Martin, E.; Obregón, M.J. Potent Thermogenic Action of Triiodothyroacetic Acid in Brown Adipocytes. Cell. Mol. Life Sci. C 2003, 60, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Senese, R.; Cioffi, F.; de Lange, P.; Goglia, F.; Lanni, A. Thyroid: Biological Actions of ‘Nonclassical’ Thyroid Hormones. J. Endocrinol. 2014, 221, R1–R12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiellini, G.; Frascarelli, S.; Ghelardoni, S.; Carnicelli, V.; Tobias, S.C.; DeBarber, A.; Brogioni, S.; Ronca-Testoni, S.; Cerbai, E.; Grandy, D.K.; et al. Cardiac Effects of 3-Iodothyronamine: A New Aminergic System Modulating Cardiac Function. FASEB J. 2007, 21, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Giammanco, M.; Di Liegro, C.M.; Schiera, G.; Di Liegro, I. Genomic and Non-Genomic Mechanisms of Action of Thyroid Hormones and Their Catabolite 3,5-Diiodo-L-Thyronine in Mammals. Int. J. Mol. Sci. 2020, 21, 4140. [Google Scholar] [CrossRef] [PubMed]
- Manzon, R.G.; Manzon, L.A. Lamprey Metamorphosis: Thyroid Hormone Signaling in a Basal Vertebrate. Mol. Cell. Endocrinol. 2017, 459, 28–42. [Google Scholar] [CrossRef]
- Klootwijk, W.; Friesema, E.C.H.; Visser, T.J. A Nonselenoprotein from Amphioxus Deiodinates Triac But Not T3: Is Triac the Primordial Bioactive Thyroid Hormone? Endocrinology 2011, 152, 3259–3267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, E.; Heyland, A. Evolution of Thyroid Hormone Signaling in Animals: Non-Genomic and Genomic Modes of Action. Mol. Cell. Endocrinol. 2017, 459, 14–20. [Google Scholar] [CrossRef]
- Bertrand, S.; Brunet, F.G.; Escriva, H.; Parmentier, G.; Laudet, V.; Robinson-Rechavi, M. Evolutionary Genomics of Nuclear Receptors: From Twenty-Five Ancestral Genes to Derived Endocrine Systems. Mol. Biol. Evol. 2004, 21, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Niles, E.G.; LoVerde, P.T. Thyroid Hormone Receptor Orthologues from Invertebrate Species with Emphasis on Schistosoma Mansoni. BMC Evol. Biol. 2007, 7, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berking, S.; Czech, N.; Gerharz, M.; Herrmann, K.; Hoffmann, U.; Raifer, H.; Sekul, G.; Siefker, B.; Sommerei, A.; Vedder, F. A Newly Discovered Oxidant Defence System and Its Involvement in the Development of Aurelia Aurita (Scyphozoa, Cnidaria): Reactive Oxygen Species and Elemental Iodine Control Medusa Formation. Int. J. Dev. Biol. 2005, 49, 969–976. [Google Scholar] [CrossRef] [Green Version]
- Eales, J.G. Iodine Metabolism and Thyroid-Related Functions in Organisms Lacking Thyroid Follicles: Are Thyroid Hormones Also Vitamins? Exp. Biol. Med. 1997, 214, 302–317. [Google Scholar] [CrossRef]
- Mourouzis, I.; Lavecchia, A.M.; Xinaris, C. Thyroid Hormone Signalling: From the Dawn of Life to the Bedside. J. Mol. Evol. 2020, 88, 88–103. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Kochman, J.; Jakubczyk, K.; Bargiel, P.; Janda-Milczarek, K. The Influence of Oxidative Stress on Thyroid Diseases. Antioxidants 2021, 10, 1442. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free Radicals, Reactive Oxygen Species, Oxidative Stress and Its Classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Mohanty, J.G.; Friedman, J.S.; Rifkind, J.M. Role of Peroxiredoxin-2 in Protecting RBCs from Hydrogen Peroxide-Induced Oxidative Stress. Free Radic. Res. 2013, 47, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.; Rong, Y.; Doctrow, S.; Baudry, M.; Malfroy, B.; Xu, Z. Synthetic Superoxide Dismutase/Catalase Mimetics Reduce Oxidative Stress and Prolong Survival in a Mouse Amyotrophic Lateral Sclerosis Model. Neurosci. Lett. 2001, 304, 157–160. [Google Scholar] [CrossRef]
- Polefka, T.G.; Meyer, T.A.; Agin, P.P.; Bianchini, R.J. Cutaneous Oxidative Stress. J. Cosmet. Dermatol. 2012, 11, 55–64. [Google Scholar] [CrossRef] [PubMed]
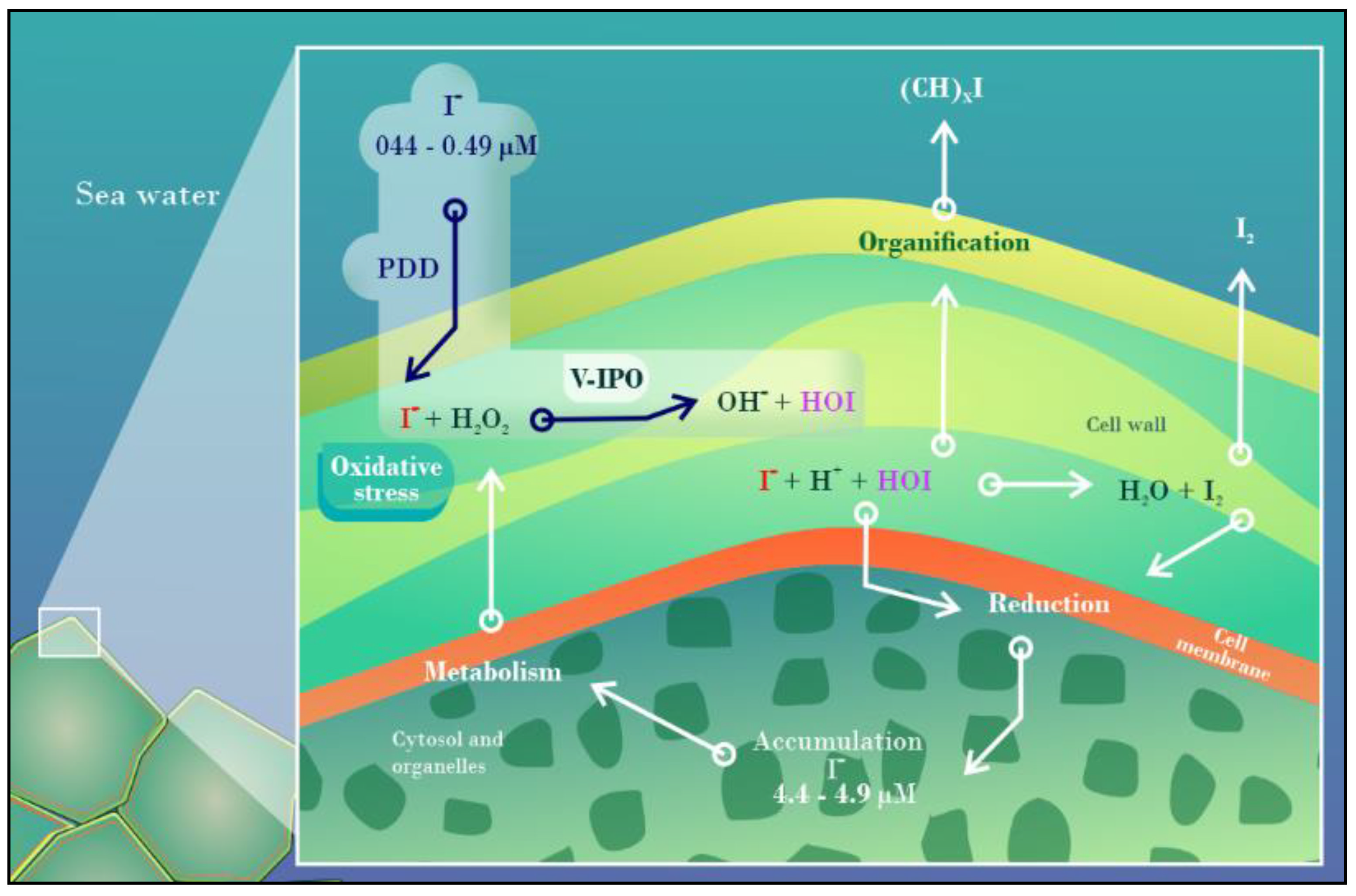
- Küpper, F.C.; Schweigert, N.; Gall, E.A.; Legendre, J.; Vilter, H.; Kloareg, B. Iodine Uptake in Laminariales Involves Extracellular, Haloperoxidase-Mediated Oxidation of Iodide. Planta 1998, 207, 163–171. [Google Scholar] [CrossRef]
- Mohammad, A.; Liebhafsky, H.A. The Kinetics of the Reduction of Hydrogen Peroxide by the Halides. J. Am. Chem. Soc. 1934, 56, 1680–1685. [Google Scholar] [CrossRef]
- Stanbury, D.M. Reduction Potentials Involving Inorganic Free Radicals in Aqueous Solution. In Advances in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 1989; Volume 33, pp. 69–138. [Google Scholar]
- Küpper, F.C.; Carrano, C.J. Key Aspects of the Iodine Metabolism in Brown Algae: A Brief Critical Review. Metallomics 2019, 11, 756–764. [Google Scholar] [CrossRef]
- Küpper, F.C.; Carpenter, L.J.; Leblanc, C.; Toyama, C.; Uchida, Y.; Maskrey, B.H.; Robinson, J.; Verhaeghe, E.F.; Malin, G.; Luther, G.W.; et al. In Vivo Speciation Studies and Antioxidant Properties of Bromine in Laminaria Digitata Reinforce the Significance of Iodine Accumulation for Kelps. J. Exp. Bot. 2013, 64, 2653–2664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosse, A.; Potin, P.; Leblanc, C. Patterns of Gene Expression Induced by Oligoguluronates Reveal Conserved and Environment-specific Molecular Defense Responses in the Brown Alga Laminaria Digitata. New Phytol. 2009, 182, 239–250. [Google Scholar] [CrossRef]
- Nitschke, U.; Stengel, D.B. Iodine Contributes to Osmotic Acclimatisation in the Kelp Laminaria Digitata (Phaeophyceae). Planta 2014, 239, 521–530. [Google Scholar] [CrossRef]
- Miller, A.E.M.M.; Heyland, A. Iodine Accumulation in Sea Urchin Larvae Is Dependent on Peroxide. J. Exp. Biol. 2012, 216, 915–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; Benavides-Mendoza, A. Use of Iodine to Biofortify and Promote Growth and Stress Tolerance in Crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef] [Green Version]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K. V Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91 Spec No, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Khanna-Chopra, R.; Semwal, V.K. Superoxide Dismutase and Ascorbate Peroxidase Are Constitutively More Thermotolerant than Other Antioxidant Enzymes in Chenopodium Album. Physiol. Mol. Biol. Plants 2011, 17, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Shukla Bajpai, M.; Singh Majumdar, R.; Mishra, P.K. Response of Iodine on Antioxidant Levels of Glycine Max L. Grown under Cd Stress 2+. Adv. Biol. Res. (Rennes). 2015, 9, 40–48. [Google Scholar]
- Halka, M.; Smoleń, S.; Ledwożyw-Smoleń, I.; Sady, W. Comparison of Effects of Potassium Iodide and Iodosalicylates on the Antioxidant Potential and Iodine Accumulation in Young Tomato Plants. J. Plant Growth Regul. 2020, 39, 282–295. [Google Scholar] [CrossRef] [Green Version]
- Weng, H.-X.; Yan, A.-L.; Hong, C.-L.; Xie, L.-L.; Qin, Y.-C.; Cheng, C.Q. Uptake of Different Species of Iodine by Water Spinach and Its Effect to Growth. Biol. Trace Elem. Res. 2008, 124, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Ameziane El Hassani, R.; Buffet, C.; Leboulleux, S.; Dupuy, C. Oxidative Stress in Thyroid Carcinomas: Biological and Clinical Significance. Endocr. Relat. Cancer 2019, 26, R131–R143. [Google Scholar] [CrossRef] [Green Version]
- Bartosz, G. Total Antioxidant Capacity. Adv. Clin. Chem. 2003, 37, 219–292. [Google Scholar] [PubMed]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef]
- Letai, A. Apoptosis and Cancer. Annu. Rev. Cancer Biol. 2017, 1, 275–294. [Google Scholar] [CrossRef]
- Alfaro, Y.; Delgado, G.; Cárabez, A.; Anguiano, B.; Aceves, C. Iodine and Doxorubicin, a Good Combination for Mammary Cancer Treatment: Antineoplastic Adjuvancy, Chemoresistance Inhibition, and Cardioprotection. Mol. Cancer 2013, 12, 45. [Google Scholar] [CrossRef] [Green Version]
- Quintero-García, M.; Delgado-González, E.; Sánchez-Tusie, A.; Vázquez, M.; Aceves, C.; Anguiano, B. Iodine Prevents the Increase of Testosterone-Induced Oxidative Stress in a Model of Rat Prostatic Hyperplasia. Free Radic. Biol. Med. 2018, 115, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Winkler, R.; Griebenow, S.; Wonisch, W. Effect of Iodide on Total Antioxidant Status of Human Serum. Cell Biochem. Funct. 2000, 18, 143–146. [Google Scholar] [CrossRef]
- Habibi, N.; Labrinidis, A.; Leemaqz, S.Y.L.; Jankovic-Karasoulos, T.; McCullough, D.; Grieger, J.A.; Gilbert, S.; Ricciardelli, C.; Zhou, S.J.; Perkins, A.V.; et al. Effect of Selenium and Iodine on Oxidative Stress in the First Trimester Human Placenta Explants. Nutrients 2021, 13, 800. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Tiwari, S.; Banakar, P.S.; Bhakat, M.; Mani, V.; Mohanty, T.K.; Mondal, G. Iodine Supplementation Improved Antioxidant Status, Hormonal Status, Sexual Behavior, and Semen Production Performance of Bos Indicus Bulls Under Tropical Climatic Condition. Biol. Trace Elem. Res. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fomichev, Y.P.; Nikanova, L.A. Increasing of the Reproductive Properties of Boar Semen While Using Organic Iodine in Feeding. Russ. Agric. Sci. 2017, 43, 419–422. [Google Scholar] [CrossRef]
- Chakraborty, A.; Singh, V.; Singh, K.; Rajender, S. Excess Iodine Impairs Spermatogenesis by Inducing Oxidative Stress and Perturbing the Blood Testis Barrier. Reprod. Toxicol. 2020, 96, 128–140. [Google Scholar] [CrossRef]
- Chakraborty, A.; Mandal, J.; Mondal, C.; Sinha, S.; Chandra, A.K. Effect of Excess Iodine on Oxidative Stress Markers, Steroidogenic-Enzyme Activities, Testicular Morphology, and Functions in Adult Male Rats. Biol. Trace Elem. Res. 2016, 172, 380–394. [Google Scholar] [CrossRef]
- Lee, J.-M.; Johnson, J.A. An Important Role of Nrf2-ARE Pathway in the Cellular Defense Mechanism. BMB Rep. 2004, 37, 139–143. [Google Scholar] [CrossRef]
- Kim, H.J.; Vaziri, N.D. Contribution of Impaired Nrf2-Keap1 Pathway to Oxidative Stress and Inflammation in Chronic Renal Failure. Am. J. Physiol.-Ren. Physiol. 2010, 298, 662–671. [Google Scholar] [CrossRef] [Green Version]
- Ben-Yehuda Greenwald, M.; Frušić-Zlotkin, M.; Soroka, Y.; Ben-Sasson, S.; Bianco-Peled, H.; Kohen, R. A Novel Role of Topical Iodine in Skin: Activation of the Nrf2 Pathway. Free Radic. Biol. Med. 2017, 104, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Aceves, C.; Mendieta, I.; Anguiano, B.; Delgado-González, E. Molecular Iodine Has Extrathyroidal Effects as an Antioxidant, Differentiator, and Immunomodulator. Int. J. Mol. Sci. 2021, 22, 1228. [Google Scholar] [CrossRef] [PubMed]
- Brash, A.R. Arachidonic Acid as a Bioactive Molecule. J. Clin. Invest. 2001, 107, 1339–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dugrillon, A.; Uedelhoven, W.; Pisarev, M.; Bechtner, G.; Gärtner, R. Identification of δ-Iodolactone in Iodide Treated Human Goiter and Its Inhibitory Effect on Proliferation of Human Thyroid Follicles. Horm. Metab. Res. 1994, 26, 465–469. [Google Scholar] [CrossRef]
- Schlorke, D.; Flemmig, J.; Birkemeyer, C.; Arnhold, J. Formation of Cyanogen Iodide by Lactoperoxidase. J. Inorg. Biochem. 2016, 154, 35–41. [Google Scholar] [CrossRef]
- Williams, R. Killing Controversy. J. Exp. Med. 2006, 203, 2404. [Google Scholar] [CrossRef] [Green Version]
- Bilal, M.Y.; Dambaeva, S.; Kwak-Kim, J.; Gilman-Sachs, A.; Beaman, K.D. A Role for Iodide and Thyroglobulin in Modulating the Function of Human Immune Cells. Front. Immunol. 2017, 8, 1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, T.J.; Onishi, K.G.; Bradley, S.P.; Prendergast, B.J. Cell-Autonomous Iodothyronine Deiodinase Expression Mediates Seasonal Plasticity in Immune Function. Brain. Behav. Immun. 2014, 36, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Weetman, A.P.; McGregor, A.M.; Campbell, H.; Lazarus, J.H.; Ibbertson, H.K.; Hall, R. Iodide Enhances IgG Synthesis by Human Peripheral Blood Lymphocytes in Vitro. Eur. J. Endocrinol. 1983, 103, 210–215. [Google Scholar] [CrossRef]
- Langer, R.; Burzler, C.; Bechtner, G.; Gärtner, R. Influence of Iodide and Iodolactones on Thyroid Apoptosis. Evidence That Apoptosis Induced by Iodide Is Mediated by Iodolactones in Intact Porcine Thyroid Follicles. Exp. Clin. Endocrinol. Diabetes 2003, 111, 325–329. [Google Scholar] [CrossRef]
- Gärtner, R.; Dugrillon, A.; Bechtner, G. Evidence That Iodolactones Are the Mediators of Growth Inhibition by Iodine on the Thyroid. Acta Med. Austriaca 1996, 23, 47–51. [Google Scholar]
- Gärtner, R.; Rank, P.; Ander, B. The Role of Iodine and δ-Iodolactone in Growth and Apoptosis of Malignant Thyroid Epithelial Cells and Breast Cancer Cells. Hormones 2010, 9, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, R.; Schopohl, D.; Schaefer, S.; Dugrillon, A.; Erdmann, A.; Toda, S.; Bechtner, G. Regulation of Transforming Growth Factor Beta 1 Messenger Ribonucleic Acid Expression in Porcine Thyroid Follicles in Vitro by Growth Factors, Iodine, or Delta-Iodolactone. Thyroid 1997, 7, 633–640. [Google Scholar] [CrossRef]
- Dugrillon, A. Iodolactones and Iodoaldehydes—Mediators of Iodine in Thyroid Autoregulation. Exp. Clin. Endocrinol. Diabetes 2009, 104, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Sassone-Corsi, P. The Cyclic AMP Pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011148. [Google Scholar] [CrossRef]
- Rodríguez-Castelán, J.; Delgado-González, E.; Rodríguez-Benítez, E.; Castelán, F.; Cuevas-Romero, E.; Aceves, C. SAT-561 Protective Effect of Moderated Dose of Iodine in Pancreatic Alterations during Hypothyroidism. J. Endocr. Soc. 2019, 3, SAT-561. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ Signaling and Metabolism: The Good, the Bad and the Future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Nava-Villalba, M.; Nuñez-Anita, R.E.; Bontempo, A.; Aceves, C. Activation of Peroxisome Proliferator-Activated Receptor Gamma Is Crucial for Antitumoral Effects of 6-Iodolactone. Mol. Cancer 2015, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Vitale, M.; Di Matola, T.; D’Ascoli, F.; Salzano, S.; Bogazzi, F.; Fenzi, G.; Martino, E.; Rossi, G. Iodide Excess Induces Apoptosis in Thyroid Cells through a P53-Independent Mechanism Involving Oxidative Stress1. Endocrinology 2000, 141, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Mendieta, I.; Nuñez-Anita, R.E.; Nava-Villalba, M.; Zambrano-Estrada, X.; Delgado-González, E.; Anguiano, B.; Aceves, C. Molecular Iodine Exerts Antineoplastic Effects by Diminishing Proliferation and Invasive Potential and Activating the Immune Response in Mammary Cancer Xenografts. BMC Cancer 2019, 19, 261. [Google Scholar] [CrossRef]
- Shrivastava, A.; Tiwari, M.; Sinha, R.A.; Kumar, A.; Balapure, A.K.; Bajpai, V.K.; Sharma, R.; Mitra, K.; Tandon, A.; Godbole, M.M. Molecular Iodine Induces Caspase-Independent Apoptosis in Human Breast Carcinoma Cells Involving the Mitochondria-Mediated Pathway. J. Biol. Chem. 2006, 281, 19762–19771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulda, S.; Debatin, K.-M. Extrinsic versus Intrinsic Apoptosis Pathways in Anticancer Chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef] [Green Version]
- Arroyo-Helguera, O.; Anguiano, B.; Delgado, G.; Aceves, C. Uptake and Antiproliterative Effect of Molecular Iodine in the MCF-7 Breast Cancer Cell Line. Endocr. Relat. Cancer 2006, 13, 1147–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aceves, C.; García-Solís, P.; Arroyo-Helguera, O.; Vega-Riveroll, L.; Delgado, G.; Anguiano, B. Antineoplastic Effect of Iodine in Mammary Cancer: Participation of 6-Iodolactone (6-IL) and Peroxisome Proliferator-Activated Receptors (PPAR). Mol. Cancer 2009, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Bigoni-Ordóñez, G.D.; Ortiz-Sánchez, E.; Rosendo-Chalma, P.; Valencia-González, H.A.; Aceves, C.; García-Carrancá, A. Molecular Iodine Inhibits the Expression of Stemness Markers on Cancer Stem-like Cells of Established Cell Lines Derived from Cervical Cancer. BMC Cancer 2018, 18, 1–12. [Google Scholar] [CrossRef]
- García-Solís, P.; Alfaro, Y.; Anguiano, B.; Delgado, G.; Guzman, R.C.; Nandi, S.; Díaz-Muñoz, M.; Vázquez-Martínez, O.; Aceves, C. Inhibition of N-Methyl-N-Nitrosourea-Induced Mammary Carcinogenesis by Molecular Iodine (I2) but Not by Iodide (I−) Treatment. Mol. Cell. Endocrinol. 2005, 236, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Juríková, M.; Danihel, Ľ.; Polák, Š.; Varga, I. Ki67, PCNA, and MCM Proteins: Markers of Proliferation in the Diagnosis of Breast Cancer. Acta Histochem. 2016, 118, 544–552. [Google Scholar] [CrossRef]
- Álvarez-León, W.; Mendieta, I.; Delgado-González, E.; Anguiano, B.; Aceves, C. Molecular Iodine/Cyclophosphamide Synergism on Chemoresistant Neuroblastoma Models. Int. J. Mol. Sci. 2021, 22, 8936. [Google Scholar]
- FitzGerald, G.A. COX-2 and beyond: Approaches to Prostaglandin Inhibition in Human Disease. Nat. Rev. Drug Discov. 2003, 2, 879–890. [Google Scholar] [CrossRef]
- Hussain, T.; Gupta, S.; Mukhtar, H. Cyclooxygenase-2 and Prostate Carcinogenesis. Cancer Lett. 2003, 191, 125–135. [Google Scholar] [CrossRef]
- Moreno-Vega, A.; Vega-Riveroll, L.; Ayala, T.; Peralta, G.; Torres-Martel, J.M.; Rojas, J.; Mondragón, P.; Domínguez, A.; De Obaldía, R.; Avecilla-Guerrero, C.; et al. Adjuvant Effect of Molecular Iodine in Conventional Chemotherapy for Breast Cancer. Randomized Pilot Study. Nutrients 2019, 11, 1623. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Micó, O.; Delgado-González, E.; Anguiano, B.; Vaca-Paniagua, F.; Medina-Rivera, A.; Rodríguez-Dorantes, M.; Aceves, C. Effects of Molecular Iodine/Chemotherapy in the Immune Component of Breast Cancer Tumoral Microenvironment. Biomolecules 2021, 11, 1501. [Google Scholar] [CrossRef] [PubMed]
- Aceves, C.; Anguiano, B.; Delgado, G. The Extrathyronine Actions of Iodine as Antioxidant, Apoptotic, and Differentiation Factor in Various Tissues. Thyroid 2013, 23, 938–946. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yong, M.; Li, J.; Dong, X.; Yu, T.; Fu, X.; Hu, L. High Level of CFTR Expression Is Associated with Tumor Aggression and Knockdown of CFTR Suppresses Proliferation of Ovarian Cancer in Vitro and in Vivo. Oncol. Rep. 2015, 33, 2227–2234. [Google Scholar] [CrossRef] [Green Version]
- Tu, Z.; Chen, Q.; Zhang, J.T.; Jiang, X.; Xia, Y.; Chan, H.C. CFTR Is a Potential Marker for Nasopharyngeal Carcinoma Prognosis and Metastasis. Oncotarget 2016, 7, 76955. [Google Scholar] [CrossRef] [Green Version]
- Bilal, M.Y.; Dambaeva, S.; Brownstein, D.; Kwak-Kim, J.; Gilman-Sachs, A.; Beaman, K.D. Iodide Transporters in the Endometrium: A Potential Diagnostic Marker for Women with Recurrent Pregnancy Failures. Med. Princ. Pract. 2020, 29, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated PH: A Perfect Storm for Cancer Progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Chang, N.W.; Wu, C.T.; Chen, D.R.; Yeh, C.Y.; Lin, C. High Levels of Arachidonic Acid and Peroxisome Proliferator-Activated Receptor-Alpha in Breast Cancer Tissues Are Associated with Promoting Cancer Cell Proliferation. J. Nutr. Biochem. 2013, 24, 274–281. [Google Scholar] [CrossRef]
- Wang, X.; Yan, F.; Wang, Q. Molecular Iodine: Catalysis in Heterocyclic Synthesis. Synth. Commun. 2021, 51, 1763–1781. [Google Scholar]
- Bürgi, H.; Schaffner, T.; Seiler, J.P. The Toxicology of Iodate: A Review of the Literature. Thyroid 2001, 11, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Tekale, S.U.; Kauthale, S.S.; Dake, S.A.; Sarda, S.R.; Pawar, R.P. Molecular Iodine: An Efficient and Versatile Reagent for Organic Synthesis. Curr. Org. Chem. 2012, 16, 1485–1501. [Google Scholar] [CrossRef]
- Dohi, T.; Kita, Y. Oxidizing Agents. In Iodine Chemistry and Applications; Kaiho, T., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 277–301. [Google Scholar]
- World Health Organization. WHO Model List of Essential Medicines—22nd List; World Health Organization: Geneva, Switzerland, 2021; p. 66. [Google Scholar]
- Lugol, J.G.A. Mémoire Sur l’Emploi de l’Iode Dans Les Maladies Scrofuleuses. Glasgow Med. J. 1832, 5, 83–92. [Google Scholar]
- Fernández, L. Global Market Value of Iodine 2017 & 2024. Available online: https://www.statista.com/statistics/1001959/market-value-iodine-worldwide/ (accessed on 2 March 2022).
- Garside, M. Iodine Global Reserves by Countries 2020. Available online: https://www.statista.com/statistics/264946/global-iodine-reserves-by-countries/ (accessed on 2 March 2022).
- Garside, M. Major Countries in Iodine Production 2010–2020. Available online: https://www.statista.com/statistics/264945/major-countries-in-iodine-production/ (accessed on 2 March 2022).
- Rayyes, A.; Hamid, A. Technical Meeting of Project Counterparts on Cyclotron Production of I-123. In Proceedings of the Cyclotron production of Iodine-123, Sao Paulo, Brazil, 8–10 August 2001; International Atomic Energy Agency: Vienna, Brazil, 2002; pp. 81–89. [Google Scholar]
- Chattopadhyay, S.; Saha Das, S. Recovery of 131I from Alkaline Solution of N-Irradiated Tellurium Target Using a Tiny Dowex-1 Column. Appl. Radiat. Isot. 2010, 68, 1967–1969. [Google Scholar] [CrossRef] [PubMed]
- Statista Research Department Global Demand for Iodine by Application 2016. Available online: https://www.statista.com/statistics/862097/global-iodine-demand-share-by-application/ (accessed on 2 March 2022).
- Tang, Y.; Xu, Y.; Li, F.; Jmaiff, L.; Hrudey, S.E.; Li, X.-F. Nontargeted Identification of Peptides and Disinfection Byproducts in Water. J. Environ. Sci. 2016, 42, 259–266. [Google Scholar] [CrossRef]
- Gao, Y.; Qiu, J.; Ji, Y.; Wawryk, N.J.P.; An, T.; Li, X.-F. Formation Mechanism of Iodinated Aromatic Disinfection Byproducts: Acid Catalysis with H2OI+. Environ. Sci. Technol. 2022, 56, 1791–1800. [Google Scholar] [CrossRef]
- Prütz, W.A.; Kissner, R.; Koppenol, W.H.; Rüegger, H. On the Irreversible Destruction of Reduced Nicotinamide Nucleotides by Hypohalous Acids. Arch. Biochem. Biophys. 2000, 380, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.M.; Knox, K.; Robin, M.B. Crystal Structure of HI 3 ·2C 6 H 5 CONH 2: A Model of the Starch—Iodine Complex. J. Chem. Phys. 1964, 40, 1082–1089. [Google Scholar] [CrossRef]
- Makhayeva, D.N.; Irmukhametova, G.S.; Khutoryanskiy, V.V. Polymeric Iodophors: Preparation, Properties, and Biomedical Applications. Rev. J. Chem. 2020, 10, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Sukawa, H.; Yoda, Y.; Sugimoto, H.; Yoshida, S.; Yamamoto, T.; Kuroda, S.; Sanechika, K.; Nishinuma, M. Absorption of Iodine by Polymers and Electrochemical Response of Polymer Film in Aqueous Solution of Iodine. Polym. J. 1989, 21, 403–408. [Google Scholar] [CrossRef] [Green Version]
- French, E.A.; Mukai, M.; Zurakowski, M.; Rauch, B.; Gioia, G.; Hillebrandt, J.R.; Henderson, M.; Schukken, Y.H.; Hemling, T.C. Iodide Residues in Milk Vary between Iodine-Based Teat Disinfectants. J. Food Sci. 2016, 81, T1864–T1870. [Google Scholar] [CrossRef]
- Panlilio, A.L.; Beck-Sague, C.M.; Siegel, J.D.; Anderson, R.L.; Yetts, S.Y.; Clark, N.C.; Duer, P.N.; Thomassen, K.A.; Vess, R.W.; Hill, B.C.; et al. Infections and Pseudoinfections Due to Povidone-Iodine Solution Contaminated with Pseudomonas Cepacia. Clin. Infect. Dis. 1992, 14, 1078–1083. [Google Scholar] [CrossRef]
- Berkelman, R.L.; Lewin, S.; Allen, J.R.; Anderson, R.L.; Budnick, L.D.; Shapiro, S.; Friedman, S.M.; Nicholas, P.; Holzman, R.S.; Haley, R.W. Pseudobacteremia Attributed to Contamination of Povidone-Iodine with Pseudomonas Cepacia. Ann. Intern. Med. 1981, 95, 32–36. [Google Scholar] [CrossRef]
- Craven, D.E.; Moody, B.; Connolly, M.G.; Kollisch, N.R.; Stottmeier, K.D.; McCabe, W.R. Pseudobacteremia Caused by Povidone-Iodine Solution Contaminated with Pseudomonas Cepacia. N. Engl. J. Med. 2010, 305, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Berkelman, R.L.; Holland, B.W.; Anderson, R.L. Increased Bactericidal Activity of Dilute Preparations of Povidone-Iodine Solutions. J. Clin. Microbiol. 1982, 15, 635–639. [Google Scholar] [CrossRef] [Green Version]
- Weber, D.J.; Rutala, W.A.; Sickbert-Bennett, E.E. Outbreaks Associated with Contaminated Antiseptics and Disinfectants. Antimicrob. Agents Chemother. 2007, 51, 4217–4224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rösner, H.; Möller, W.; Groebner, S.; Torremante, P. Antiproliferative/Cytotoxic Effects of Molecular Iodine, Povidone-Iodine and Lugol’s Solution in Different Human Carcinoma Cell Lines. Oncol. Lett. 2016, 12, 2159–2162. [Google Scholar] [CrossRef] [Green Version]
- Piérard, G.E.; Piérard-Franchimont, C.; Arrese, J.E. Povidone-Iodine Wash Solutions in the Prevention of Superficial Fungal Infections; Predictive Evaluation Using the Corneofungimetry Bioassay. Eur. J. Clin. Pharmacol. 1997, 53, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tak-Yin, M. The Efficacy of Povidone-Iodine Pessaries in a Short, Low-Dose Treatment Regime on Candidal, Trichomonal and Non-Specific Vaginitis. Postgrad. Med. J. 1993, 69 (Suppl. S3), S58–S61. [Google Scholar] [PubMed]
- Gatti, S.; Cevini, C.; Bruno, A.; Penso, G.; Rama, P.; Scaglia, M. In Vitro Effectiveness of Povidone-Iodine on Acanthamoeba Isolates from Human Cornea. Antimicrob. Agents Chemother. 1998, 42, 2232–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawana, R.; Kitamura, T.; Nakagomi, O.; Matsumoto, I.; Arita, M.; Yoshihara, N.; Yanagi, K.; Yamada, A.; Morita, O.; Yoshida, Y.; et al. Inactivation of Human Viruses by Povidone-Iodine in Comparison with Other Antiseptics. Dermatology 1997, 195, 29–35. [Google Scholar] [CrossRef]
- Asanaka, M. In Vitro Study on Inactivation of Various Viruses Including Human Immunodeficiency Virus (HIV) by PVP-I. Proc. First Asian/Pacific Congr. Antisepsis. 1988. Available online: https://cir.nii.ac.jp/crid/1570009750369814784?lang=en (accessed on 15 March 2022).
- Sriwilaijaroen, N.; Wilairat, P.; Hiramatsu, H.; Takahashi, T.; Suzuki, T.; Ito, M.; Ito, Y.; Tashiro, M.; Suzuki, Y. Mechanisms of the Action of Povidone-Iodine against Human and Avian Influenza A Viruses: Its Effects on Hemagglutination and Sialidase Activities. Virol. J. 2009, 6, 124. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.E.; Sivalingam, V.; Kang, A.E.Z.; Ananthanarayanan, A.; Arumugam, H.; Jenkins, T.M.; Hadjiat, Y.; Eggers, M. Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease. Infect. Dis. Ther. 2020, 9, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Yuan, X.; Wei, G.; Wang, W.; Zhang, M.; Peng, H.; Javer, A.; Mendenhall, M.; Julander, J.; Huang, S.; et al. In-Vivo Toxicity Studies and In-Vitro Inactivation of SARS-CoV-2 by Povidone-Iodine In-Situ Gel Forming Formulations. bioRxiv 2020. bioRxiv:2020.05.18.103184. [Google Scholar]
- Garcia-Sanchez, A.; Peña-Cardelles, J.F.; Ruiz, S.; Robles, F.; Ordonez-Fernandez, E.; Salgado-Peralvo, A.O.; Balloch, J.; Simon, J.C. Efficacy of Pre-Procedural Mouthwashes against SARS-CoV-2: A Systematic Review of Randomized Controlled Trials. J. Clin. Med. 2022, 11, 1692. [Google Scholar] [CrossRef] [PubMed]
- Muppalaneni, S.; Omidian, H. Polyvinyl Alcohol in Medicine and Pharmacy: A Perspective. J. Dev. Drugs 2013, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Miao, Z.; Sun, Y.; Tao, Z.; Chen, Y.; Ma, Y.; Zhu, D.; Huang, X.; Zha, Z. Thermochromic Polyvinyl Alcohol-Iodine Hydrogels with Safe Threshold Temperature for Infectious Wound Healing. Adv. Healthc. Mater. 2021, 10, 2100722. [Google Scholar] [CrossRef]
- Montaser, A.S.; Rehan, M.; El-Naggar, M.E. PH-Thermosensitive Hydrogel Based on Polyvinyl Alcohol/Sodium Alginate/N-Isopropyl Acrylamide Composite for Treating Re-Infected Wounds. Int. J. Biol. Macromol. 2019, 124, 1016–1024. [Google Scholar] [CrossRef]
- World Health Organization/United Nations Children’s Fund (UNICEF)/International Council for Control of Iodine Deficiency Disorders. Review of Findings from 7-Country Study in Africa on Levels of Salt Idodization in Relation to Iodine Deficiency Disorders, Including Iodine-Induced Hyperthyroidism; WHO, UNICEF, ICCIDD: Geneva, Switzerland, 1996. [Google Scholar]
- Nagataki, S. The Average of Dietary Iodine Intake Due to the Ingestion of Seaweeds Is 1.2 Mg/Day in Japan. Thyroid 2008, 18, 667–668. [Google Scholar] [CrossRef]
- Farebrother, J.; Zimmermann, M.B.; Andersson, M. Excess Iodine Intake: Sources, Assessment, and Effects on Thyroid Function. Ann. N. Y. Acad. Sci. 2019, 1446, 44–65. [Google Scholar] [CrossRef]
- Backer, H.; Hollowell, J. Use of Iodine for Water Disinfection: Iodine Toxicity and Maximum Recommended Dose. Environ. Health Perspect. 2000, 108, 679–684. [Google Scholar] [CrossRef]
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- World Health Organization. Urinary Iodine Concentrations for Determining Iodine Status in Populations; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Ristic-Medic, D.; Piskackova, Z.; Hooper, L.; Ruprich, J.; Casgrain, A.; Ashton, K.; Pavlovic, M.; Glibetic, M. Methods of Assessment of Iodine Status in Humans: A Systematic Review. Am. J. Clin. Nutr. 2009, 89, 2052S–2069S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milagres, R.C.; de Souza, E.C.G.; Peluzio, M.; Franceschini, S.; Duarte, M.S.L. Food Iodine Content Table Compiled from International Databases. Rev. Nutr. 2020, 33, 1–12. [Google Scholar] [CrossRef]
- Venkatesh Mannar, M.G.; Dunn, J.T.; World Health Organization. Salt Iodization for the Elimination of Iodine Deficiency; International Council for Control of Iodine Deficiency Disorders: Brussels; Belgium, 1995. [Google Scholar]
- World Health Organization Guideline. In Fortification of Food-Grade Salt with Iodine for the Prevention and Control of Iodine Deficiency Disorders; World Health Organization: Geneva, Switzerland, 2014.
- UNICEF Worldwide, 89 per Cent of People Consume Iodized Salt. Available online: https://data.unicef.org/topic/nutrition/iodine/ (accessed on 15 March 2022).
- Chavasit, V.; Malaivongse, P.; Judprasong, K. Study on Stability of Iodine in Iodated Salt by Use of Different Cooking Model Conditions. J. Food Compos. Anal. 2002, 15, 265–276. [Google Scholar] [CrossRef]
- Diosady, L.L.; Alberti, J.O.; Mannar, M.G.V.; FitzGerald, S. Stability of Iodine in Iodized Salt Used for Correction of Iodine-Deficiency Disorders. II. Food Nutr. Bull. 1998, 19, 240–250. [Google Scholar] [CrossRef]
- Stansbury, J.; Saunders, P.; Winston, D. Promoting Healthy Thyroid Function with Iodine, Bladderwrack, Guggul and Iris. J. Restor. Med. 2012, 1, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.; Delange, F. Iodine Supplementation of Pregnant Women in Europe: A Review and Recommendations. Eur. J. Clin. Nutr. 2004, 58, 979–984. [Google Scholar]
- Leung, A.M.; Pearce, E.N.; Braverman, L.E. Iodine Content of Prenatal Multivitamins in the United States. N. Engl. J. Med. 2009, 360, 939–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennessey, J.V. The Emergence of Levothyroxine as a Treatment for Hypothyroidism. Endocr. 2016 551 2016, 55, 6–18. [Google Scholar]
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar]
- Agrawal, V.; Ghaznavi, S.A.; Paschke, R. Environmental Goitrogens. In Encyclopedia of Endocrine Diseases; Elsevier: Amsterdam, The Netherlands, 2018; pp. 506–511. [Google Scholar]
- López-Moreno, M.; Garcés-Rimón, M.; Miguel, M. Antinutrients: Lectins, Goitrogens, Phytates and Oxalates, Friends or Foe? J. Funct. Foods 2022, 89. [Google Scholar]
- Gaitan, E. 9 Goitrogens. Baillieres. Clin. Endocrinol. Metab. 1988, 2, 683–702. [Google Scholar] [CrossRef]
- Speck, U. X-ray Contrast Media: Overview, Use and Pharmaceutical Aspects; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Bourin, M.; Jolliet, P.; Ballereau, F. An Overview of the Clinical Pharmacokinetics of X-ray Contrast Media. Clin. Pharmacokinet. 1997, 32, 180–193. [Google Scholar] [CrossRef]
- Krause, W. Iodinated X-ray Contrast Agents. In Iodine Chemistry and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; Volume 9781118466, pp. 353–374. [Google Scholar]
- Zhibin, Y. FDA Approved Radiopharmaceuticals. Foreign Med. Sci. Sect. Radiat. Med. Nucl. Med. 2000, 24, 161–163. [Google Scholar]
- Bartolozzi, C.; Lencioni, R.; Caramella, D.; Palla, A.; Bassi, A.M.; Di Candio, G. Small Hepatocellular Carcinoma. Acta Radiol. 1996, 37, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Wallers, K.J.; McDermott, P.; James, W.B. Intravenous Cholangiography by Bolus Injection of Meglumine Iotroxamate and Meglumine Iodoxamate: A Comparative Trial of Two New Contrast Media. Clin. Radiol. 1981, 32, 457–459. [Google Scholar] [CrossRef]
- Miszczuk, M.A.; Chapiro, J.; Geschwind, J.-F.H.; Thakur, V.; Nezami, N.; Laage-Gaupp, F.; Kulon, M.; van Breugel, J.M.M.; Fereydooni, A.; Lin, M.; et al. Lipiodol as an Imaging Biomarker of Tumor Response After Conventional Transarterial Chemoembolization: Prospective Clinical Validation in Patients with Primary and Secondary Liver Cancer. Transl. Oncol. 2020, 13, 100742. [Google Scholar] [CrossRef]
- Karmaker, N.; Maraz, K.M.; Islam, F.; Haque, M.M.; Razzak, M.; Mollah, M.Z.I.; Faruque, M.R.I.; Ruhul, A. Khan Fundamental Characteristics and Application of Radiation. GSC Adv. Res. Rev. 2021, 7, 064–072. [Google Scholar]
- Shirakami, Y. Radioactive Iodine. In Iodine Chemistry and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; Volume 9781118466, pp. 603–624. [Google Scholar]
- Zerdoud, S.; Giraudet, A.-L.; Leboulleux, S.; Leenhardt, L.; Bardet, S.; Clerc, J.; Toubert, M.-E.; Al Ghuzlan, A.; Lamy, P.-J.; Bournaud, C.; et al. Radioactive Iodine Therapy, Molecular Imaging and Serum Biomarkers for Differentiated Thyroid Cancer: 2017 Guidelines of the French Societies of Nuclear Medicine, Endocrinology, Pathology, Biology, Endocrine Surgery and Head and Neck Surgery. Ann. Endocrinol. 2017, 78, 162–175. [Google Scholar] [CrossRef]
- Braghirolli, A.M.S.; Waissmann, W.; Da Silva, J.B.; Dos Santos, G.R. Production of Iodine-124 and Its Applications in Nuclear Medicine. Appl. Radiat. Isot. 2014, 90, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Piel, M.; Rösch, F. Radiopharmaceutical Chemistry; Springer: Berlin/Heidelberg, Germany, 2012; Volume 71. [Google Scholar]
- Crișan, G.; Moldovean-Cioroianu, N.S.; Timaru, D.-G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022, 23, 5023. [Google Scholar] [CrossRef]
- Kondev, F.G.; Wang, M.; Huang, W.J.; Naimi, S.; Audi, G. The NUBASE2020 Evaluation of Nuclear Physics Properties. Chinese Phys. C 2021, 45, 030001. [Google Scholar] [CrossRef]
- Rasmussen, T.; de Nijs, R.; Olsen, L.K.; Kamper, A.L.; Bang, L.E.; Frimodt-Møller, M.; Kelbæk, H.; Sørensen, S.S.; Kjær, A.; Feldt-Rasmussen, B.; et al. Renal 123I-MIBG Uptake before and after Live-Donor Kidney Transplantation. Diagnostics 2020, 10, 802. [Google Scholar] [CrossRef]
- Qaim, S.M.; Scholten, B.; Neumaier, B. New Developments in the Production of Theranostic Pairs of Radionuclides. J. Radioanal. Nucl. Chem. 2018, 318, 1493–1509. [Google Scholar] [CrossRef] [Green Version]
- Barca, C.; Griessinger, C.; Faust, A.; Depke, D.; Essler, M.; Windhorst, A.; Devoogdt, N.; Brindle, K.; Schäfers, M.; Zinnhardt, B.; et al. Expanding Theranostic Radiopharmaceuticals for Tumor Diagnosis and Therapy. Pharmaceuticals 2021, 15, 13. [Google Scholar] [CrossRef]
- Spitzweg, C.; Dietz, A.; O’Connor, M.; Bergert, E.; Tindall, D.; Young, C.; Morris, J. In Vivo Sodium Iodide Symporter Gene Therapy of Prostate Cancer. Gene Ther. 2001, 8, 1524–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boland, A.; Ricard, M.; Opolon, P.; Bidart, J.M.; Yeh, P.; Filetti, S.; Schlumberger, M.; Perricaudet, M. Adenovirus-Mediated Transfer of the Thyroid Sodium/Iodide Symporter Gene into Tumors for a Targeted Radiotherapy. Cancer Res. 2000, 60, 3484–3492. [Google Scholar]
- Chen, E.; Wang, J.; Zhang, H.; Zhang, Y.; Jia, C.; Min, X.; Liang, Y. Analysis of the Efficacy and Safety of Iodine-125 Seeds Implantation in the Treatment of Patients with Inoperable Early-Stage Non-Small Cell Lung Cancer. J. Contemp. Brachytherapy 2021, 13, 347–357. [Google Scholar] [CrossRef]
- Pavlicek, W.; Walton, H.A.; Karstaedt, P.J.; Gray, R.J. Radiation Safety with Use of I-125 Seeds for Localization of Nonpalpable Breast Lesions. Acad. Radiol. 2006, 13, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.; Shahbazi-Gahrouei, D. A Review on Theranostic Applications of Iodine Nanoparticles: Recent Findings and Perspectives. Nanomedicine J. 2021, 8, 234–240. [Google Scholar]
- Zimmermann, M.B. Iodine Deficiency Disorders and Their Correction Using Iodized Salt and/or Iodine Supplements. In Iodine Chemistry and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; Volume 9781118466, pp. 421–431. [Google Scholar]
- Yoshida, S.; Ojino, M.; Ozaki, T.; Hatanaka, T.; Nomura, K.; Ishii, M.; Koriyama, K.; Akashi, M. Guidelines for Iodine Prophylaxis as a Protective Measure: Information for Physicians. Japan Med. Assoc. J. JMAJ 2014, 57, 113–123. [Google Scholar]
- Küpper, F.C.; Kroneck, P.M.H. Iodine Bioinorganic Chemistry. In Iodine Chemistry and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 555–589. [Google Scholar]
- Markou, K.; Georgopoulos, N.; Kyriazopoulou, V.; Vagenakis, A.G. Iodine-Induced Hypothyroidism. Thyroid 2001, 11, 501–510. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Protection of the Human Environment. Guidelines for Iodine Prophylaxis Following Nuclear Accidents; Update 1999; World Health Organization: Geneva, Switzerland, 1999; pp. 1–45. [Google Scholar]


| Isotope | Abundance | Atomic Mass | Half-Life | Decay Mode (%) | keV | Production | Application |
|---|---|---|---|---|---|---|---|
| 123I | Synthetic | 122.9056 | 13.2232 h | β+ (100%) | 159 | Cyclotron | Diagnosis (SPECT) and therapy |
| 124I | Synthetic | 123.9062 | 4.176 d | β+ (100%) | 603 | Cyclotron | Research and diagnosis (PET) |
| 125I | Synthetic | 124.9046 | 59.392 d | ε (100%) | 27.5 | Nuclear reactor and cyclotron | Therapy and radioimmunoassay |
| 127I | Natural (1) | 126.9045 | Stable | Natural | Diagnosis (X-rays and CT) and therapy | ||
| 131I | Synthetic | 130.9061 | 8.0249 d | β− (100) | 364.5 | Nuclear reactor | Diagnosis, therapy, and RIA |
| Age Group | I2 (mg) | KI (mg) | KIO3 (mg) | Fraction of a Tablet (100 mg) |
|---|---|---|---|---|
| Adults and adolescents, including lactating women (>12 years) | 100 | 130 | 170 | 1 |
| Children (3–12 years) | 50 | 65 | 85 | 1/2 |
| Infants (1 month–3 years) | 25 | 32 | 42 | 1/4 |
| Neonates (birth–1 month) | 12.5 | 16 | 21 | 1/8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espino-Vázquez, A.N.; Rojas-Castro, F.C.; Fajardo-Yamamoto, L.M. Implications and Practical Applications of the Chemical Speciation of Iodine in the Biological Context. Future Pharmacol. 2022, 2, 377-414. https://doi.org/10.3390/futurepharmacol2040026
Espino-Vázquez AN, Rojas-Castro FC, Fajardo-Yamamoto LM. Implications and Practical Applications of the Chemical Speciation of Iodine in the Biological Context. Future Pharmacology. 2022; 2(4):377-414. https://doi.org/10.3390/futurepharmacol2040026
Chicago/Turabian StyleEspino-Vázquez, Astrid N., Flor C. Rojas-Castro, and Liria Mitzuko Fajardo-Yamamoto. 2022. "Implications and Practical Applications of the Chemical Speciation of Iodine in the Biological Context" Future Pharmacology 2, no. 4: 377-414. https://doi.org/10.3390/futurepharmacol2040026
APA StyleEspino-Vázquez, A. N., Rojas-Castro, F. C., & Fajardo-Yamamoto, L. M. (2022). Implications and Practical Applications of the Chemical Speciation of Iodine in the Biological Context. Future Pharmacology, 2(4), 377-414. https://doi.org/10.3390/futurepharmacol2040026