YOLO Algorithm for Long-Term Tracking and Detection of Escherichia Coli at Different Depths of Microchannels Based on Microsphere Positioning Assistance
Abstract
1. Introduction
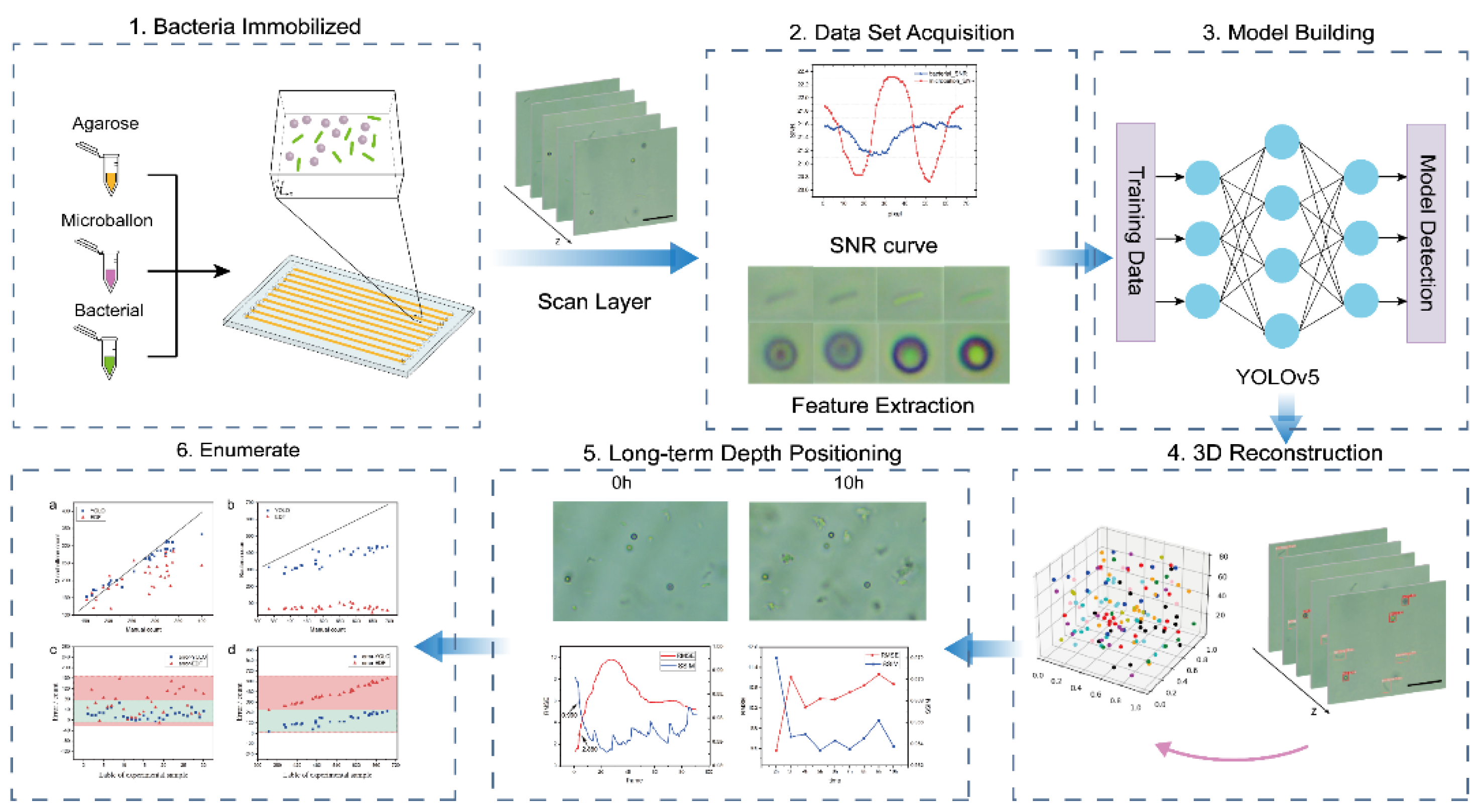
2. Method
2.1. Video Recording
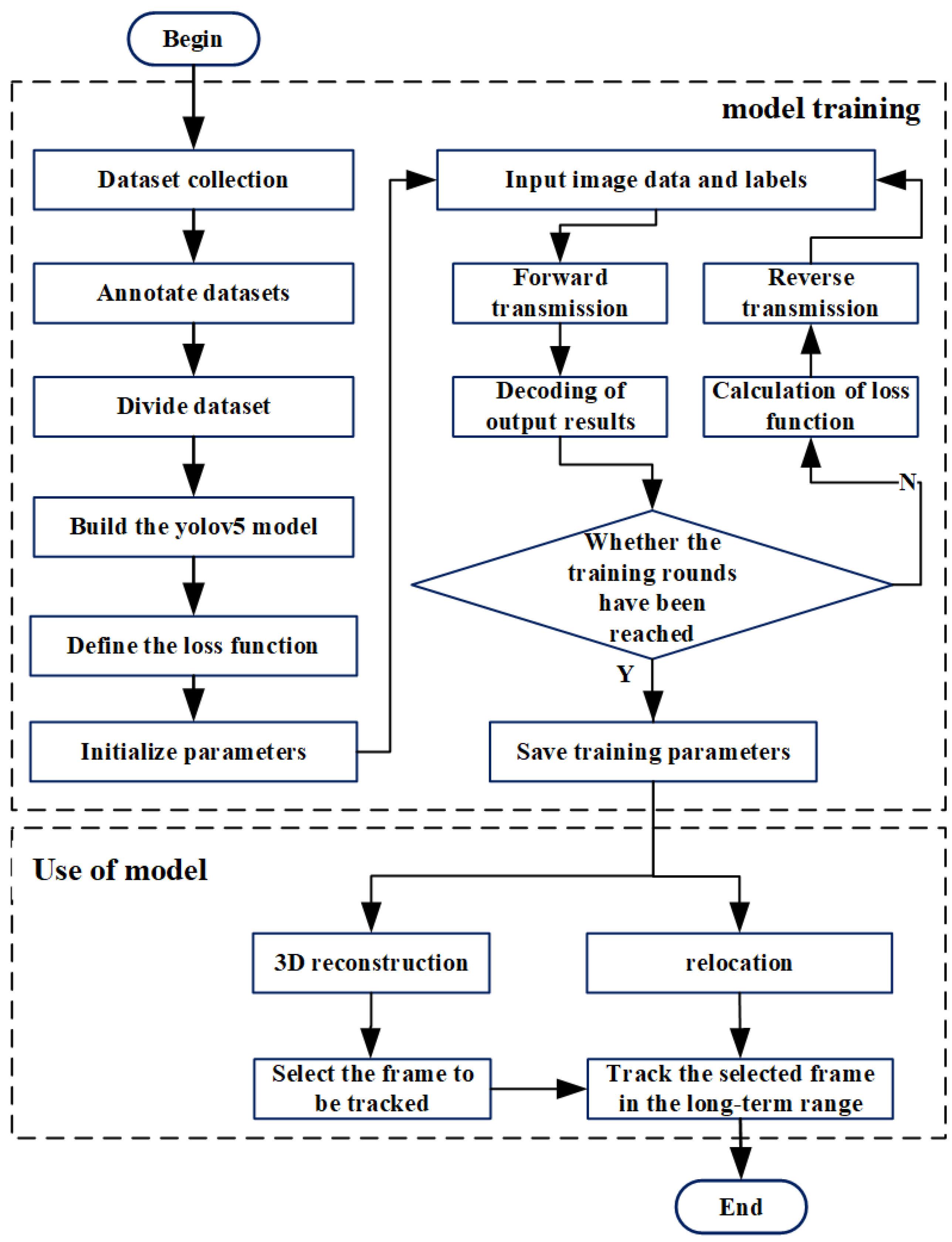
2.2. Model Design
2.3. Comparison of 2D and 3D Detection Methods
2.4. 3D Reconstruction and Tracking Algorithm
3. Results
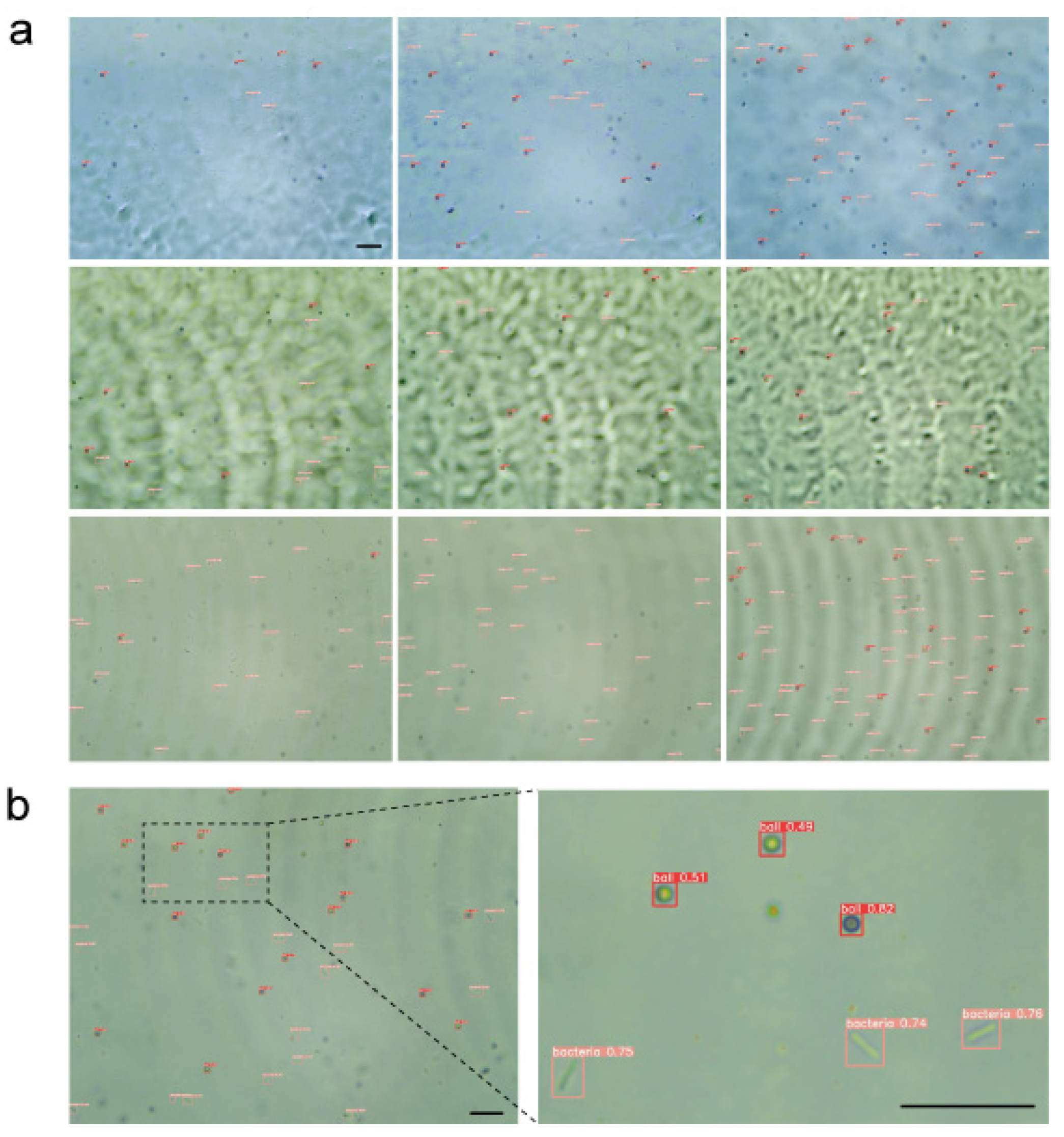
3.1. Performance Comparison with Other Models
3.2. Performance Comparison of 2D and 3D Detection Methods
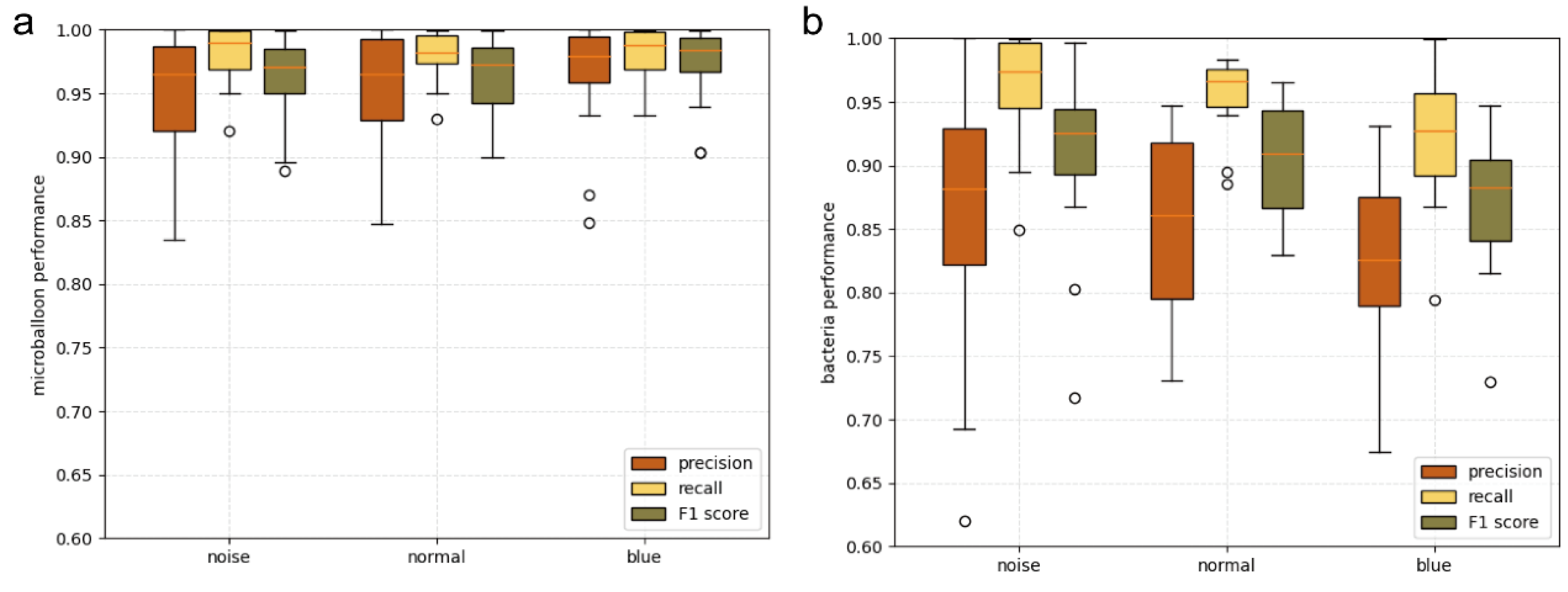
3.3. Denoising Performance Evaluation
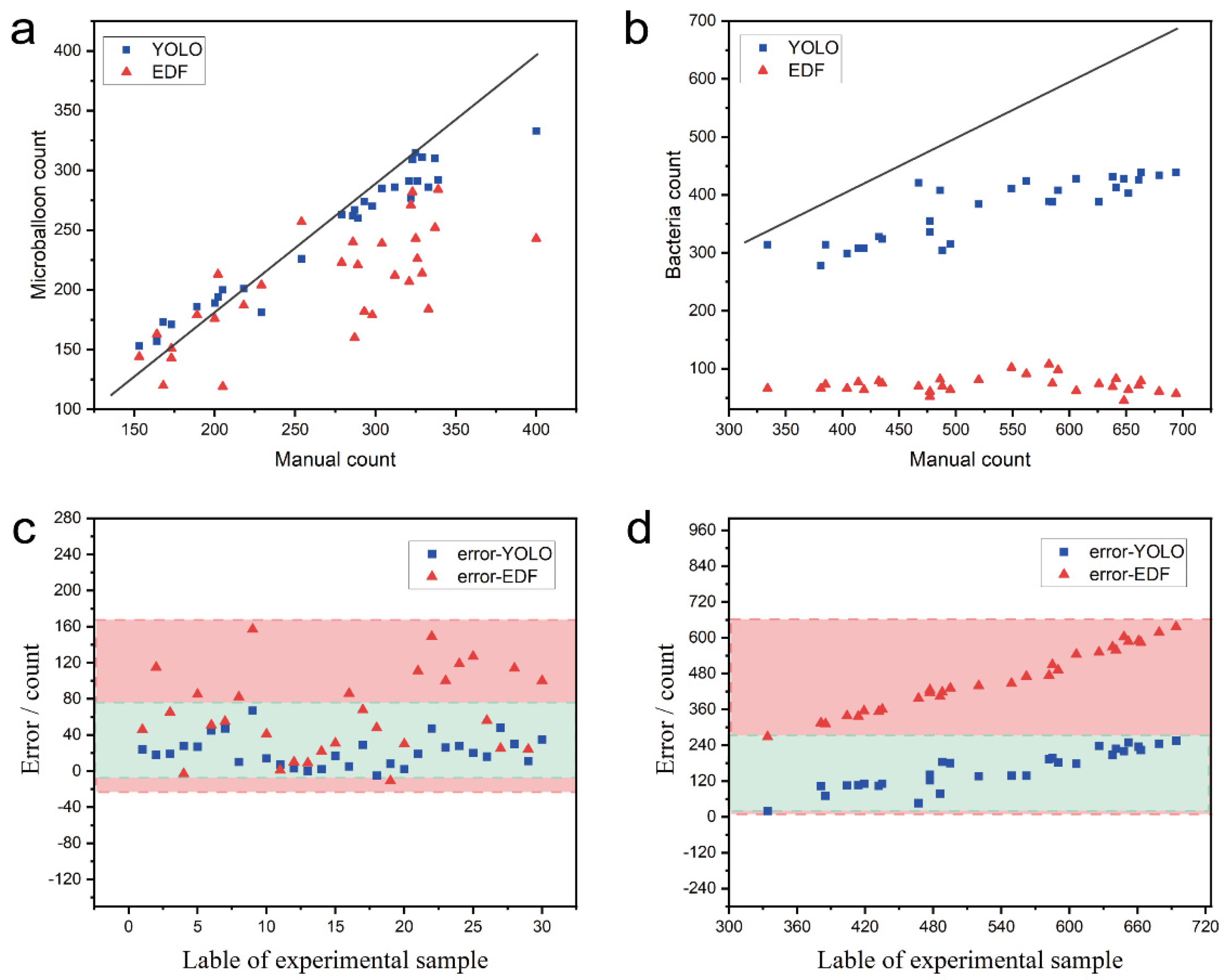
3.4. Counting Performance Evaluation
3.5. 3D Reconstruction and Bacterial Tracking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Svoboda, A.; Shaw, A.; Dzubak, J.; Mendonca, A.; Wilson, L.; Nair, A. Effectiveness of broad-spectrum chemical produce sanitizers against foodborne pathogens as in vitro planktonic cells and on the surface of whole cantaloupes and watermelons. J. Food Prot. 2016, 79, 524–530. [Google Scholar] [CrossRef]
- Fitzpatrick, K.J.; Rohlf, H.J.; Sutherland, T.D.; Koo, K.M.; Beckett, S.; Okelo, W.O.; Keyburn, A.L.; Morgan, B.S.; Drigo, B.; Trau, M. Progressing Antimicrobial Resistance Sensing Technologies across Human, Animal, and Environmental Health Domains. ACS Sens. 2021, 6, 4283–4296. [Google Scholar] [CrossRef]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial susceptibility testing: A comprehensive review of currently used methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Kállai, A.; Kelemen, M.; Molnár, N.; Tropotei, A.; Hauser, B.; Iványi, Z.; Gál, J.; Ligeti, E.; Kristóf, K.; Lőrincz, Á.M. MICy: A Novel Flow Cytometric Method for Rapid Determination of Minimal Inhibitory Concentration. Microbiol. Spectr. 2021, 9, e00901-21. [Google Scholar] [CrossRef]
- Abram, T.J.; Cherukury, H.; Ou, C.-Y.; Vu, T.; Toledano, M.; Li, Y.; Grunwald, J.T.; Toosky, M.N.; Tifrea, D.F.; Slepenkin, A. Rapid bacterial detection and antibiotic susceptibility testing in whole blood using one-step, high throughput blood digital PCR. Lab A Chip 2020, 20, 477–489. [Google Scholar] [CrossRef]
- Han, Y.-Y.; Lin, Y.-C.; Cheng, W.-C.; Lin, Y.-T.; Teng, L.-J.; Wang, J.-K.; Wang, Y.-L. Rapid antibiotic susceptibility testing of bacteria from patients’ blood via assaying bacterial metabolic response with surface-enhanced Raman spectroscopy. Sci. Rep. 2020, 10, 12538. [Google Scholar] [CrossRef]
- Savela, E.S.; Schoepp, N.G.; Cooper, M.M.; Rolando, J.C.; Klausner, J.D.; Soge, O.O.; Ismagilov, R.F. Surfactant-enhanced DNA accessibility to nuclease accelerates phenotypic β-lactam antibiotic susceptibility testing of Neisseria gonorrhoeae. PLoS Biol. 2020, 18, e3000651. [Google Scholar] [CrossRef]
- Torres-Simón, A.; Marino, M.H.; Gómez-Cruz, C.; Cañadas, M.; Marco, M.; Ripoll, J.; Vaquero, J.J.; Muñoz-Barrutia, A. Development of an inverted epifluorescence microscope for long-term monitoring of bacteria in multiplexed microfluidic devices. Sensors 2020, 20, 4140. [Google Scholar] [CrossRef]
- Syal, K.; Shen, S.; Yang, Y.; Wang, S.; Haydel, S.E.; Tao, N. Rapid antibiotic susceptibility testing of uropathogenic E. coli by tracking submicron scale motion of single bacterial cells. ACS Sens. 2017, 2, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Dietvorst, J.; Vilaplana, L.; Uria, N.; Marco, M.-P.; Muñoz-Berbel, X. Current and near-future technologies for antibiotic susceptibility testing and resistant bacteria detection. TrAC Trends Anal. Chem. 2020, 127, 115891. [Google Scholar] [CrossRef]
- Zahir, T.; Camacho, R.; Vitale, R.; Ruckebusch, C.; Hofkens, J.; Fauvart, M.; Michiels, J. High-throughput time-resolved morphology screening in bacteria reveals phenotypic responses to antibiotics. Commun. Biol. 2019, 2, 269. [Google Scholar] [CrossRef]
- Lim, T.; Kim, E.-G.; Choi, J.; Kwon, S. A high-throughput cell culture system based on capillary and centrifugal actions for rapid antimicrobial susceptibility testing. Lab A Chip 2020, 20, 4552–4560. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.; Kim, J.-K.; Chung, H.J.; Jeon, J.S. Microfluidic-based observation of local bacterial density under antimicrobial concentration gradient for rapid antibiotic susceptibility testing. Biomicrofluidics 2019, 13, 014108. [Google Scholar] [CrossRef]
- Mohan, R.; Mukherjee, A.; Sevgen, S.E.; Sanpitakseree, C.; Lee, J.; Schroeder, C.M.; Kenis, P.J. A multiplexed microfluidic platform for rapid antibiotic susceptibility testing. Biosens. Bioelectron. 2013, 49, 118–125. [Google Scholar] [CrossRef]
- Hou, Z.; An, Y.; Hjort, K.; Hjort, K.; Sandegren, L.; Wu, Z. Time lapse investigation of antibiotic susceptibility using a microfluidic linear gradient 3D culture device. Lab A Chip 2014, 14, 3409–3418. [Google Scholar] [CrossRef] [PubMed]
- Osaid, M.; Chen, Y.-S.; Wang, C.-H.; Sinha, A.; Lee, W.-B.; Gopinathan, P.; Wu, H.-B.; Lee, G.-B. A multiplexed nanoliter array-based microfluidic platform for quick, automatic antimicrobial susceptibility testing. Lab Chip 2021, 21, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Cestellos-Blanco, S.; Inoue, K.; Zare, R.N. Miniaturized antimicrobial susceptibility test by combining concentration gradient generation and rapid cell culturing. Antibiotics 2015, 4, 455–466. [Google Scholar] [CrossRef]
- Lee, W.-B.; Fu, C.-Y.; Chang, W.-H.; You, H.-L.; Wang, C.-H.; Lee, M.S.; Lee, G.-B. A microfluidic device for antimicrobial susceptibility testing based on a broth dilution method. Biosens. Bioelectron. 2017, 87, 669–678. [Google Scholar] [CrossRef]
- Choi, J.; Yoo, J.; Lee, M.; Kim, E.-G.; Lee, J.S.; Lee, S.; Joo, S.; Song, S.H.; Kim, E.-C.; Lee, J.C. A rapid antimicrobial susceptibility test based on single-cell morphological analysis. Sci. Transl. Med. 2014, 6, 267ra174. [Google Scholar] [CrossRef]
- Choi, J.; Jung, Y.-G.; Kim, J.; Kim, S.; Jung, Y.; Na, H.; Kwon, S. Rapid antibiotic susceptibility testing by tracking single cell growth in a microfluidic agarose channel system. Lab A Chip 2013, 13, 280–287. [Google Scholar] [CrossRef]
- Choi, J.; Yoo, J.; Kim, K.-j.; Kim, E.-G.; Park, K.O.; Kim, H.; Kim, H.; Jung, H.; Kim, T.; Choi, M. Rapid drug susceptibility test of Mycobacterium tuberculosis using microscopic time-lapse imaging in an agarose matrix. Appl. Microbiol. Biotechnol. 2016, 100, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Syal, K.; Mo, M.; Yu, H.; Iriya, R.; Jing, W.; Guodong, S.; Wang, S.; Grys, T.E.; Haydel, S.E.; Tao, N. Current and emerging techniques for antibiotic susceptibility tests. Theranostics 2017, 7, 1795. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, E.; Wang, Z.; Van Gasse, K.; Hänsch, T.W.; Picqué, N. Dual-comb hyperspectral digital holography. Nat. Photonics 2021, 15, 890–894. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, R.; Chen, X.; Waller, L.; Kau, A.; Fung, A.A.; Gutierrez, B.; An, C.; Cho, S.H.; Shi, L. A high-throughput technique to map cell images to cell positions using a 3D imaging flow cytometer. Proc. Natl. Acad. Sci. USA 2022, 119, e2118068119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, H.; Ye, T.; Juhas, M. Deep learning for imaging and detection of microorganisms. Trends Microbiol. 2021, 29, 569–572. [Google Scholar] [CrossRef]
- Bennett, M.R.; Hasty, J. Microfluidic devices for measuring gene network dynamics in single cells. Nat. Rev. Genet. 2009, 10, 628–638. [Google Scholar] [CrossRef]
- Kreft, M.; Stenovec, M.; Zorec, R. Focus-drift correction in time-lapse confocal imaging. Ann. N. Y. Acad. Sci. 2005, 1048, 321–330. [Google Scholar] [CrossRef]
- Zhang, R.; Wen, C. SOD-YOLO: A Small Target Defect Detection Algorithm for Wind Turbine Blades Based on Improved YOLOv5. Adv. Theory Simul. 2022, 2100631. [Google Scholar] [CrossRef]
- Sità, L.; Brondi, M.; Lagomarsino de Leon Roig, P.; Curreli, S.; Panniello, M.; Vecchia, D.; Fellin, T. A deep-learning approach for online cell identification and trace extraction in functional two-photon calcium imaging. Nat. Commun. 2022, 13, 1529. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ren, Z. Application of Yolo on mask detection task. In Proceedings of the 2021 IEEE 13th International Conference on Computer Research and Development (ICCRD), Beijing, China, 5–7 January 2021; pp. 130–136. [Google Scholar]
- Buric, M.; Pobar, M.; Ivasic-Kos, M. Ball detection using YOLO and Mask R-CNN. In Proceedings of the 2018 IEEE: International Conference on Computational Science and Computational Intelligence (CSCI), Las Vegas, NV, USA, 12–14 December 2018; pp. 319–323. [Google Scholar]
- Liu, L.; Özsu, M.T. Encyclopedia of Database Systems; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2009; Volume 6. [Google Scholar]
- Baltekin, Ö.; Boucharin, A.; Tano, E.; Andersson, D.I.; Elf, J. Antibiotic susceptibility testing in less than 30 min using direct single-cell imaging. Proc. Natl. Acad. Sci. USA 2017, 114, 9170–9175. [Google Scholar] [CrossRef]
- Wang, Z.; Bovik, A.C.; Sheikh, H.R.; Simoncelli, E.P. Image quality assessment: From error visibility to structural similarity. IEEE Trans. Image Process. 2004, 13, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Sara, U.; Akter, M.; Uddin, M.S. Image quality assessment through FSIM, SSIM, MSE and PSNR—A comparative study. J. Comput. Commun. 2019, 7, 8–18. [Google Scholar] [CrossRef]


| Model | Precision/Ball | Precision/Bacterial | mAp@0.5 |
|---|---|---|---|
| YOLOv3 | 0.820 | 0.686 | 0.754 |
| YOLOv4 | 0.849 | 0.729 | 0.789 |
| YOLOv5 | 0.91 | 0.734 | 0.822 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Xu, Y.; Rao, Z.; Chen, J.; Liu, Z.; Lu, N. YOLO Algorithm for Long-Term Tracking and Detection of Escherichia Coli at Different Depths of Microchannels Based on Microsphere Positioning Assistance. Sensors 2022, 22, 7454. https://doi.org/10.3390/s22197454
Sun L, Xu Y, Rao Z, Chen J, Liu Z, Lu N. YOLO Algorithm for Long-Term Tracking and Detection of Escherichia Coli at Different Depths of Microchannels Based on Microsphere Positioning Assistance. Sensors. 2022; 22(19):7454. https://doi.org/10.3390/s22197454
Chicago/Turabian StyleSun, Lesheng, Ying Xu, Zhikang Rao, Juntao Chen, Zhe Liu, and Ning Lu. 2022. "YOLO Algorithm for Long-Term Tracking and Detection of Escherichia Coli at Different Depths of Microchannels Based on Microsphere Positioning Assistance" Sensors 22, no. 19: 7454. https://doi.org/10.3390/s22197454
APA StyleSun, L., Xu, Y., Rao, Z., Chen, J., Liu, Z., & Lu, N. (2022). YOLO Algorithm for Long-Term Tracking and Detection of Escherichia Coli at Different Depths of Microchannels Based on Microsphere Positioning Assistance. Sensors, 22(19), 7454. https://doi.org/10.3390/s22197454






