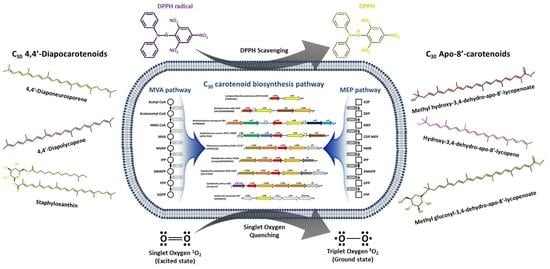
Antioxidant Potential and Capacity of Microorganism-Sourced C30 Carotenoids—A Review
Abstract
:1. Introduction
2. Microbial C30 Carotenoids and Their Derivatives
| C30 Carotenoid | Quenching Type | Antioxidant Activity | Conjugated Double Bond | Group Contained | H Bond Donor /Acceptor | Microbial Sources [Ref.] | |
|---|---|---|---|---|---|---|---|
| Carotenoid Content | Antioxidant Control | ||||||
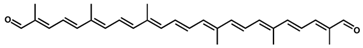 4,4′-Diapolycopenedial (4,4′-diapolycopen-4,4′-dial)/C30H36O2 | DPPH scavenging | 7.5 µM (IC50) | DL-α-tocopherol, 33.2 µM (IC50) | 13 | Aldehyde (2) | 0/2 | Staphylococcus spp. [36], Methylomonas spp. [37] |
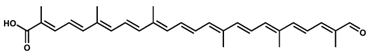 4,4′-Diapocaroten-4′-al-4-oic acid/C30H36O3 4,4′-Diapocaroten-4′-al-4-oic acid/C30H36O3 | - | - | - | 13 | Carboxylic acid, aldehyde | 1/3 | Pseudomonas rhodos [21] |
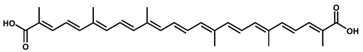 4,4′-Diapolycopene-4,4′-dioic acid/C30H36O4 | Singlet oxygen quenching | 5.8 µM | Astaxanthin, 9.3 µM | 13 | Carboxylic acid (2) | 2/4 | Cytobacillus firmus [16] |
 4,4′-Diapolycopen-4-al (4, 4′-Diapolycopenal)/C30H38O | - | - | - | 12 | Aldehyde | 0/1 | Staphylococcus aureus [24] |
 4,4′-Diapocarotenoic acid/C30H38O2 | - | - | - | 12 | Carboxylic acid | 1/2 | Pseudomonas rhodos [21] |
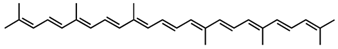 4,4′-Diapolycopene/C30H40 | DPPH scavenging | 8.7 µM (IC50) | DL-α-tocopherol, 33.2 µM (IC50) | 11 | Hydrocarbon | 0/0 | Heliobacteria spp. [36] |
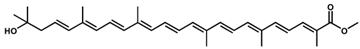 Methyl hydroxy-3,4-dehydro-apo-8′-lycopenoate /C31H42O3 Methyl hydroxy-3,4-dehydro-apo-8′-lycopenoate /C31H42O3 | Singlet oxygen quenching | 44 μM (IC50) | Astaxanthin, 8.9 µM (IC50); β-carotene, >100 µM (IC50) | 11 | Ester, hydroxyl | 1/3 | Halobacillus halophilus [27] |
 Methyl glucosyl-3,4-dehydro-apo-8′-lycopenoate /C37H52O8 Methyl glucosyl-3,4-dehydro-apo-8′-lycopenoate /C37H52O8 | Singlet oxygen quenching | 5.1 μM (IC50) | 11 | Ester, glucosyl | 4/8 | Planococcus maritimus [26], Halobacillus halophilus [27] | |
 Hydroxy-3,4-dehydro-apo-8′-lycopene /C30H42O | Singlet oxygen quenching | 79 μM (IC50) | 10 | Hydroxyl | 1/1 | Halobacillus Halophilus [27] | |
 4,4′-Diaponeurosporen-4-al (Diaponeurosporenal)/C30H40O | DPPH scavenging | 10.2 µM (IC50) | DL-α-tocopherol, 33.2 µM (IC50) | 10 | Aldehyde | 0/1 | Staphylococcus aureus [36] |
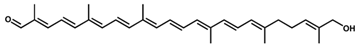 Hydroxy-diaponeurosporenal/C30H40O2 Hydroxy-diaponeurosporenal/C30H40O2 | - | - | - | 10 | Aldehyde, hydroxyl | 1/2 | Staphylococcus aureus [38] |
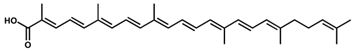 4,4′-Diaponeurosporen-4-oic acid/C30H40O2 | DPPH scavenging | 9.7 µM (IC50) | DL-α-tocopherol, 33.2 µM (IC50) | 10 | Carboxylic acid | 1/2 | Staphylococcus aureus [36] |
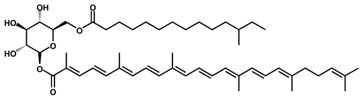 Staphyloxanthin/C51H78O8 | DPPH scavenging | 54.22 µg/mL (IC50) | Ascorbic acid, 35.54 µg/mL (IC50) | 10 | Ketone | 3/8 | Staphylococcus spp. [34] |
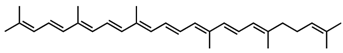 4,4′-Diaponeurosporene/C30H42 | DPPH scavenging, singlet oxygen quenching | 27.1% 11.6 µM (IC50) 45 µM | BHT, 50 µg/mL DL-α-tocopherol, 33.2 µM (IC50) astaxanthin, 3.7 µM | 9 | Hydrocarbon | 0/0 | Staphylococcus aureus [17], Heliobacteria spp. [22], Lactiplantibacillus plantarum [29,36] |
 4-Hydroxy-4,4′-diaponeurosporene/C30H42O | - | - | - | 9 | Hydroxyl | 1/1 | Streptococcus faecium [39] |
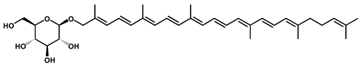 4-(D-Glucopyranosyloxy)-4,4′-diaponeurosporene/C36H52O6 | - | - | - | 9 | Ester, glycosyl | 4/6 | Streptococcus faecium [39] |
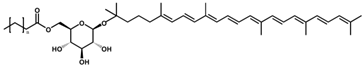 OH-Diaponeurosporene glucoside ester OH-Diaponeurosporene glucoside ester/C40H60O7 | - | - | - | 9 | Ester | 3/7 | Heliobacteria spp. [23] |
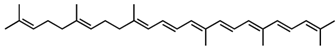 4,4′-Diapo-7,8,11,12-tetrahydrolycopene /C30H44 | - | - | - | 7 | Hydrocarbon | 0/0 | Staphylococcus aureus [24] |
 4,4′-Diapo-ζ-carotene (4,4′-Diapo-zeta-carotene) /C30H44 | - | - | - | 7 | Hydrocarbon | 0/0 | Staphylococcus aureus [24] |
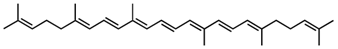 4,4′-Diapophytofluene/C30H46 | - | - | - | 5 | Hydrocarbon | 0/0 | Staphylococcus aureus [24] |
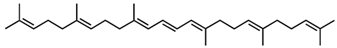 4,4′-Diapophytoene (Dehydrosqualene) /C30H48 | - | - | - | 3 | Hydrocarbon | 0/0 | Staphylococcus aureus [24], Bacillus spp. [19] |
3. Antioxidant Properties of C30 Carotenoids
4. Microbial Fermentation for Commercial Production of C30 Carotenoids
4.1. Growth Factor Effects on C30 Carotenoid Production
4.2. Abiotic Stresses for the Optimization of C30 Carotenoid Production
5. Biosynthetic Pathway Engineering for Novel C30 Carotenoid Generation
6. Future Predictions and Expectations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alcaíno, J.; Baeza, M.; Cifuentes, V. Carotenoid distribution in nature. Subcell. Biochem. 2016, 79, 3–33. [Google Scholar]
- Maoka, T.; Kawase, N.; Hironaka, M.; Nishida, R. Carotenoids of Hemipteran insects, from the perspective of chemo-systematic and chemical ecological studies. Biochem. Syst. Ecol. 2021, 95, 104241. [Google Scholar] [CrossRef]
- Nováková, E.; Moran, N.A. Diversification of genes for carotenoid biosynthesis in aphids following an ancient transfer from a fungus. Mol. Biol. Evol. 2012, 29, 313–323. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H.; Mercadante, A.; Egeland, E. Carotenoids-Handbook; Birkhäuser Verlag: Basel, Switzerland, 2004; pp. 1–625. [Google Scholar]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Mezzomo, N.; Ferreira, S.R. Carotenoids functionality, sources, and processing by supercritical technology: A review. J. Chem. 2016, 2016, 3164312. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Carle, R. Carotenoid production by bacteria, microalgae, and fungi. In Carotenoids: Nutrition, Analysis and Technology; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 217–240. [Google Scholar]
- Armstrong, G.A.; Hearst, J.E. Genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J. 1996, 10, 228–237. [Google Scholar] [CrossRef]
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C.-W. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12. [Google Scholar] [CrossRef]
- Cardoso, L.A.; Karp, S.G.; Vendruscolo, F.; Kanno, K.Y.; Zoz, L.I.; Carvalho, J.C. Biotechnological production of carotenoids and their applications in food and pharmaceutical products. Carotenoids 2017, 8, 125–141. [Google Scholar]
- Fernandes, A.S.; do Nascimento, T.C.; Jacob-Lopes, E.; De Rosso, V.V.; Zepka, L.Q. Carotenoids: A brief overview on its structure, biosynthesis, synthesis, and applications. Prog. Carotenoid Res. 2018, 1, 1–17. [Google Scholar]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef]
- Garrido-Fernández, J.; Maldonado-Barragán, A.; Caballero-Guerrero, B.; Hornero-Méndez, D.; Ruiz-Barba, J.L. Carotenoid production in Lactobacillus plantarum. Int. J. Food Microbiol. 2010, 140, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Steiger, S.; Perez-Fons, L.; Fraser, P.; Sandmann, G. Biosynthesis of a novel C30 carotenoid in Bacillus firmus isolates. J. Appl. Microbiol. 2012, 113, 888–895. [Google Scholar] [CrossRef]
- Wieland, B.; Feil, C.; Gloria-Maercker, E.; Thumm, G.; Lechner, M.; Bravo, J.-M.; Poralla, K.; Götz, F. Genetic and biochemical analyses of the biosynthesis of the yellow carotenoid 4,4′-diaponeurosporene of Staphylococcus aureus. J. Bacteriol. 1994, 176, 7719–7726. [Google Scholar] [CrossRef]
- Carruthers, D.N.; Lee, T.S. Diversifying Isoprenoid Platforms via Atypical Carbon Substrates and Non-model Microorganisms. Front. Microbiol. 2021, 12, 3725. [Google Scholar] [CrossRef]
- Perez-Fons, L.; Steiger, S.; Khaneja, R.; Bramley, P.M.; Cutting, S.M.; Sandmann, G.; Fraser, P.D. Identification and the developmental formation of carotenoid pigments in the yellow/orange Bacillus spore-formers. Biochim. Biophys. Acta 2011, 1811, 177–185. [Google Scholar] [CrossRef]
- Steiger, S.; Perez-Fons, L.; Cutting, S.M.; Fraser, P.D.; Sandmann, G. Annotation and functional assignment of the genes for the C30 carotenoid pathways from the genomes of two bacteria: Bacillus indicus and Bacillus firmus. Microbiology 2015, 161, 194–202. [Google Scholar] [CrossRef]
- Kleinig, H.; Schmitt, R.; Meister, W.; Englert, G.; Thommen, H. New C30-carotenoic acid glucosyl esters from Pseudomonas rhodos. Z. Naturforsch. 1979, 34, 181–185. [Google Scholar] [CrossRef]
- Takaichi, S.; Inoue, K.; Akaike, M.; Kobayashi, M.; Oh-oka, H.; Madigan, M.T. The major carotenoid in all known species of heliobacteria is the C30 carotenoid 4,4′-diaponeurosporene, not neurosporene. Arch. Microbiol. 1997, 168, 277–281. [Google Scholar] [CrossRef]
- Takaichi, S.; Oh-Oka, H.; Maoka, T.; Jung, D.O.; Madigan, M.T. Novel carotenoid glucoside esters from alkaliphilic heliobacteria. Arch. Microbiol. 2003, 179, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.H.; Wilmoth, G.J. Pigments of Staphylococcus aureus, a series of triterpenoid carotenoids. J. Bacteriol. 1981, 147, 900–913. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.H.; Wilmoth, G.J. Proposed pathway of triterpenoid carotenoid biosynthesis in Staphylococcus aureus: Evidence from a study of mutants. J. Bacteriol. 1981, 147, 914–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shindo, K.; Endo, M.; Miyake, Y.; Wakasugi, K.; Morritt, D.; Bramley, P.M.; Fraser, P.D.; Kasai, H.; Misawa, N. Methyl glucosyl-3,4-dehydro-apo-8′-lycopenoate, a novel antioxidative glyco-C30-carotenoic acid produced by a marine bacterium Planococcus maritimus. J. Antibiot. 2008, 61, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Osawa, A.; Ishii, Y.; Sasamura, N.; Morita, M.; Koecher, S.; Mueller, V.; Sandmann, G.; Shindo, K. Hydroxy-3,4-dehydro-apo-8′-lycopene and methyl hydroxy-3,4-dehydro-apo-8′-lycopenoate, novel C30 carotenoids produced by a mutant of marine bacterium Halobacillus halophilus. J. Antibiot. 2010, 63, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Liu, H.; Xu, W.; Yang, Q. 4,4′-Diaponeurosporene-producing Bacillus subtilis promotes the development of the mucosal immune system of the piglet gut. Anat. Rec. 2019, 302, 1800–1807. [Google Scholar] [CrossRef]
- Kim, M.; Seo, D.-H.; Park, Y.-S.; Cha, I.-T.; Seo, M.-J. Isolation of Lactobacillus plantarum subsp. plantarum producing C30 carotenoid 4,4′-diaponeurosporene and the assessment of its antioxidant activity. J. Microbiol. Biotechnol. 2019, 29, 1925–1930. [Google Scholar]
- Liu, H.; Xu, W.; Yu, Q.; Yang, Q. 4,4′-Diaponeurosporene-producing Bacillus subtilis increased mouse resistance against Salmonella typhimurium infection in a CD36-dependent manner. Front. Immunol. 2017, 8, 483. [Google Scholar] [CrossRef]
- Kim, M.; Jung, D.-H.; Seo, D.-H.; Park, Y.-S.; Seo, M.-J. 4,4′-Diaponeurosporene from Lactobacillus plantarum subsp. plantarum KCCP11226: Low Temperature Stress-Induced Production Enhancement and in Vitro Antioxidant Activity. J. Microbiol. Biotechnol. 2021, 31, 63–69. [Google Scholar]
- Liu, H.; Xu, W.; Chang, X.; Qin, T.; Yin, Y.; Yang, Q. 4,4′-diaponeurosporene, a C30 carotenoid, effectively activates dendritic cells via CD36 and NF-κB signaling in a ROS independent manner. Oncotarget 2016, 7, 40978–40991. [Google Scholar] [CrossRef]
- Takemura, M.; Takagi, C.; Aikawa, M.; Araki, K.; Choi, S.-K.; Itaya, M.; Shindo, K.; Misawa, N. Heterologous production of novel and rare C30-carotenoids using Planococcus carotenoid biosynthesis genes. Microb. Cell Fact. 2021, 20, 194. [Google Scholar] [CrossRef] [PubMed]
- Barretto, D.A.; Vootla, S.K. In vitro anticancer activity of staphyloxanthin pigment extracted from Staphylococcus gallinarum KX912244, a gut microbe of Bombyx mori. Indian J. Microbiol. 2018, 58, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Pelz, A.; Wieland, K.-P.; Putzbach, K.; Hentschel, P.; Albert, K.; Götz, F. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J. Biol. Chem. 2005, 280, 32493–32498. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, M.S.; Lee, B.Y.; Lee, P.C. Generation of structurally novel short carotenoids and study of their biological activity. Sci. Rep. 2016, 6, 21987. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Schenzle, A.; Odom, J.M.; Cheng, Q. Novel carotenoid oxidase involved in biosynthesis of 4,4′-diapolycopene dialdehyde. Appl. Environ. Microbiol. 2005, 71, 3294–3301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mijts, B.N.; Lee, P.C.; Schmidt-Dannert, C. Identification of a carotenoid oxygenase synthesizing acyclic xanthophylls: Combinatorial biosynthesis and directed evolution. Chem. Biol. 2005, 12, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.F.; Davies, B.H. Triterpenoid carotenoids and related lipids. Triterpenoid carotenoid aldehydes from Streptococcus faecium UNH 564P. Biochem. J. 1976, 153, 233–239. [Google Scholar] [CrossRef]
- Maeda, I. Genetic modification in Bacillus subtilis for production of C30 carotenoids. Methods Mol. Biol. 2012, 892, 197–205. [Google Scholar]
- Kleinig, H.; Schmitt, R. On the biosynthesis of C30 carotenoic acid glucosyl esters in Pseudomonas rhodos. Analysis of car-mutants. Z. Naturforsch. 1982, 37, 758–760. [Google Scholar] [CrossRef]
- Butnariu, M. Methods of analysis (extraction, separation, identification and quantification) of carotenoids from natural products. J. Ecosyst. Ecograph. 2016, 6, 193. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.; Mitra, M.; Shah, F.; Tirkey, S.R.; Mishra, S. Bacteria as an alternate biofactory for carotenoid production: A review of its applications, opportunities and challenges. J. Func. Foods 2020, 67, 103867. [Google Scholar] [CrossRef]
- Valla, A.R.; Cartier, D.L.; Labia, R. Chemistry of natural retinoids and carotenoids: Challenges for the future. Curr. Org. Syn. 2004, 1, 167–209. [Google Scholar] [CrossRef]
- Osawa, A.; Iki, K.; Sandmann, G.; Shindo, K. Isolation and Identification of 4,4′-diapolycopene-4,4′-dioic acid produced by Bacillus firmus GB1 and its singlet oxygen quenching activity. J. Oleo Sci. 2013, 62, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Focsan, A.L.; Kispert, L.D. Antioxidant activity in supramolecular carotenoid complexes favored by nonpolar environment and disfavored by hydrogen bonding. Antioxidants 2020, 9, 625. [Google Scholar] [CrossRef]
- Han, R.-M.; Zhang, J.-P.; Skibsted, L.H. Reaction dynamics of flavonoids and carotenoids as antioxidants. Molecules 2012, 17, 2140–2160. [Google Scholar] [CrossRef] [Green Version]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Liu, D.; Shi, J.; Ibarra, A.C.; Kakuda, Y.; Xue, S.J. The scavenging capacity and synergistic effects of lycopene, vitamin E, vitamin C, and β-carotene mixtures on the DPPH free radical. LWT-Food Sci. Technol. 2008, 41, 1344–1349. [Google Scholar] [CrossRef]
- Martínez, A.; Barbosa, A. Antiradical power of carotenoids and vitamin E: Testing the hydrogen atom transfer mechanism. J. Phys. Chem. B 2008, 112, 16945–16951. [Google Scholar] [CrossRef]
- Nakanishi, I.; Miyazaki, K.; Shimada, T.; Ohkubo, K.; Urano, S.; Ikota, N.; Ozawa, T.; Fukuzumi, S.; Fukuhara, K. Effects of metal ions distinguishing between one-step hydrogen-and electron-transfer mechanisms for the radical-scavenging reaction of (+)-catechin. J. Phys. Chem. A 2002, 106, 11123–11126. [Google Scholar] [CrossRef]
- Perera, C.O.; Yen, G.M. Functional properties of carotenoids in human health. Int. J. Food Prop. 2007, 10, 201–230. [Google Scholar] [CrossRef]
- D’Alessandro, E.B.; Antoniosi Filho, N.R. Concepts and studies on lipid and pigments of microalgae: A review. Renew. Sustain. Energy Rev. 2016, 58, 832–841. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Kimura, M. HarvestPlus Handbook for Carotenoid Analysis; International Food Policy Research Institute (IFPRI): Washington, DC, USA; International Center for Tropical Agriculture (CIAT): Cali, CO, USA, 2004. [Google Scholar]
- Shimidzu, N.; Goto, M.; Miki, W. Carotenoids as singlet oxygen quenchers in marine organisms. Fish. Sci. 1996, 62, 134–137. [Google Scholar] [CrossRef]
- Chae, H.S.; Kim, K.-H.; Kim, S.C.; Lee, P.C. Strain-dependent carotenoid productions in metabolically engineered Escherichia coli. Appl. Biochem. Biotechnol. 2010, 162, 2333–2344. [Google Scholar] [CrossRef]
- Lee, P.C.; Momen, A.Z.R.; Mijts, B.N.; Schmidt-Dannert, C. Biosynthesis of structurally novel carotenoids in Escherichia coli. Chem. Biol. 2003, 10, 453–462. [Google Scholar] [CrossRef]
- Shindo, K.; Asagi, E.; Sano, A.; Hotta, E.; Minemura, N.; Mikami, K.; Tamesada, E.; Misawa, N.; Maoka, T. Diapolycopenedioic acid xylosyl esters A, B, and C, novel antioxidative glyco-C30-carotenoic acids produced by a new marine bacterium Rubritalea squalenifaciens. J. Antibiot. 2008, 61, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Osawa, A.; Kaseya, Y.; Koue, N.; Schrader, J.; Knief, C.; Vorholt, J.A.; Sandmann, G.; Shindo, K. 4-[2-O-11Z-Octadecenoyl-β-glucopyranosyl]-4,4′-diapolycopene-4,4′-dioic acid and 4-[2-O-9Z-hexadecenoyl-β-glucopyranosyl]-4,4′-diapolycopene-4,4′-dioic acid: New C30-carotenoids produced by Methylobacterium. Tetrahedron Lett. 2015, 56, 2791–2794. [Google Scholar] [CrossRef]
- Shindo, K.; Misawa, N. New and rare carotenoids isolated from marine bacteria and their antioxidant activities. Mar. Drugs 2014, 12, 1690–1698. [Google Scholar] [CrossRef]
- Gultekin, F.; Doguc, D.K. Allergic and immunologic reactions to food additives. Clin. Rev. Allergy Immunol. 2013, 45, 6–29. [Google Scholar] [CrossRef]
- Brauch, J. Underutilized fruits and vegetables as potential novel pigment sources. In Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color; Carle, R., Schweiggert, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 305–335. [Google Scholar]
- Córdova, P.; Baeza, M.; Cifuentes, V.; Alcaíno, J. Microbiological synthesis of carotenoids: Pathways and regulation. In Progress in Carotenoid Research Pigments; Queiroz Zepka, L., Jacob-Lopes, E., Rosso, V.V., Eds.; IntechOpen: London, UK, 2018; pp. 63–83. [Google Scholar]
- McWilliams, A. The Global Market for Carotenoids|BCC Research. Available online: https://www.bccresearch.com/marketresearch/food-and-beverage/the-global-market-for-carotenoids.html (accessed on 16 February 2022).
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the food industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Pirwitz, K.; Flassig, R.J.; Rihko-Struckmann, L.K.; Sundmacher, K. Energy and operating cost assessment of competing harvesting methods for D. salina in a β-carotene production process. Algal Res. 2015, 12, 161–169. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef]
- Igreja, W.S.; Maia, F.d.A.; Lopes, A.S.; Chisté, R.C. Biotechnological production of carotenoids using low cost-substrates is influenced by cultivation parameters: A review. Int. J. Mol. Sci. 2021, 22, 8819. [Google Scholar] [CrossRef]
- da Costa Cardoso, L.A.; Kanno, K.Y.F.; Karp, S.G. Microbial production of carotenoids a review. Afr. J. Biotechnol. 2017, 16, 139–146. [Google Scholar]
- Frigaard, N.-U. Biotechnology of anoxygenic phototrophic bacteria. Adv. Biochem. Eng. Biotechnol. 2016, 156, 139–154. [Google Scholar]
- Seckbach, J.; Oren, A. Oxygenic photosynthetic microorganisms in extreme environments. In Algae and Cyanobacteria in Extreme Environments; Springer: Berlin/Heidelberg, Germany, 2007; pp. 3–25. [Google Scholar]
- Latha, B.; Jeevaratnam, K.; Murali, H.; Manja, K. Influence of growth factors on carotenoid pigmentation of Rhodotorula glutinis DFR-PDY from natural source. Indian J. Biotechnol. 2005, 4, 353–357. [Google Scholar]
- Kim, M.; Jung, D.-H.; Seo, D.-H.; Chung, W.-H.; Seo, M.-J. Genome analysis of Lactobacillus plantarum subsp. plantarum KCCP11226 reveals a well-conserved C30 carotenoid biosynthetic pathway. 3 Biotech 2020, 10, 150. [Google Scholar]
- Li, S.; Zhao, Y.; Zhang, L.; Zhang, X.; Huang, L.; Li, D.; Niu, C.; Yang, Z.; Wang, Q. Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food Chem. 2012, 135, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Panesar, R.; Kaur, S.; Panesar, P.S. Production of microbial pigments utilizing agro-industrial waste: A review. Curr. Opin. Food Sci. 2015, 1, 70–76. [Google Scholar] [CrossRef]
- Köcher, S.; Breitenbach, J.; Müller, V.; Sandmann, G. Structure, function and biosynthesis of carotenoids in the moderately halophilic bacterium Halobacillus halophilus. Arch. Microbiol. 2009, 191, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Umeno, D.; Tobias, A.V.; Arnold, F.H. Evolution of the C30 carotenoid synthase CrtM for function in a C40 pathway. J. Bacteriol. 2002, 184, 6690–6699. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Choi, B.H.; Kim, J.H.; Kang, H.-J.; Ryu, H.; Lee, P.C. Complete genome sequence of Planococcus faecalis AJ003T, the type species of the genus Planococcus and a microbial C30 carotenoid producer. J. Biotechnol. 2018, 266, 72–76. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014, 13, 12. [Google Scholar] [CrossRef]
- Frengova, G.; Simova, E.; Pavlova, K.; Beshkova, D.; Grigorova, D. Formation of carotenoids by Rhodotorula glutinis in whey ultrafiltrate. Biotechnol. Bioeng. 1994, 44, 888–894. [Google Scholar] [CrossRef]
- Gallego-Cartagena, E.; Castillo-Ramírez, M.; Martínez-Burgos, W. Effect of stressful conditions on the carotenogenic activity of a Colombian strain of Dunaliella salina. Saudi J. Biol. Sci. 2019, 26, 1325–1330. [Google Scholar] [CrossRef]
- Sowmya, R.; Sachindra, N. Carotenoid production by Formosa sp. KMW, a marine bacteria of Flavobacteriaceae family: Influence of culture conditions and nutrient composition. Biocatal. Agric. Biotechnol. 2015, 4, 559–567. [Google Scholar] [CrossRef]
- Hayman, E.P.; Yokoyama, H.; Chichester, C.O.; Simpson, K.L. Carotenoid biosynthesis in Rhodotorula glutinis. J. Bacteriol. 1974, 120, 1339–1343. [Google Scholar] [CrossRef] [Green Version]
- Mihalcea, A.; Ungureanu, C.; Ferdes, M.; Chirvase, A.A.; Tanase, C. The influence of operating conditions on the growth of the yeast Rhodotorula rubra ICCF 209 and on torularhodin formation. Rev. Chim. 2011, 11, 12. [Google Scholar]
- Takaichi, S.; Ishidsu, J.-I. Influence of Growth Temperature on Compositions of Carotenoids and Fatty Acids from Carotenoid Glucoside Ester and from Cellular Lipids in Rhodococcus rhodochrous RNMSI. Biosci. Biotechnol. Biochem. 1993, 57, 1886–1889. [Google Scholar] [CrossRef]
- Simova, E.D.; Frengova, G.I.; Beshkova, D.M. Effect of aeration on the production of carotenoid pigments by Rhodotorula rubra-lactobacillus casei subsp. casei co-cultures in whey ultrafiltrate. Z. Naturforsch. C 2003, 58, 225–229. [Google Scholar] [CrossRef]
- Tinoi, J.; Rakariyatham, N.; Deming, R. Simplex optimization of carotenoid production by Rhodotorula glutinis using hydrolyzed mung bean waste flour as substrate. Process Biochem. 2005, 40, 2551–2557. [Google Scholar] [CrossRef]
- Marova, I.; Carnecka, M.; Halienova, A.; Certik, M.; Dvorakova, T.; Haronikova, A. Use of several waste substrates for carotenoid-rich yeast biomass production. J. Environ. Manag. 2012, 95, S338–S342. [Google Scholar] [CrossRef] [PubMed]
- Valduga, E.; Tatsch, P.; Vanzo, L.T.; Rauber, F.; Di Luccio, M.; Treichel, H. Assessment of hydrolysis of cheese whey and use of hydrolysate for bioproduction of carotenoids by Sporidiobolus salmonicolor CBS 2636. J. Sci. Food Agric. 2009, 89, 1060–1065. [Google Scholar] [CrossRef]
- Bhosale, P. Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl. Microbiol. Biotechnol. 2004, 63, 351–361. [Google Scholar] [CrossRef]
- Shi, F.; Zhan, W.; Li, Y.; Wang, X. Temperature influences β-carotene production in recombinant Saccharomyces cerevisiae expressing carotenogenic genes from Phaffia rhodozyma. World J. Microbiol. Biotechnol. 2014, 30, 125–133. [Google Scholar] [CrossRef]
- El-Banna, A.A.; El-Razek, A.M.A.; El-Mahdy, A.R. Some factors affecting the production of carotenoids by Rhodotorula glutinis var. glutinis. Food Nutr. Sci. 2012, 2012, 17082. [Google Scholar]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef]
- Ram, S.; Paliwal, C.; Mishra, S. Growth medium and nitrogen stress sparked biochemical and carotenogenic alterations in Scenedesmus sp. CCNM 1028. Bioresour. Technol. Rep. 2019, 7, 100194. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, J.G.; Chen, Q. Progress on molecular breeding and metabolic engineering of biosynthesis pathways of C30, C35, C40, C45, C50 carotenoids. Biotechnol. Adv. 2007, 25, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Schmidt-Dannert, C. Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl. Microbiol. Biotechnol. 2002, 60, 1–11. [Google Scholar] [PubMed]
- Takaichi, S.; Sandmann, G.; Schnurr, G.; Satomi, Y.; Suzuki, A.; Misawa, N. The carotenoid 7,8-dihydro-Ψ end group can be cyclized by the lycopene cyclases from the bacterium Erwinia uredovora and the higher plant Capsicum annuum. Eur. J. Biochem. 1996, 241, 291–296. [Google Scholar] [CrossRef] [PubMed]
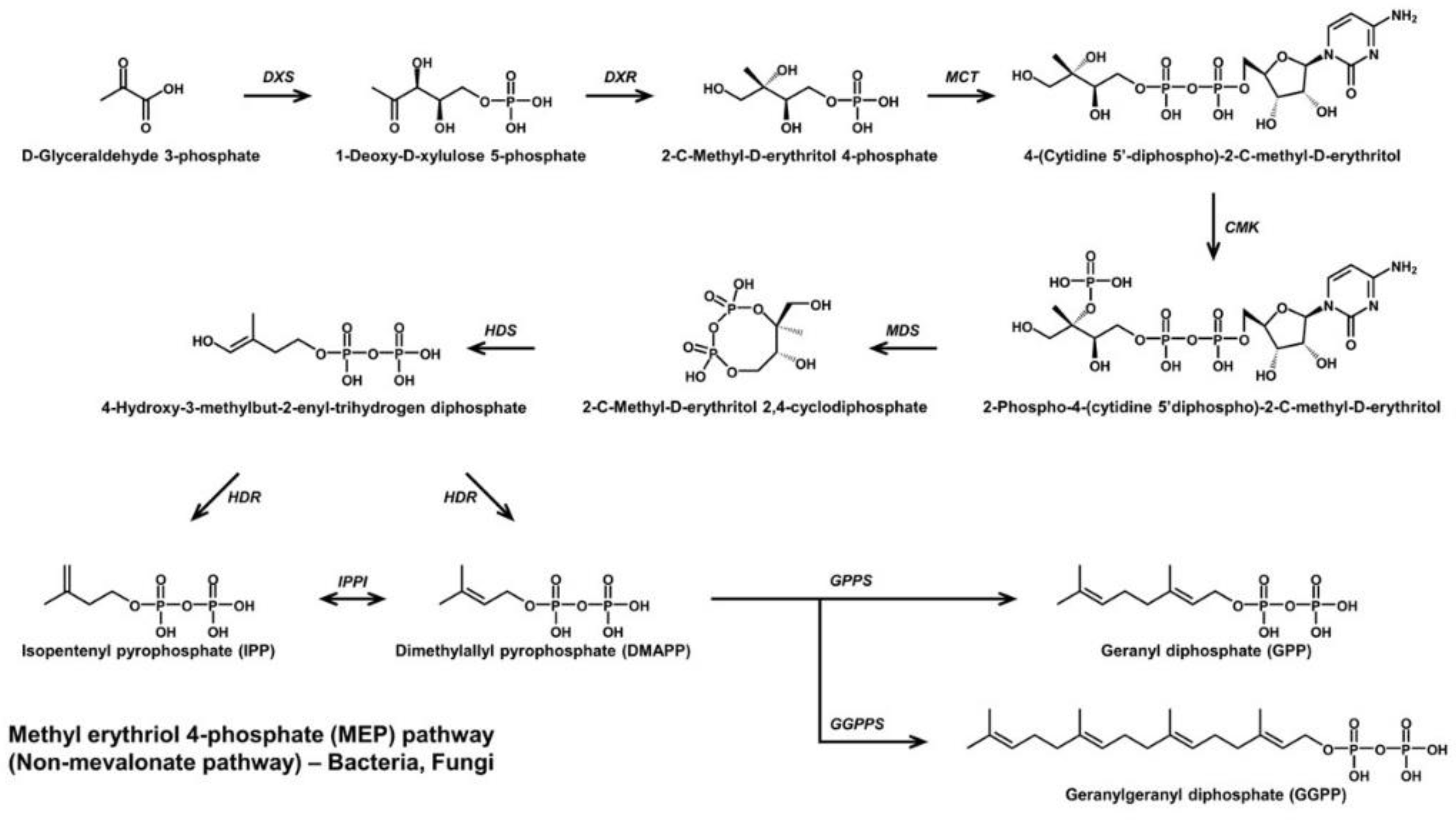
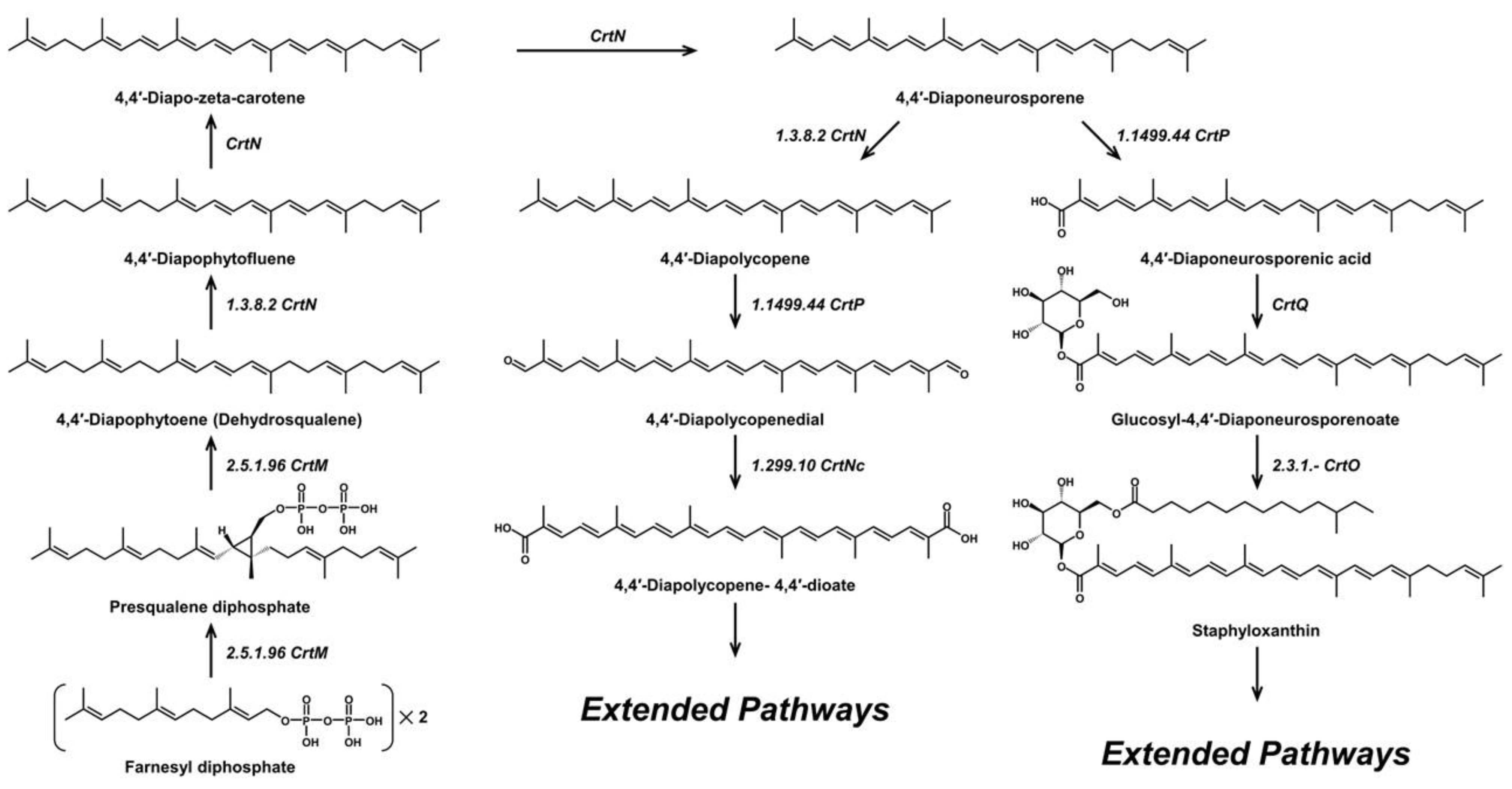
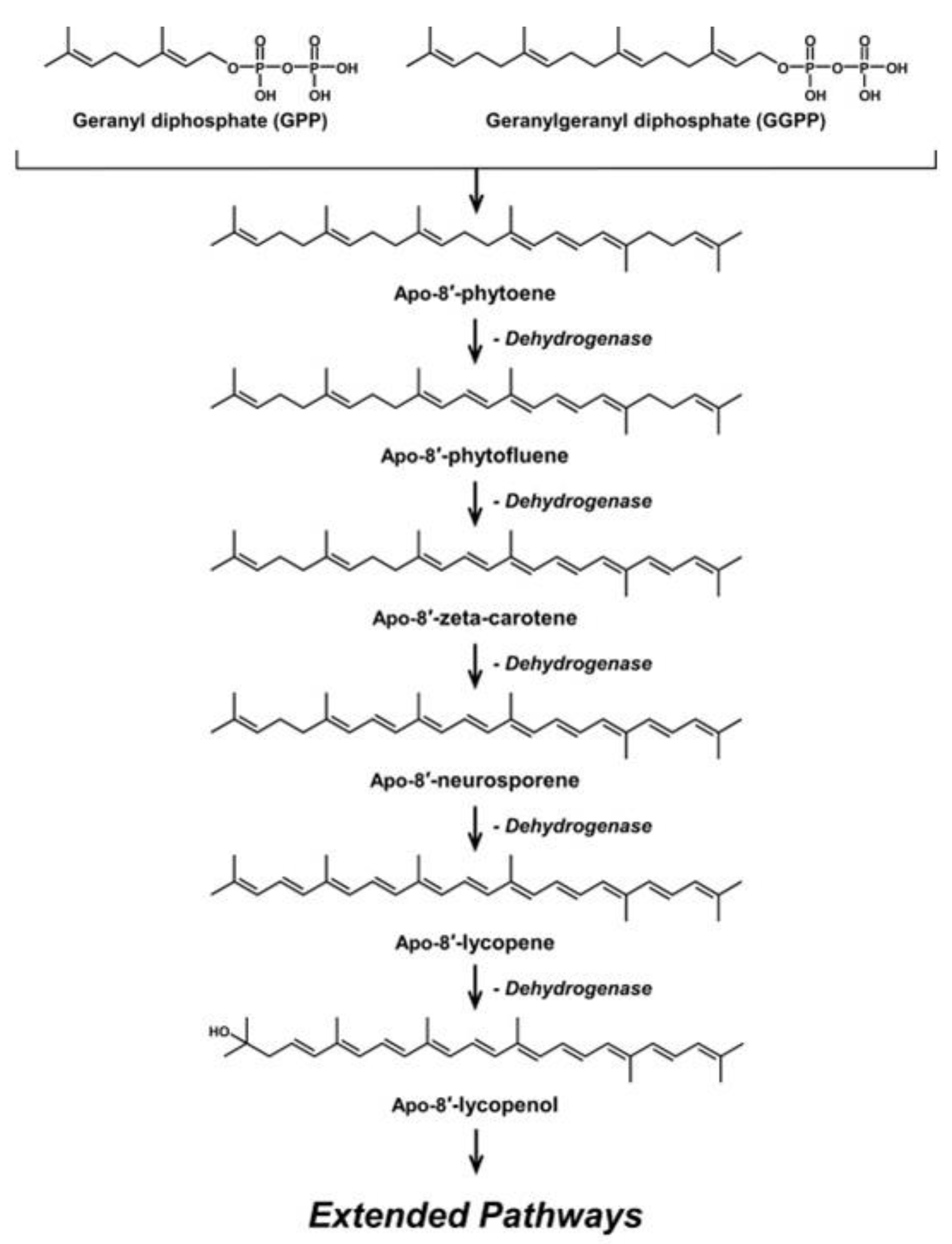
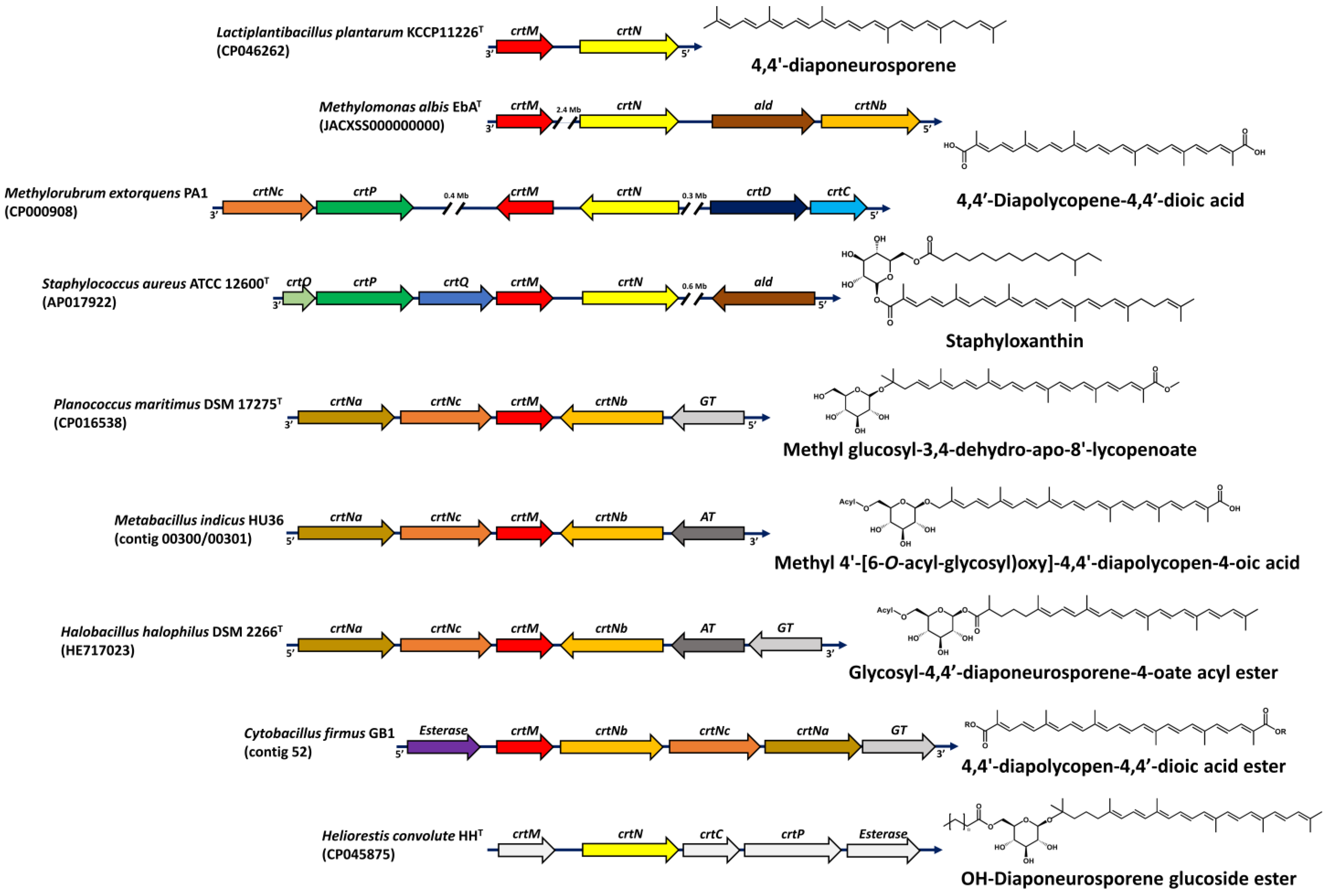
| Controlled Factor | Action | Effect | Ref. |
|---|---|---|---|
| Oxidative stress | Diphenylamine and duroquinone application | Complete inhibition of C30 carotenoids | [80] |
| Oxidative stress | Diphenylamine application | Complete inhibition of C30 carotenoids | [16] |
| Oxidative stress | Hydrogen peroxide exposure | 5.9-fold increase in carotenoid production | [29] |
| Temperature | Low temperature | Increased 4,4′-diaponeurosporene antioxidant activity– DPPH: 1.7-fold vs BHT; ABTS: 7.5-fold vs BHT; FRAP: 8-fold vs BHT | [31] |
| Recombinant technique | Constitutive lac promoter | Increased 4,4′-diapolycopene production | [81] |
| Recombinant technique | Optimizing engineered pathways in heterologous host | Increased 4,4′-diapolycopene and 4,4′-diaponeurosporene production | [82] |
| Recombinant technique | Gene coexpression | 20-fold increase in 4,4′-diapolycopene dialdehyde production | [37] |
| Recombinant technique | Farnesyl diphosphate (FDP) synthase overexpression | 3- to 5-fold increase in diapotorulene-to-diaponeurosporene ratio | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siziya, I.N.; Hwang, C.Y.; Seo, M.-J. Antioxidant Potential and Capacity of Microorganism-Sourced C30 Carotenoids—A Review. Antioxidants 2022, 11, 1963. https://doi.org/10.3390/antiox11101963
Siziya IN, Hwang CY, Seo M-J. Antioxidant Potential and Capacity of Microorganism-Sourced C30 Carotenoids—A Review. Antioxidants. 2022; 11(10):1963. https://doi.org/10.3390/antiox11101963
Chicago/Turabian StyleSiziya, Inonge Noni, Chi Young Hwang, and Myung-Ji Seo. 2022. "Antioxidant Potential and Capacity of Microorganism-Sourced C30 Carotenoids—A Review" Antioxidants 11, no. 10: 1963. https://doi.org/10.3390/antiox11101963
APA StyleSiziya, I. N., Hwang, C. Y., & Seo, M.-J. (2022). Antioxidant Potential and Capacity of Microorganism-Sourced C30 Carotenoids—A Review. Antioxidants, 11(10), 1963. https://doi.org/10.3390/antiox11101963