Biological Effects of Hydrogen Water on Subjects with NAFLD: A Randomized, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Subjects and Study Design
2.2. Clinical Biochemical/Hematological Parameters
2.3. Markers of Oxidative Stress
2.3.1. Malondialdehyde Measurement
2.3.2. 8-hydroxydeoxyguanosine Measurement
2.4. Lactate Dehydrogenase Assay
2.5. Western Blot
2.6. Measurement of Matrix Metalloproteinases Activities by Gelatin Zymography
2.7. Statistical Analysis
3. Results
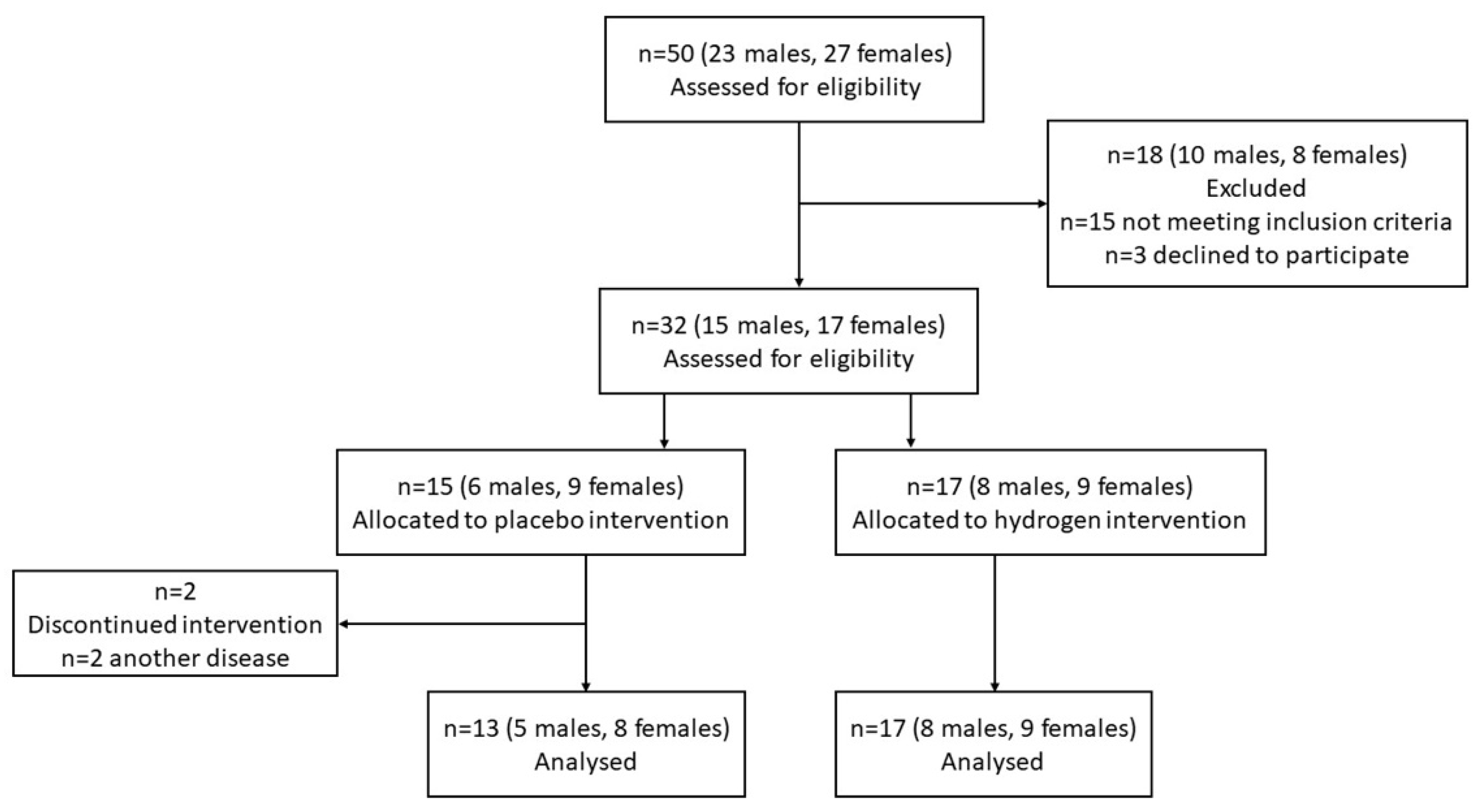
3.1. Subjects
3.2. Consumption of HRW Improved Plasma Biomarkers
3.3. Biomarkers of Redox Status
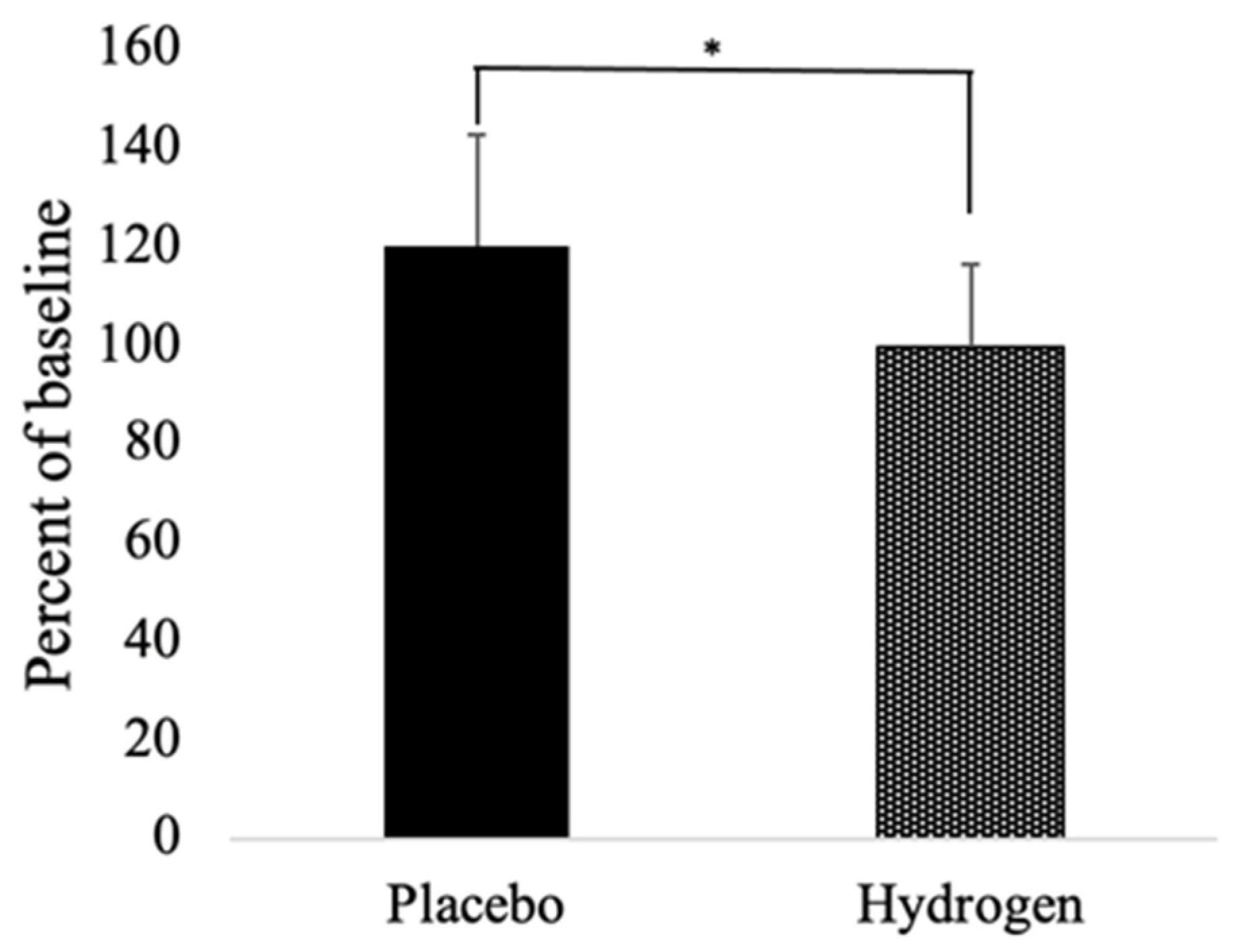
3.4. Inflammatory Markers and Heat Shock Proteins
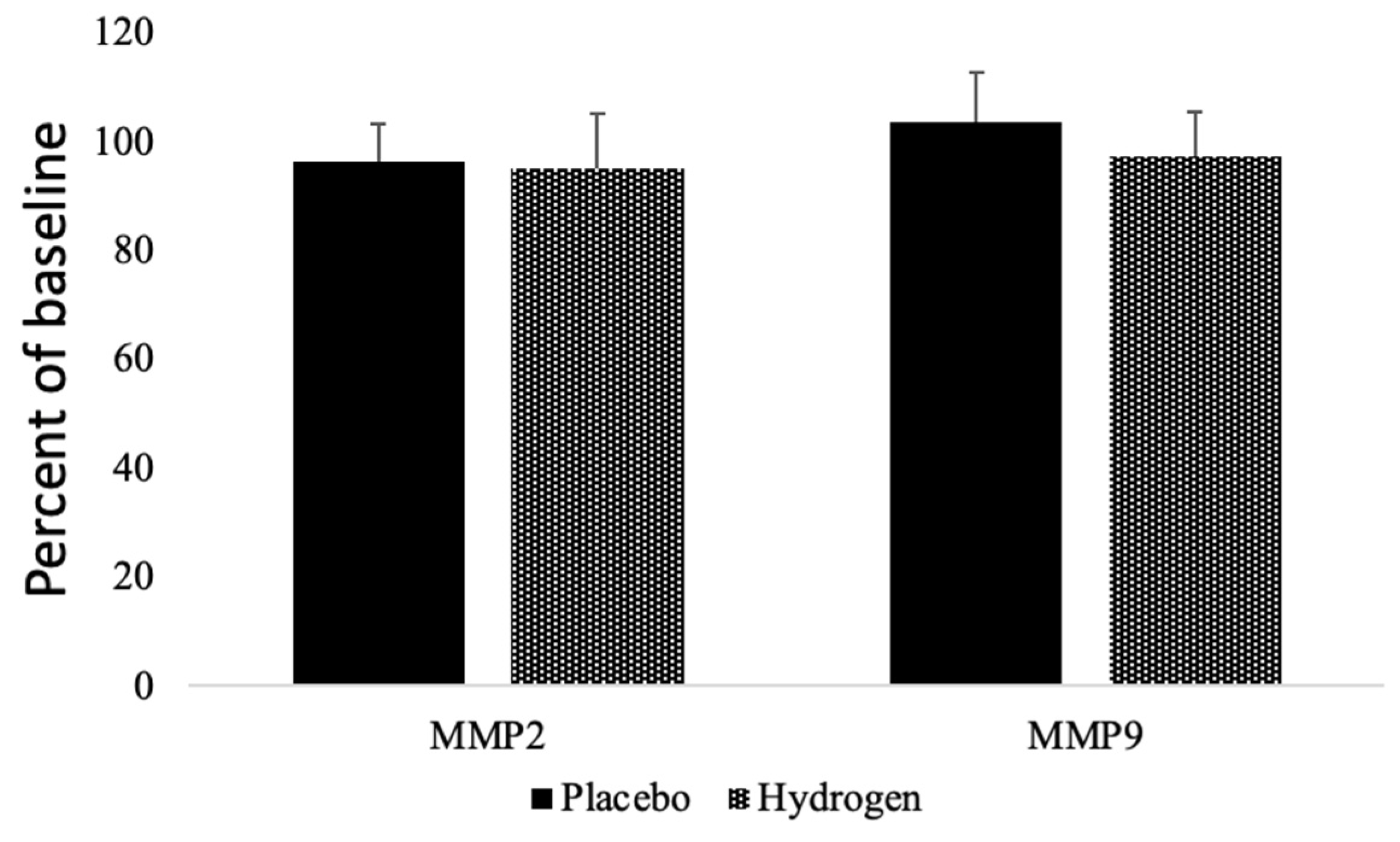
3.5. Matrix Metalloproteinases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver dis-ease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Soret, P.-A.; Magusto, J.; Housset, C.; Gautheron, J. In Vitro and In Vivo Models of Non-Alcoholic Fatty Liver Disease: A Critical Appraisal. J. Clin. Med. 2020, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- LeBaron, T.W.; Kura, B.; Kalocayova, B.; Tribulova, N.; Slezak, J. A New Approach for the Prevention and Treatment of Cardio-vascular Disorders. Molecular Hydrogen Significantly Reduces the Effects of Oxidative Stress. Molecules 2019, 24, 2076. [Google Scholar] [CrossRef]
- Hancock, J.T.; LeBaron, T.W.; May, J.; Thomas, A.; Russell, G. Molecular Hydrogen: A new treatment for plants in the UK? Plants 2021, 10, 2270. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Hirayama, M.; Yamai, K.; Goto, S.; Ito, M.; Ichihara, M.; Ohno, K. Drinking hydrogen water and intermittent hydrogen gas exposure, but not lactulose or continuous hydrogen gas exposure, prevent 6-hydorxydopamine-induced Parkinson’s disease in rats. Med. Gas Res. 2012; 2, 15. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Asgharzadeh, F.; Khazei, M.; Kura, B.; Tarnava, A.; Slezak, J. Molecular hydrogen is comparable to sulfasalazine as a treatment for DSS-induced colitis in mice. EXCLI J. 2021, 20, 1106–1117. [Google Scholar] [CrossRef]
- Koyama, Y.; Taura, K.; Hatano, E.; Tanabe, K.; Yamamoto, G.; Nakamura, K.; Yamanaka, K.; Kitamura, K.; Narita, M.; Nagata, H.; et al. Effects of oral intake of hydrogen water on liver fibrogenesis in mice. Hepatol. Res. 2013, 44, 663–677. [Google Scholar] [CrossRef]
- Zhai, X.; Chen, X.; Lu, J.; Zhang, Y.; Sun, X.; Huang, Q.; Wang, Q. Hydrogen-rich saline improves nonalcoholic fatty liver disease by alleviating oxidative stress and activating hepatic PPARalpha and PPARgamma. Mol. Med. Rep. 2017, 15, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- LeBaron, T.W.; Singh, R.B.; Fatima, G.; Kartikey, K.; Sharma, J.P.; Ostojic, S.M.; Gvozdjakova, A.; Kura, B.; Noda, M.; Mojto, V.; et al. The Effects of 24-Week, High-Concentration Hydrogen-Rich Water on Body Composition, Blood Lipid Profiles and Inflammation Biomarkers in Men and Women with Metabolic Syndrome: A Randomized Controlled Trial. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Toyoda, Y.; Sharma, P.; Evans, M.; Guthrie, N. Effectiveness of Hydrogen Rich Water on Antioxidant Status of Subjects with Potential Metabolic Syndrome—An Open Label Pilot Study. J. Clin. Biochem. Nutr. 2010, 46, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Korovljev, D.; Stajer, V.; Ostojic, J.; LeBaron, T.W.; Ostojic, S.M. Hydrogen-rich water reduces liver fat accumulation and improves liver enzyme profiles in patients with non-alcoholic fatty liver disease: A randomized controlled pilot trial. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 688–693. [Google Scholar] [CrossRef] [PubMed]
- LeBaron, T.W.; Larson, A.J.; Ohta, S.; Mikami, T.; Barlow, J.; Bulloch, J.; DeBeliso, M. Acute Supplementation with Molecular Hydrogen Benefits Submaximal Exercise Indices. Randomized, Double-Blinded, Placebo-Controlled Crossover Pilot Study. J. Lifestyle Med. 2019, 9, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Cohen, J.; Hebert, P.R.; O Taylor, J.; Hennekens, C.H. Implications of small reductions in diastolic blood pressure for primary prevention. Arch. Intern. Med. 1995, 155, 701–709. [Google Scholar] [CrossRef]
- Da, G.A.K.; Kelley, K.A.; Tran, Z. Aerobic Exercise and Resting Blood Pressure: A Meta-Analytic Review of Randomized, Controlled Trials. Prev. Cardiol. 2001, 4, 73–80. [Google Scholar] [CrossRef]
- Song, G.; Li, M.; Sang, H.; Zhang, L.; Li, X.; Yao, S.; Yu, Y.; Zong, C.; Xue, Y.; Qin, S. Hydrogen-rich water decreases serum LDL-cholesterol levels and improves HDL function in patients with potential metabolic syndrome. J. Lipid Res. 2013, 54, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of hydrogen-rich water im-proves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- da Luz, P.L.; Favarato, D.; Faria-Neto, J.R., Jr.; Lemos, P.; Chagas, A.C.P. High ratio of triglycerides to HDL-cholesterol predicts extensive coronary disease. Clinics 2008, 63, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Sultani, R.; Tong, D.C.; Peverelle, M.; Lee, Y.S.; Baradi, A.; Wilson, A.M. Elevated Triglycerides to High-Density Lipoprotein Cholesterol (TG/HDL-C) Ratio Predicts Long-Term Mortality in High-Risk Patients. Heart Lung Circ. 2020, 29, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.-W.; Yi, J.-J.; Ohrr, H. Total cholesterol and all-cause mortality by sex and age: A prospective cohort study among 12.8 million adults. Sci. Rep. 2019, 9, 1596. [Google Scholar] [CrossRef]
- Petursson, H.; Sigurdsson, J.A.; Bengtsson, C.; Nilsen, T.I.L.; Getz, L. Is the use of cholesterol in mortality risk algorithms in clinical guidelines valid? Ten years prospective data from the Norwegian HUNT 2 study. J. Eval. Clin. Pract. 2012, 18, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Tanaka, H.; Miyamatsu, N.; Hayakawa, T.; Kadowaki, T.; Kita, Y.; Nakamura, Y.; Okayama, A.; Ueshima, H. The relationship between serum total cholesterol and all-cause or cause-specific mortality in a 17.3-year study of a Japanese cohort. Atherosclerosis 2007, 190, 216–223. [Google Scholar] [CrossRef]
- Child, R.B.; Wilkinson, D.M.; Fallowfield, J.L.; Donnelly, A. Elevated serum antioxidant capacity and plasma malondialdehyde concentration in response to a simulated half-marathon run. Med. Sci. Sports Exerc. 1998, 30, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Orhan, H.; van Holland, B.; Krab, B.; Moeken, J.; Vermeulen, N.P.; Hollander, P.; Meerman, J.H. Evaluation of a Multi-parameter Biomarker Set for Oxidative Damage in Man: Increased Urinary Excretion of Lipid, Protein and DNA Oxidation Products after One Hour of Exercise. Free Radic. Res. 2004, 38, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Thusu, K.; Cook, S.; Snyder, B.; Makowski, J.; Armstrong, D.; Nicotera, T. Oxidative damage to DNA in diabetes mellitus. Lancet 1996, 347, 444–445. [Google Scholar] [CrossRef]
- Han, A.L.; Park, S.-H.; Park, M.S. Hydrogen Treatment Protects against Cell Death and Senescence Induced by Oxidative Damage. J. Microbiol. Biotechnol. 2017, 27, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Kawamura, T.; Masutani, K.; Peng, X.; Sun, Q.; Stolz, D.B.; Pribis, J.P.; Billiar, T.R.; Sun, X.; Bermudez, C.A.; et al. Oral intake of hydrogen-rich water inhibits intimal hy-perplasia in arterialized vein grafts in rats. Cardiovasc. Res. 2012, 94, 144–153. [Google Scholar] [CrossRef]
- Trojanek, J.B.; Michalkiewicz, J.; Grzywa-Czuba, R.; Janczyk, W.; Gackowska, L.; Kubiszewska, I.; Helmin-Basa, A.; Wierzbicka-Rucińska, A.; Szalecki, M.; Socha, P. Expression of Matrix Met-alloproteinases and Their Tissue Inhibitors in Peripheral Blood Leukocytes and Plasma of Children with Nonalcoholic Fatty Liver Disease. Mediat. Inflamm. 2020, 2020, 8327945. [Google Scholar] [CrossRef]
- Barancik, M.; Kura, B.; LeBaron, T.W.; Bolli, R.; Buday, J.; Slezak, J. Molecular and Cellular Mechanisms Associated with Effects of Molecular Hydrogen in Cardiovascular and Central Nervous Systems. Antioxidants 2020, 9, 1281. [Google Scholar] [CrossRef]
- Alwazeer, D.; Liu, F.F.; Wu, X.Y.; LeBaron, T.W. Combating Oxidative Stress and Inflammation in COVID-19 by Molecular Hy-drogen Therapy: Mechanisms and Perspectives. Oxidative Med. Cell. Longev. 2021, 2021, 5513868. [Google Scholar] [CrossRef]
- Russell, G.; Rehman, M.; LeBaron, T.W.; Veal, D.; Adukwu, E.; Hancock, J.T. An Overview of SARS-CoV-2 (COVID-19) Infection: The Importance of Molecular Hydrogen as an Adjunctive Therapy. React. Oxyg. Species 2020, 28, 150–165. [Google Scholar] [CrossRef]
- Slezak, J.; Kura, B.; LeBaron, T.W.; Singal, P.K.; Buday, J.; Barancik, M. Oxidative Stress and Pathways of Molecular Hydrogen Effects in Medicine. Curr. Pharm. Des. 2021, 27, 610–625. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.; Dressler, N.; Ben-Shushan, R.S.; Meerson, A.; LeBaron, T.W.; Tamir, S. Effects of alkaline-electrolyzed and hydrogen-rich water, in a high-fat-diet nonalcoholic fatty liver disease mouse model. World J. Gastroenterol. 2018, 24, 5095–5108. [Google Scholar] [CrossRef]
- Hou, C.; Wang, Y.; Zhu, E.; Yan, C.; Zhao, L.; Wang, X.; Qiu, Y.; Shen, H.; Sun, X.; Feng, Z.; et al. Coral calcium hydride prevents hepatic steatosis in high fat diet-induced obese rats: A potent mitochondrial nutrient and phase II enzyme inducer. Biochem. Pharmacol. 2016, 103, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Hirayama, M.; Ito, M.; Minato, T.; Yoritaka, A.; LeBaron, T.W. Inhalation of hydrogen gas elevates urinary 8-hydroxy-2′-deoxyguanine in Parkinson’s disease. Med. Gas Res. 2018, 8, 144–149. [Google Scholar] [CrossRef]
- Eckermann, J.M.; Chen, W.; Jadhav, V.; Hsu, F.P.; Colohan, A.R.; Tang, J.; Zhang, J.H. Hydrogen is neuroprotective against surgically induced brain injury. Med. Gas Res. 2011, 1, 7. [Google Scholar] [CrossRef]
- Matchett, G.A.; Fathali, N.; Hasegawa, Y.; Jadhav, V.; Ostrowski, R.P.; Martin, R.D.; Dorotta, I.R.; Sun, X.; Zhang, J.H. Hydrogen gas is ineffective in moderate and severe neonatal hypoxia–ischemia rat models. Brain Res. 2009, 1259, 90–97. [Google Scholar] [CrossRef]
- Kura, B.; Kalocayova, B.; LeBaron, T.W.; Frimmel, K.; Buday, J.; Surovy, J.; Slezak, J. Regulation of microRNAs by molecular hydrogen contributes to the prevention of radiation-induced damage in the rat myocardium. Mol. Cell. Biochem. 2019, 457, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Mao, Y.; Zhang, W.; Lai, D.; Wang, Q.; Shen, W. Reactive Oxygen Species-Dependent Nitric Oxide Production Contributes to Hydrogen-Promoted Stomatal Closure in Arabidopsis. Plant Physiol. 2014, 165, 759–773. [Google Scholar] [CrossRef]
- Murakami, Y.; Ito, M.; Ohsawa, I. Molecular hydrogen protects against oxidative stress-induced SH-SY5Y neuroblastoma cell death through the process of mitohormesis. PLoS ONE 2017, 12, e0176992. [Google Scholar] [CrossRef] [PubMed]
- LeBaron, T.W.; Laher, I.; Kura, B.; Slezak, J. Hydrogen gas: From clinical medicine to an emerging ergogenic molecule for sports athletes. Can. J. Physiol. Pharmacol. 2019, 97, 797–807. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Ito, M.; Negishi, S.; Sobue, S.; Ichihara, M.; Ohno, K. Molecular hydrogen upregulates heat shock response and col-lagen biosynthesis, and downregulates cell cycles—Meta-analyses of gene expression profiles. Free Radic. Res. 2018, 52, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Ito, M.; Hasegawa, S.; Teranishi, M.; Takeda, K.; Negishi, S.; Nishiwaki, H.; Takeda, J.-I.; LeBaron, T.W.; Ohno, K. Molecular Hydrogen Enhances Proliferation of Cancer Cells That Exhibit Potent Mitochondrial Unfolded Protein Response. Int. J. Mol. Sci. 2022, 23, 2888. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T.; LeBaron, T.W.; Russell, G. Molecular Hydrogen: Redox Reactions and Possible Biological Interactions. React. Oxyg. Species 2021, 11, m17–m25. [Google Scholar] [CrossRef]

| Baseline Characteristics | Placebo | Hydrogen | p-Value |
|---|---|---|---|
| Age (years) | 53.23 ± 9.13 | 52.65 ± 11.9 | >0.1 |
| Height (cm) | 170.46 ± 10.41 | 168.94 ± 11.08 | >0.1 |
| Weight (kg) | 95.77 ± 14.21 | 101.35 ± 15.14 | >0.1 |
| Body mass index (BMI) | 32.8 ± 3.37 | 35.52 ± 4.03 | >0.1 |
| Systolic blood pressure | 125.38 ± 11.98 | 128.47 ± 9.37 | >0.1 |
| Diastolic blood pressure | 77.69 ± 8.32 | 77.94 ± 6.86 | >0.1 |
| Heart rate (beats/min) | 73.31 ± 8.22 | 69.65 ± 8.96 | >0.1 |
| Characteristics | Placebo | Hydrogen | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Weight | 95.77 ± 14.21 | 96.00 ± 14.98 | 101.35 ± 15.14 | 100.26 ± 15.66 |
| BMI | 32.8 ± 3.37 | 32.91 ± 3.03 | 35.52 ± 4.03 | 35.16 ± 4.33 * |
| Systolic bp | 125.38 ± 11.98 | 126.15 ± 11.93 | 128.47 ± 9.37 | 126.18 ± 9.28 |
| Diastolic bp | 77.69 ± 8.32 | 74.62 ± 7.76 | 77.94 ± 6.86 | 77.06 ± 4.70 |
| Pulse | 73.31 ± 8.22 | 71.64 ± 6.05 | 69.65 ± 8.96 | 71.29 ± 7.28 |
| Marker | Placebo | Hydrogen | ||
|---|---|---|---|---|
| Baseline | Follow Up | Baseline | Follow Up | |
| Albumin (ALB) (g/L) | 43.22 ± 2.6 | 44.47 ± 2.90 | 42.68 ± 2.36 | 44.58 ± 2.04 |
| Alkaline phosphatase (ALP) (ukat/L) | 1.07 ± 0.33 | 1.09 ± 0.37 | 1.28 ± 0.53 | 1.32 ± 0.55 |
| Alanine transferase (ALT) (ukat/L) | 0.69 ± 0.34 | 0.58 ± 0.24 | 0.77 ± 0.48 | 0.75 ± 0.30 |
| Aspartate aminotransferase (AST) (ukat/L) | 0.57 ± 0.22 | 0.48 ± 0.14 | 0.62 ±0.41 | 0.53 ± 0.22 |
| C-reactive protein (CRP) (mg/L) | 3.26 ± 1.50 | 4.49 ± 2.50 | 4.52 ± 3.55 | 4.57 ± 3.20 |
| MDA (µM/µL) | 3.11 ± 1.53 | 2.34 ± 0.60 | 2.67 ± 0.82 | 3.13 ± 1.60 * |
| 8-OHdG (ng/mL) | 26.93 ± 8.70 | 27.87 ± 7.67 | 24.36 ± 9.51 | 26.39 ± 9.72 |
| LDH (mU/mL) | 71.23 ±32.50 | 73.10 ± 39.16 | 68.53 ± 28.91 | 64.43 ± 32.82 |
| Total cholesterol (TC) (mg/dL) | 179.34 ± 31.60 | 187.76 ± 41.06 | 180.32 ± 39.28 | 190.06 ± 38.85 |
| High density lipoprotein (HDL) (mg/dL) | 44.50 ± 9.29 | 46.05 ± 8.70 | 43.38 ± 4.19 | 46.52 ± 3.96 |
| Low density lipoprotein (LDL) (mg/dL) | 114.34 ± 24.40 | 119.91 ± 30.70 | 116.33 ± 31.21 | 120.54 ± 30.63 |
| Triglycerides (TG) (mg/dL) | 170.19 ± 58.59 | 195.60 ± 69.62 | 156.88 ± 39.12 | 169.45 ± 74.47 |
| TG/HDL ratio | 3.82 ± 6.31 | 4.25 ± 8.00 | 3.62 ± 9.33 | 3.64 ± 18.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kura, B.; Szantova, M.; LeBaron, T.W.; Mojto, V.; Barancik, M.; Szeiffova Bacova, B.; Kalocayova, B.; Sykora, M.; Okruhlicova, L.; Tribulova, N.; et al. Biological Effects of Hydrogen Water on Subjects with NAFLD: A Randomized, Placebo-Controlled Trial. Antioxidants 2022, 11, 1935. https://doi.org/10.3390/antiox11101935
Kura B, Szantova M, LeBaron TW, Mojto V, Barancik M, Szeiffova Bacova B, Kalocayova B, Sykora M, Okruhlicova L, Tribulova N, et al. Biological Effects of Hydrogen Water on Subjects with NAFLD: A Randomized, Placebo-Controlled Trial. Antioxidants. 2022; 11(10):1935. https://doi.org/10.3390/antiox11101935
Chicago/Turabian StyleKura, Branislav, Maria Szantova, Tyler W. LeBaron, Viliam Mojto, Miroslav Barancik, Barbara Szeiffova Bacova, Barbora Kalocayova, Matus Sykora, Ludmila Okruhlicova, Narcisa Tribulova, and et al. 2022. "Biological Effects of Hydrogen Water on Subjects with NAFLD: A Randomized, Placebo-Controlled Trial" Antioxidants 11, no. 10: 1935. https://doi.org/10.3390/antiox11101935
APA StyleKura, B., Szantova, M., LeBaron, T. W., Mojto, V., Barancik, M., Szeiffova Bacova, B., Kalocayova, B., Sykora, M., Okruhlicova, L., Tribulova, N., Gvozdjakova, A., Sumbalova, Z., Kucharska, J., Faktorova, X., Jakabovicova, M., Durkovicová, Z., Macutek, J., Koscová, M., & Slezak, J. (2022). Biological Effects of Hydrogen Water on Subjects with NAFLD: A Randomized, Placebo-Controlled Trial. Antioxidants, 11(10), 1935. https://doi.org/10.3390/antiox11101935









