Antibiotic-Resistant Bacteria in Drinking Water from the Greater Accra Region, Ghana: A Cross-Sectional Study, December 2021–March 2022
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. General Setting
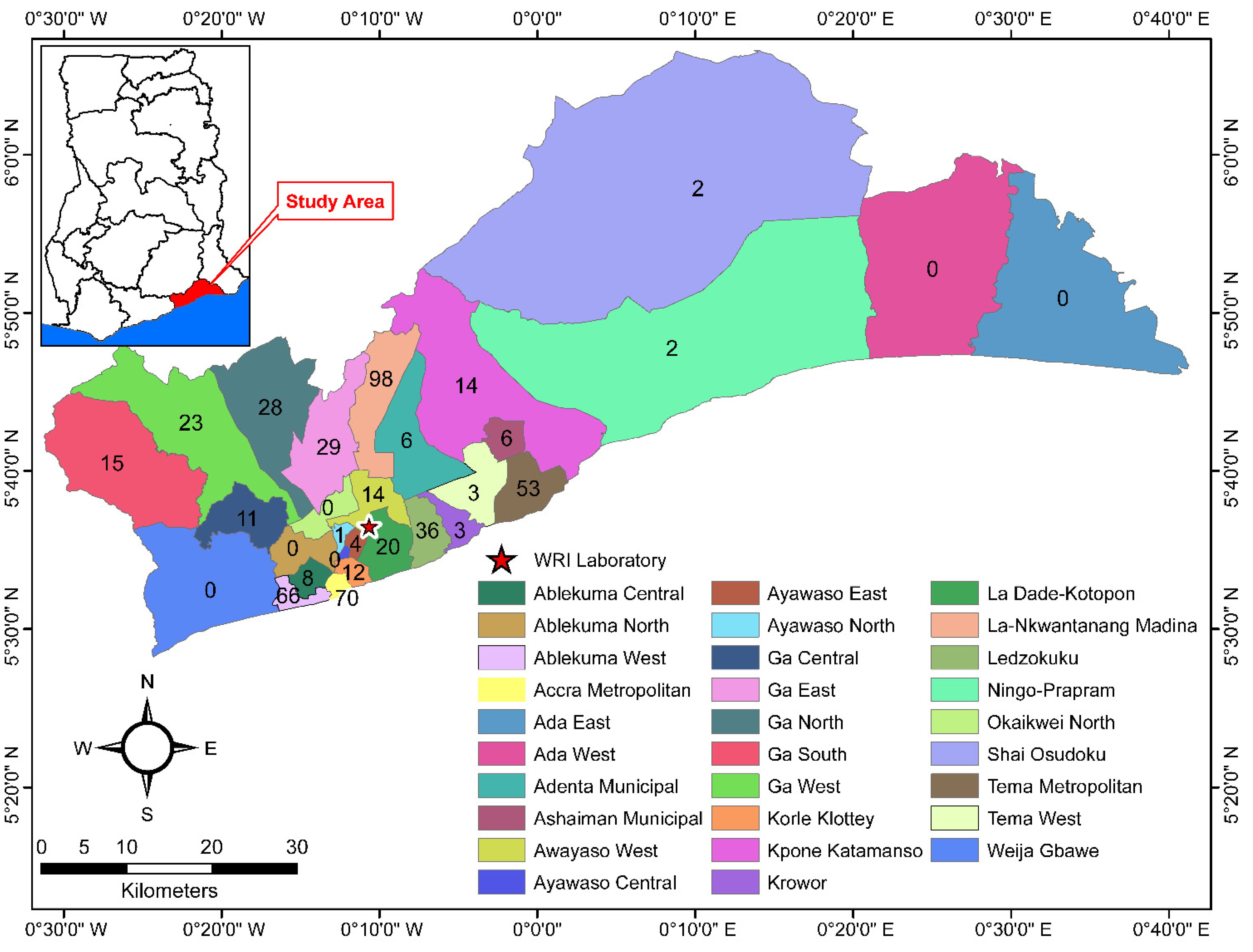
2.3. Specific Setting
2.4. Sample Collection
2.5. Sample Analysis
2.6. Antibiotic Susceptibility Testing
2.7. Quality-Control Procedures
2.8. Data Collection and Validation
2.9. Statistical Analysis
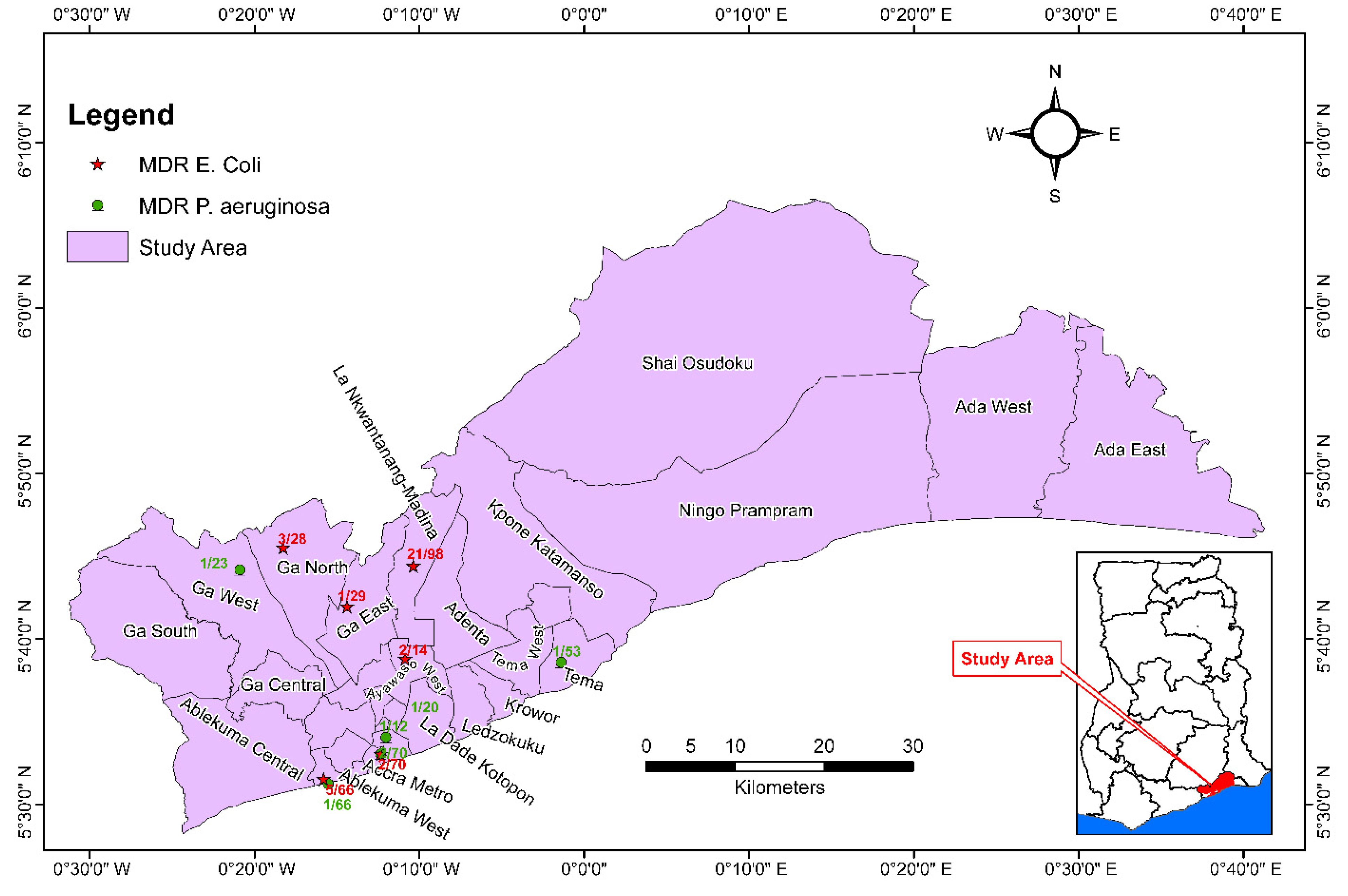
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development; UN General Assembly: New York, NY, USA, 2015; Volume A/RES/70/1, Available online: https://www.refworld.org/docid/57b6e3e44.html (accessed on 10 August 2022).
- Bain, R.; Cronk, R.; Hossain, R.; Bonjour, S.; Onda, K.; Wright, J.; Yang, H.; Slaymaker, T.; Hunter, P.; Prüss-Ustün, A.; et al. Global Assessment of Exposure to Faecal Contamination through Drinking Water Based on a Systematic Review. Trop. Med. Int. Heal. 2014, 19, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Dekker, D.M.; Krumkamp, R.; Sarpong, N.; Frickmann, H.; Boahen, K.G.; Frimpong, M.; Asare, R.; Larbi, R.; Hagen, R.M.; Poppert, S.; et al. Drinking Water from Dug Wells in Rural Ghana — Salmonella Contamination, Environmental Factors, and Genotypes. Int. J. Environ. Res. Public Health 2015, 12, 3535–3546. [Google Scholar] [CrossRef] [PubMed]
- Sanganyado, E.; Gwenzi, W. Antibiotic Resistance in Drinking Water Systems: Occurrence, Removal, and Human Health Risks. Sci. Total Environ. 2019, 669, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Ghana Statistical Service. Ghana Living Standards Survey Round 7 (GLSS7), Main Report; Ghana Statistical Service: Accra, Ghana, 2019; pp. 1–343.
- Ghana Statistical Service. Multiple Indicator Cluster Survey (MICS 2017/2018), Survey Findings Report; Ghana Statistical Service: Accra, Ghana, 2018.
- Banu, R.A.; Alvarez, J.M.; Reid, A.J.; Enbiale, W.; Labi, A.K.; Ansa, E.D.O.; Annan, E.A.; Akrong, M.O.; Borbor, S.; Adomako, L.A.B.; et al. Extended Spectrum Beta-Lactamase Escherichia Coli in River Waters Collected from Two Cities in Ghana, 2018–2020. Trop. Med. Infect. Dis. 2021, 6, 2018–2020. [Google Scholar] [CrossRef]
- Sakkas, H.; Bozidis, P.; Ilia, A.; Mpekoulis, G.; Papadopoulou, C. Antimicrobial Resistance in Bacterial Pathogens and Detection of Carbapenemases in Klebsiella Pneumoniae Isolates from Hospital Wastewater. Antibiotics 2019, 8, 85. [Google Scholar] [CrossRef]
- He, Y.; Yuan, Q.; Mathieu, J.; Stadler, L.; Senehi, N.; Sun, R.; Alvarez, P.J.J. Antibiotic Resistance Genes from Livestock Waste: Occurrence, Dissemination, and Treatment. npj Clean Water 2020, 3, 1–11. [Google Scholar] [CrossRef]
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and Economic Impact of Antibiotic Resistance in Developing Countries: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef]
- Augustin, J.C.; Carlier, V. Lessons from the Organization of a Proficiency Testing Program in Food Microbiology by Interlaboratory Comparison: Analytical Methods in Use, Impact of Methods on Bacterial Counts and Measurement Uncertainty of Bacterial Counts. Food Microbiol. 2006, 23, 1–38. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality—Fourth Edition, Incorporating the 1st Addendum; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- GSA. GHANA STANDARD GS 175:2017 Water Quality Specification for Drinking Water; Ghana Standards Authority: Accra, Ghana, 2017.
- Flores Ribeiro, A.; Bodilis, J.; Alonso, L.; Buquet, S.; Feuilloley, M.; Dupont, J.P.; Pawlak, B. Occurrence of Multi-Antibiotic Resistant Pseudomonas Spp. in Drinking Water Produced from Karstic Hydrosystems. Sci. Total Environ. 2014, 490, 370–378. [Google Scholar] [CrossRef]
- Odonkor, S.T.; Addo, K.K. Prevalence of Multidrug-Resistant Escherichia Coli Isolated from Drinking Water Sources. Int. J. Microbiol. 2018, 2018, 7204013. [Google Scholar] [CrossRef]
- Kichana, E.; Addy, F.; Dufailu, O.A. Genetic Characterization and Antimicrobial Susceptibility of Escherichia Coli Isolated from Household Water Sources in Northern Ghana. J. Water Health 2022, 20, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Asokan, G.V.; Ramadhan, T.; Ahmed, E.; Sanad, H. WHO Global Priority Pathogens List: A Bibliometric Analysis of Medline-Pubmed for Knowledge Mobilization to Infection Prevention and Control Practices in Bahrain. Oman Med. J. 2019, 34, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Stoler, J.; Ahmed, H.; Asantewa Frimpong, L.; Bello, M. Presence of Pseudomonas Aeruginosa in Coliform-Free Sachet Drinking Water in Ghana. Food Control 2015, 55, 242–247. [Google Scholar] [CrossRef]
- García-Vello, P.; González-Zorn, B.; Saba, C.K.S. Antibiotic Resistance Patterns in Human, Animal, Food and Environmental Isolates in Ghana: A Review. Pan Afr. Med. J. 2020, 35, 1–15. [Google Scholar] [CrossRef]
- Ghana Statistical Service. Ghana 2021 Population and Housing Census General Report, Volume 3B; Ghana Standards Authority: Accra, Ghana, 2021.
- Appiah-Effah, E.; Ahenkorah, E.N.; Duku, G.A.; Nyarko, K.B. Domestic Drinking Water Management: Quality Assessment in Oforikrom Municipality, Ghana. Sci. Prog. 2021, 104, 1–26. [Google Scholar] [CrossRef]
- Carranzo, I.V. Standard Methods for Examination of Water and Wastewater. In Anales De Hidrología Médica; Universidad Complutense de Madrid: Madrid, Spain, 2012; ISBN 978-087553-013-0. [Google Scholar]
- M100Ed32|Performance Standards for Antimicrobial Susceptibility Testing, 32nd Edition. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 9 July 2022).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Dzodzomenyo, M.; Fink, G.; Dotse-Gborgbortsi, W.; Wardrop, N.; Aryeetey, G.; Coleman, N.; Hill, A.; Wright, J. Sachet Water Quality and Product Registration: A Cross-Sectional Study in Accra, Ghana. J. Water Health 2018, 16, 646–656. [Google Scholar] [CrossRef]
- Stoler, J.; Tutu, R.A.; Ahmed, H.; Frimpong, L.A.; Bello, M. Sachet Water Quality and Brand Reputation in Two Low-Income Urban Communities in Greater Accra, Ghana. Am. J. Trop. Med. Hyg. 2014, 90, 272. [Google Scholar] [CrossRef]
- Tekpor, M.; Akrong, M.O.; Asmah, M.H.; Banu, R.A.; Ansa, E.D.O. Bacteriological Quality of Drinking Water in the Atebubu-Amantin District of the Brong-Ahafo Region of Ghana. Appl. Water Sci. 2017, 7, 2571–2576. [Google Scholar] [CrossRef]
- Viban, T.B.; Herman, O.N.; Layu, T.C.; Madi, O.P.; Nfor, E.N.; Kingsly, M.T.; Germanus, B.; Victor, N.N.; Albert, N. Risk Factors Contributing to Microbiological Contamination of Boreholes and Hand Dug Wells Water in the Vina Division, Adamawa, Cameroon. Adv. Microbiol. 2021, 11, 90–108. [Google Scholar] [CrossRef]
- Adomako, L.A.B.; Yirenya-Tawiah, D.; Nukpezah, D.; Abrahamya, A.; Labi, A.K.; Grigoryan, R.; Ahmed, H.; Owusu-Danquah, J.; Annang, T.Y.; Banu, R.A.; et al. Reduced Bacterial Counts from a Sewage Treatment Plant but Increased Counts and Antibiotic Resistance in the Recipient Stream in Accra, Ghana-A Cross-Sectional Study. Trop. Med. Infect. Dis. 2021, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Labi, A.K.; Obeng-Nkrumah, N.; Nartey, E.T.; Bjerrum, S.; Adu-Aryee, N.A.; Ofori-Adjei, Y.A.; Yawson, A.E.; Newman, M.J. Antibiotic Use in a Tertiary Healthcare Facility in Ghana: A Point Prevalence Survey. Antimicrob. Resist. Infect. Control 2018, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Kwabena, O.; Amponsah, O.; Buabeng, K.O.; Owusu-Ofori, A.; Kwame Ayisi-Boateng, N.; Hä Meen-Anttila, K.; Enlund, H. Point Prevalence Survey of Antibiotic Consumption across Three Hospitals in Ghana. JAC-Antimicrob. Resist. 2021, 3. [Google Scholar] [CrossRef]
- Duarte, A.C.; Rodrigues, S.; Afonso, A.; Nogueira, A.; Coutinho, P. Antibiotic Resistance in the Drinking Water: Old and New Strategies to Remove Antibiotics, Resistant Bacteria, and Resistance Genes. Pharmaceuticals 2022, 15, 393. [Google Scholar] [CrossRef] [PubMed]
- Lulani, I.; Van Der Steen, P.; Vairavamoorthy, K. Water Knowledge for the New Millennium Analysis of the Public Health Risks of the Urban Water System in Accra by Microbial Risk Assessment. Master’s Thesis, UNESCO-IHE, Delft, The Netherlands, 2007. [Google Scholar]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Ateba, C.N.; Tabi, N.M.; Fri, J.; Bissong, M.E.A.; Bezuidenhout, C.C. Occurrence of Antibiotic-Resistant Bacteria and Genes in Two Drinking Water Treatment and Distribution Systems in the North-West Province of South Africa. Antibiotics 2020, 9, 745. [Google Scholar] [CrossRef]
- Shi, P.; Jia, S.; Zhang, X.X.; Zhang, T.; Cheng, S.; Li, A. Metagenomic Insights into Chlorination Effects on Microbial Antibiotic Resistance in Drinking Water. Water Res. 2013, 47, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Hu, H.Y.; Wu, Y.H.; Wei, B.; Lu, Y. Effect of Chlorination and Ultraviolet Disinfection on TetA-Mediated Tetracycline Resistance of Escherichia Coli. Chemosphere 2013, 90, 2247–2253. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yu, X.; McDonald, T.J.; Jinadatha, C.; Dionysiou, D.D.; Feng, M. Elimination of Antibiotic Resistance Genes and Control of Horizontal Transfer Risk by UV-Based Treatment of Drinking Water: A Mini Review. Front. Environ. Sci. Eng. 2019, 13, 37. [Google Scholar] [CrossRef]
- Da Costa, P.M.; Loureiro, L.; Matos, A.J.F. Transfer of Multidrug-Resistant Bacteria Between Intermingled Ecological Niches: The Interface Between Humans, Animals and the Environment. Int. J. Environ. Res. Public Health 2013, 10, 278–294. [Google Scholar] [CrossRef]
- WHO. Critically Important Antimicrobials for Human Medicine: 5th Revision; WHO: Geneva, Switzerland, 2017; pp. 1–41. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Sagoe, G.; Danquah, F.S.; Simon Amofa-Sarkodie, E.; Appiah-Effah, E.; Ekumah, E.; Mensah, E.K.; Karikari, K.S. GIS-Aided Optimisation of Faecal Sludge Management in Developing Countries: The Case of the Greater Accra Metropolitan Area, Ghana. Heliyon 2017, 5, e02505. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | N | % | |
|---|---|---|---|
| Total | 524 | 100 | |
| Sample provider | Individual | 60 | 11.5 |
| Water or food processing company | 464 | 88.5 | |
| Sample type | Sachet | 190 | 36.3 |
| Bottled | 47 | 9.0 | |
| Tap water | 196 | 37.4 | |
| Borehole water | 77 | 14.7 | |
| Well water | 14 | 2.7 | |
| Treatment methods used | Untreated | 69 | 13.2 |
| Chlorination only | 171 | 32.6 | |
| Chlorination, Filtration | 7 | 1.3 | |
| Chlorination, Filtration, Ultrafiltration, UV | 20 | 3.8 | |
| Filtration, UV | 11 | 2.1 | |
| RO, Filtration | 14 | 2.7 | |
| Sand, Carbon, RO, Filtration, UV | 232 | 44.3 |
| Type of Bacteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample Type | No. Samples | Total Coliforms | E. coli | P. aeruginosa | THB | ||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | ||
| Sachet | 190 | 0.0 | 0–0 | 0.0 | 0–0 | 0.0 | 0–0 | 1.0 | 0–28 |
| Bottle | 47 | 0.0 | 0–0 | 0.0 | 0–0 | 0.0 | 0–0 | 0.0 | 0–14 |
| Borehole | 77 | 5.0 | 0–111 | 0.0 | 0–0 | 0.0 | 0–21 | 1196.0 | 1–2808 |
| Tap | 196 | 13.0 | 0–465 | 0.0 | 0–0 | 0.0 | 0–10 | 676.0 | 0–2808 |
| Well | 14 | 125.0 | 40–275 | 45.0 | 10–80 | 0.0 | 0–36 | 1493.5 | 744–2790 |
| E. coli | P. aeruginosa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotics (μg) | Treated (N = 82) | Untreated (N = 33) | p | Treated (N = 144) | Untreated (N = 58) | p | ||||
| n | % | n | % | n | % | n | % | |||
| Amoxicillin–clavulanate (20/10 µg) | 45 | 54.9 | 15 | 45.5 | 0.36 | - | - | - | - | - |
| Piperacillin–tazobactam (100/10 µg) | - | - | - | - | - | 14 | 9.7 | 5 | 8.6 | 0.8 |
| Gentamicin (10 µg) | 3 | 3.7 | 0 | 0.0 | 0.26 | 16 | 11.1 | 7 | 12.1 | 0.84 |
| Ciprofloxacin (5 µg) | 14 | 17.1 | 0 | 0.0 | 0.01 | 7 | 4.9 | 2 | 3.4 | 0.66 |
| Aztreonam (30 µg) | 13 | 15.9 | 8 | 24.2 | 0.29 | 75 | 52.1 | 22 | 37.9 | 0.06 |
| Cefuroxime (30 µg) | 75 | 91.5 | 27 | 81.8 | 0.14 | - | - | - | - | - |
| Ertapenem (10 µg) | 5 | 6.1 | 2 | 6.1 | 0.99 | - | - | - | - | - |
| Trimethoprim–sulfamethoxazole (1.25/23.75 µg) | 54 | 65.9 | 18 | 54.5 | 0.25 | - | - | - | - | - |
| Chloramphenicol (30 µg) | 28 | 34.1 | 8 | 24.2 | 0.3 | - | - | - | - | - |
| Ceftriaxone (30 µg) | 28 | 34.1 | 7 | 21.2 | 0.17 | - | - | - | - | - |
| E. coli | P. aeruginosa | |||
|---|---|---|---|---|
| n (N) | % | n (N) | % | |
| Total Isolates | 67 (115) | 58.3 | 9 (202) | 4.5 |
| Sachet | - | - | 2 (45) | 4.4 |
| Bottled | - | - | 0 (3) | 0.0 |
| Tap | 51 (79) | 64.6 | 3 (90) | 3.3 |
| Borehole | 8 (11) | 72.7 | 4 (58) | 6.9 |
| Well | 8 (25) | 32.0 | 0 (6) | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, H.; Zolfo, M.; Williams, A.; Ashubwe-Jalemba, J.; Tweya, H.; Adeapena, W.; Labi, A.-K.; Adomako, L.A.B.; Addico, G.N.D.; Banu, R.A.; et al. Antibiotic-Resistant Bacteria in Drinking Water from the Greater Accra Region, Ghana: A Cross-Sectional Study, December 2021–March 2022. Int. J. Environ. Res. Public Health 2022, 19, 12300. https://doi.org/10.3390/ijerph191912300
Ahmed H, Zolfo M, Williams A, Ashubwe-Jalemba J, Tweya H, Adeapena W, Labi A-K, Adomako LAB, Addico GND, Banu RA, et al. Antibiotic-Resistant Bacteria in Drinking Water from the Greater Accra Region, Ghana: A Cross-Sectional Study, December 2021–March 2022. International Journal of Environmental Research and Public Health. 2022; 19(19):12300. https://doi.org/10.3390/ijerph191912300
Chicago/Turabian StyleAhmed, Hawa, Maria Zolfo, Anita Williams, Jacklyne Ashubwe-Jalemba, Hannock Tweya, Wisdom Adeapena, Appiah-Korang Labi, Lady A. B. Adomako, Gloria N. D. Addico, Regina A. Banu, and et al. 2022. "Antibiotic-Resistant Bacteria in Drinking Water from the Greater Accra Region, Ghana: A Cross-Sectional Study, December 2021–March 2022" International Journal of Environmental Research and Public Health 19, no. 19: 12300. https://doi.org/10.3390/ijerph191912300
APA StyleAhmed, H., Zolfo, M., Williams, A., Ashubwe-Jalemba, J., Tweya, H., Adeapena, W., Labi, A.-K., Adomako, L. A. B., Addico, G. N. D., Banu, R. A., Akrong, M. O., Quarcoo, G., Borbor, S., & Osei-Atweneboana, M. Y. (2022). Antibiotic-Resistant Bacteria in Drinking Water from the Greater Accra Region, Ghana: A Cross-Sectional Study, December 2021–March 2022. International Journal of Environmental Research and Public Health, 19(19), 12300. https://doi.org/10.3390/ijerph191912300











