Assessment of Dengue and Chikungunya Infections among Febrile Patients Visiting Four Healthcare Centres in Yaoundé and Dizangué, Cameroon
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
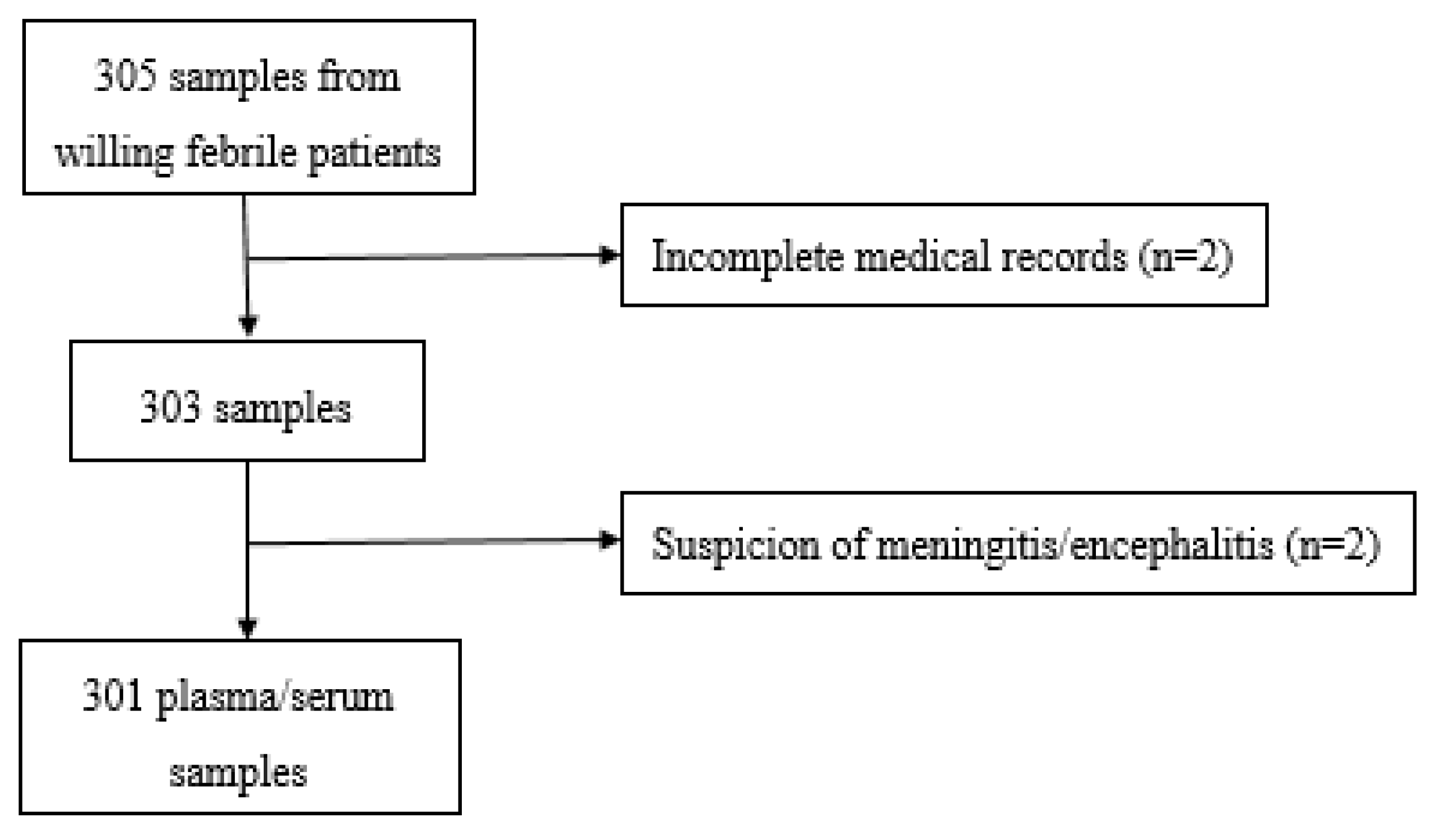
2.2. Study Design and Participants
2.3. Data Collection
2.3.1. Sample Processing
2.3.2. Laboratory Analyses
2.4. Case Definition
2.5. Statistical Analysis
3. Results
3.1. Socio-Demographic Characteristics of the Study Population
3.2. Clinical Features Associated with Acute Dengue and Chikungunya Infections
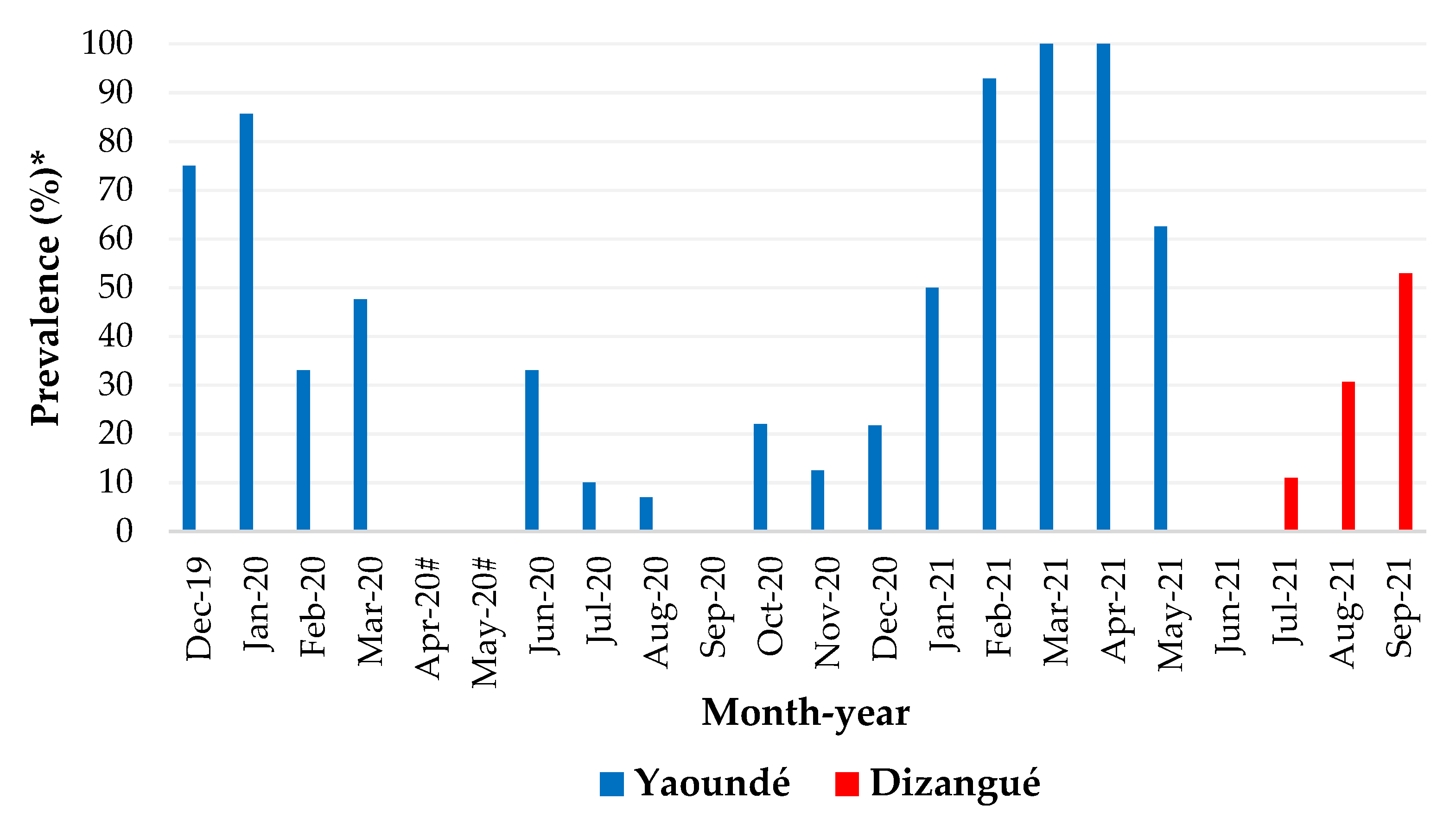
3.3. Monthly Distribution of Dengue Cases
3.4. DENV Serotypes and Co-Infections
3.5. RDTs, ELISA, and rtRT-PCR Testing Results
3.6. Sensitivity and Specificity of RDTs Compared to ELISA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Brady, O. Mapping the Emerging Burden of Dengue. eLife 2019, 8, e47458. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martínez, E.; et al. Dengue: A Continuing Global Threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Managing Epidemics: Key Facts about Major Deadly Diseases; World Health Organization: Geneva, Switzerland, 2018; ISBN 978-92-4-156553-0. [Google Scholar]
- WHO/TDR. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2009; ISBN 978-92-4-154787-1. [Google Scholar]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The Global Distribution of the Arbovirus Vectors Aedes Aegypti and Ae. Albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Demanou, M.; Antonio-Nkondjio, C.; Ngapana, E.; Rousset, D.; Paupy, C.; Manuguerra, J.-C.; Zeller, H. Chikungunya Outbreak in a Rural Area of Western Cameroon in 2006: A Retrospective Serological and Entomological Survey. BMC Res. Notes 2010, 3, 128. [Google Scholar] [CrossRef]
- Fokam, E.B.; Levai, L.D.; Guzman, H.; Amelia, P.A.; Titanji, V.P.K.; Tesh, R.B.; Weaver, S.C. Silent Circulation of Arboviruses in Cameroon. East Afr. Med. J. 2010, 87, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Ndip, L.M.; Bouyer, D.H.; Travassos Da Rosa, A.P.A.; Titanji, V.P.K.; Tesh, R.B.; Walker, D.H. Acute Spotted Fever Rickettsiosis among Febrile Patients, Cameroon. Emerg. Infect Dis. 2004, 10, 432–437. [Google Scholar] [CrossRef]
- Tchuandom, S.B.; Tchouangueu, T.F.; Antonio-Nkondjio, C.; Lissom, A.; Djang, J.O.N.; Atabonkeng, E.P.; Kechia, A.; Nchinda, G.; Kuiate, J.-R. Seroprevalence of Dengue Virus among Children Presenting with Febrile Illness in Some Public Health Facilities in Cameroon. Pan Afr. Med. J. 2018, 31, 177. [Google Scholar] [CrossRef]
- Demanou, M.; Alain, S.-M.S.; Christophe, V.; Rene, N.; Irene, K.T.; Marthe, I.N.; Richard, N. Molecular Characterization of Chikungunya Virus from Three Regions of Cameroon. Virol. Sin. 2015, 30, 470–473. [Google Scholar] [CrossRef]
- Nemg Simo, F.B.; Sado Yousseu, F.B.; Evouna Mbarga, A.; Bigna, J.J.; Melong, A.; Ntoude, A.; Kamgang, B.; Bouyne, R.; Moundipa Fewou, P.; Demanou, M. Investigation of an Outbreak of Dengue Virus Serotype 1 in a Rural Area of Kribi, South Cameroon: A Cross-Sectional Study. Intervirology 2018, 61, 265–271. [Google Scholar] [CrossRef]
- Peyrefitte, C.N.; Rousset, D.; Pastorino, B.A.M.; Pouillot, R.; Bessaud, M.; Tock, F.; Mansaray, H.; Merle, O.L.; Pascual, A.M.; Paupy, C.; et al. Chikungunya Virus, Cameroon, 2006. Emerg. Infect. Dis. 2007, 13, 768. [Google Scholar] [CrossRef]
- Yousseu, F.B.S.; Nemg, F.B.S.; Ngouanet, S.A.; Mekanda, F.M.O.; Demanou, M. Detection and Serotyping of Dengue Viruses in Febrile Patients Consulting at the New-Bell District Hospital in Douala, Cameroon. PLoS ONE 2018, 13, e0204143. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Lecuit, M. Chikungunya Virus and the Global Spread of a Mosquito-Borne Disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.; Sammon, M.; Garg, M. Dengue, Zika and Chikungunya: Emerging Arboviruses in the New World. West. J. Emerg. Med. Integr. Emerg. Care Popul. Health 2016, 17, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Yap, G.; Pok, K.-Y.; Lai, Y.-L.; Hapuarachchi, H.-C.; Chow, A.; Leo, Y.-S.; Tan, L.-K.; Ng, L.-C. Evaluation of Chikungunya Diagnostic Assays: Differences in Sensitivity of Serology Assays in Two Independent Outbreaks. PLoS Negl. Trop. Dis. 2010, 4, e753. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Fonville, J.M.; Gromowski, G.D.; Arriaga, J.B.; Green, A.; James, S.L.; Lau, L.; Montoya, M.; Wang, C.; VanBlargan, L.A.; et al. Dengue Viruses Cluster Antigenically but Not as Discrete Serotypes. Science 2015, 349, 1338–1343. [Google Scholar] [CrossRef]
- Nunes, P.C.; de Filippis, A.M.; Lima, M.Q.; Faria, N.R.; de Bruycker-Nogueira, F.; Santos, J.B.; Heringer, M.; Chouin-Carneiro, T.; Couto-Lima, D.; de Santis Gonçalves, B.; et al. 30 Years of Dengue Fatal Cases in Brazil: A Laboratorial-Based Investigation of 1047 Cases. BMC Infect Dis. 2018, 18, 346. [Google Scholar] [CrossRef]
- Chelluboina, S.; Robin, S.; Aswathyraj, S.; Arunkumar, G. Persistence of Antibody Response in Chikungunya. Virusdisease 2019, 30, 469–473. [Google Scholar] [CrossRef]
- Lima, M.d.R.Q.; de Lima, R.C.; de Azeredo, E.L.; dos Santos, F.B. Analysis of a Routinely Used Commercial Anti-Chikungunya IgM ELISA Reveals Cross-Reactivities with Dengue in Brazil: A New Challenge for Differential Diagnosis? Diagnostics 2021, 11, 819. [Google Scholar] [CrossRef]
- Natrajan, M.S.; Rojas, A.; Waggoner, J.J. Beyond Fever and Pain: Diagnostic Methods for Chikungunya Virus. J. Clin. Microbiol. 2019, 57, e00350-19. [Google Scholar] [CrossRef]
- Vignuzzi, M.; Higgs, S. The Bridges and Blockades to Evolutionary Convergence on the Road to Predicting Chikungunya Virus Evolution. Annu. Rev. Virol. 2017, 4, 181–200. [Google Scholar] [CrossRef]
- Weaver, S.C.; Forrester, N.L. Chikungunya: Evolutionary History and Recent Epidemic Spread. Antivir. Res. 2015, 120, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.W.; Russell, B.J.; Goodman, C.H. Laboratory Diagnosis of Chikungunya Virus Infections and Commercial Sources for Diagnostic Assays. J. Infect. Dis. 2016, 214, S471–S474. [Google Scholar] [CrossRef] [PubMed]
- Murtagh, M.M. Dengue Virus Infection Diagnostics Landscape. 2017. Available online: https://www.semanticscholar.org/paper/Dengue-Virus-Infection-Diagnostics-Landscape-Murtagh/0b096caea0a5a1d4291f7850de9d20d7a1ac7d50 (accessed on 16 September 2022).
- Najioullah, F.; Viron, F.; Paturel, L.; Césaire, R. Diagnostic Biologique de La Dengue. Virologie 2012, 16, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Tool for Diagnosis and Care of Patients with Suspected Arboviral Disease; Pan American Health Organization: Washington, DC, USA, 2017. [Google Scholar]
- Prat, C.M.; Flusin, O.; Panella, A.J.; Tenebray, B.; Lanciotti, R.; Leparc-Goffart, I. Evaluation of Commercially Available Serologic Diagnostic Tests for Chikungunya Virus. Emerg. Infect. Dis. 2014, 20, 2129. [Google Scholar] [CrossRef] [PubMed]
- Galani, B.R.T.; Mapouokam, D.W.; Simo, F.B.N.; Mohamadou, H.; Chuisseu, P.D.D.; Njintang, N.Y.; Moundipa, P.F. Investigation of Dengue-Malaria Coinfection among Febrile Patients Consulting at Ngaoundere Regional Hospital, Cameroon. J. Med. Virol. 2021, 93, 3350–3361. [Google Scholar] [CrossRef] [PubMed]
- Monamele, G.C.; Demanou, M. First Documented Evidence of Dengue and Malaria Co-Infection in Children Attending Two Health Centers in Yaoundé, Cameroon. Pan Afr. Med. J. 2018, 29, 227. [Google Scholar] [CrossRef]
- Simo Tchetgna, H.; Sado Yousseu, F.; Kamgang, B.; Tedjou, A.; McCall, P.J.; Wondji, C.S. Concurrent Circulation of Dengue Serotype 1, 2 and 3 among Acute Febrile Patients in Cameroon. PLoS Negl. Trop. Dis. 2021, 15, e0009860. [Google Scholar] [CrossRef] [PubMed]
- Demanou, M.; Pouillot, R.; Grandadam, M.; Boisier, P.; Kamgang, B.; Hervé, J.P.; Rogier, C.; Rousset, D.; Paupy, C. Evidence of Dengue Virus Transmission and Factors Associated with the Presence of Anti-Dengue Virus Antibodies in Humans in Three Major Towns in Cameroon. PLOS Negl. Trop. Dis. 2014, 8, e2950. [Google Scholar] [CrossRef]
- Kuniholm, M.H.; Wolfe, N.D.; Huang, C.Y.-H.; Mpoudi-Ngole, E.; Tamoufe, U.; Burke, D.S.; Gubler, D.J. Seroprevalence and Distribution of Flaviviridae, Togaviridae, and Bunyaviridae Arboviral Infections in Rural Cameroonian Adults. Am. J. Trop. Med. Hyg. 2006, 74, 1078–1083. [Google Scholar] [CrossRef]
- Nana-Ndjangwo, S.M.; Mony, R.; Bamou, R.; Ango’o, G.B.; Tchangou, D.P.W.; Awono-Ambene, P.; Bilong Bilong, C.F.; Antonio-Nkondjio, C. Knowledge of Healthcare Workers Regarding Dengue and Chikungunya in Some Health Facilities of the City of Yaoundé (Cameroon) and Its Neighbourhood. Open J. Clin. Diagn. 2021, 11, 77–91. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Panella, A.J.; Velez, J.O.; Lambert, A.J.; Campbell, G.L. Chikungunya Virus in US Travelers Returning from India, 2006. Emerg. Infect. Dis. 2007, 13, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Santiago, G.A.; Vergne, E.; Quiles, Y.; Cosme, J.; Vazquez, J.; Medina, J.F.; Medina, F.; Colón, C.; Margolis, H.; Muñoz-Jordán, J.L. Analytical and Clinical Performance of the CDC Real Time RT-PCR Assay for Detection and Typing of Dengue Virus. PLoS Negl. Trop. Dis. 2013, 7, e2311. [Google Scholar] [CrossRef] [PubMed]
- Florkowski, C.M. Sensitivity, Specificity, Receiver-Operating Characteristic (ROC) Curves and Likelihood Ratios: Communicating the Performance of Diagnostic Tests. Clin. Biochem. Rev. 2008, 29, S83–S87. [Google Scholar] [PubMed]
- Kamgang, B.; Vazeille, M.; Tedjou, A.N.; Wilson-Bahun, T.A.; Yougang, A.P.; Mousson, L.; Wondji, C.S.; Failloux, A.-B. Risk of Dengue in Central Africa: Vector Competence Studies with Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae) Populations and Dengue 2 Virus. PLoS Negl. Trop. Dis. 2019, 13, e0007985. [Google Scholar] [CrossRef] [PubMed]
- Kamgang, B.; Vazeille, M.; Yougang, A.P.; Tedjou, A.N.; Wilson-Bahun, T.A.; Mousson, L.; Wondji, C.S.; Failloux, A.-B. Potential of Aedes Albopictus and Aedes Aegypti (Diptera: Culicidae) to Transmit Yellow Fever Virus in Urban Areas in Central Africa. Emerg. Microbes Infect. 2019, 8, 1636–1641. [Google Scholar] [CrossRef]
- Djiappi-Tchamen, B.; Nana-Ndjangwo, M.S.; Tchuinkam, T.; Makoudjou, I.; Nchoutpouen, E.; Kopya, E.; Talipouo, A.; Bamou, R.; Mayi, M.P.A.; Awono-Ambene, P.; et al. Aedes Mosquito Distribution along a Transect from Rural to Urban Settings in Yaoundé, Cameroon. Insects 2021, 12, 819. [Google Scholar] [CrossRef]
- Kamgang, B.; Yougang, A.P.; Tchoupo, M.; Riveron, J.M.; Wondji, C. Temporal Distribution and Insecticide Resistance Profile of Two Major Arbovirus Vectors Aedes Aegypti and Aedes Albopictus in Yaoundé, the Capital City of Cameroon. Parasit Vectors 2017, 10, 469. [Google Scholar] [CrossRef]
- Tedjou, A.N.; Kamgang, B.; Yougang, A.P.; Njiokou, F.; Wondji, C.S. Update on the Geographical Distribution and Prevalence of Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae), Two Major Arbovirus Vectors in Cameroon. PLoS Negl. Trop. Dis. 2019, 13, e0007137. [Google Scholar] [CrossRef]
- Bamou, R.; Mayi, M.P.A.; Djiappi-Tchamen, B.; Nana-Ndjangwo, S.M.; Nchoutpouen, E.; Cornel, A.J.; Awono-Ambene, P.; Parola, P.; Tchuinkam, T.; Antonio-Nkondjio, C. An Update on the Mosquito Fauna and Mosquito-Borne Diseases Distribution in Cameroon. Parasit Vectors 2021, 14, 527. [Google Scholar] [CrossRef]
- Honório, N.A.; Câmara, D.C.P.; Calvet, G.A.; Brasil, P. Chikungunya: An Arbovirus Infection in the Process of Establishment and Expansion in Brazil. Cad Saude Publica 2015, 31, 906–908. [Google Scholar] [CrossRef]
- Pialoux, G.; Gaüzère, B.-A.; Jauréguiberry, S.; Strobel, M. Chikungunya, an Epidemic Arbovirosis. Lancet Infect. Dis. 2007, 7, 319–327. [Google Scholar] [CrossRef]
- Abroug, N.; Khairallah, M.; Zina, S.; Ksiaa, I.; Amor, H.B.; Attia, S.; Jelliti, B.; Khochtali, S.; Khairallah, M. Ocular Manifestations of Emerging Arthropod-Borne Infectious Diseases. J. Curr. Ophthalmol. 2021, 33, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Merle, H.; Donnio, A.; Jean-Charles, A.; Guyomarch, J.; Hage, R.; Najioullah, F.; Césaire, R.; Cabié, A. Ocular Manifestations of Emerging Arboviruses: Dengue Fever, Chikungunya, Zika Virus, West Nile Virus, and Yellow Fever. J. Français D’ophtalmologie 2018, 41, e235–e243. [Google Scholar] [CrossRef] [PubMed]
- Mercier, A.; Obadia, T.; Carraretto, D.; Velo, E.; Gabiane, G.; Bino, S.; Vazeille, M.; Gasperi, G.; Dauga, C.; Malacrida, A.R.; et al. Impact of Temperature on Dengue and Chikungunya Transmission by the Mosquito Aedes Albopictus. Sci. Rep. 2022, 12, 6973. [Google Scholar] [CrossRef] [PubMed]
- Colón-González, F.J.; Fezzi, C.; Lake, I.R.; Hunter, P.R. The Effects of Weather and Climate Change on Dengue. PLoS Negl. Trop. Dis. 2013, 7, e2503. [Google Scholar] [CrossRef]
- Abe, H.; Ushijima, Y.; Loembe, M.M.; Bikangui, R.; Nguema-Ondo, G.; Mpingabo, P.I.; Zadeh, V.R.; Pemba, C.M.; Kurosaki, Y.; Igasaki, Y.; et al. Re-Emergence of Dengue Virus Serotype 3 Infections in Gabon in 2016–2017, and Evidence for the Risk of Repeated Dengue Virus Infections. Int. J. Infect. Dis. 2020, 91, 129–136. [Google Scholar] [CrossRef]
- Proesmans, S.; Katshongo, F.; Milambu, J.; Fungula, B.; Muhindo Mavoko, H.; Ahuka-Mundeke, S.; Inocêncio da Luz, R.; Van Esbroeck, M.; Ariën, K.K.; Cnops, L.; et al. Dengue and Chikungunya among Outpatients with Acute Undifferentiated Fever in Kinshasa, Democratic Republic of Congo: A Cross-Sectional Study. PLoS Negl. Trop. Dis. 2019, 13, e0007047. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Scott, T.W.; Zou, C.; Pigott, D.M.; Duda, K.A.; Bhatt, S.; Katzelnick, L.; Howes, R.E.; Battle, K.E.; et al. Global Spread of Dengue Virus Types: Mapping the 70 Year History. Trends Microbiol. 2014, 22, 138–146. [Google Scholar] [CrossRef]
- Rao, M.R.K.; Padhy, R.N.; Das, M.K. Episodes of the Epidemiological Factors Correlated with Prevailing Viral Infections with Dengue Virus and Molecular Characterization of Serotype-Specific Dengue Virus Circulation in Eastern India. Infect. Genet. Evol. 2018, 58, 40–49. [Google Scholar] [CrossRef]
- Pan American Health Organization/Centers for Diseases Control and Prevention. Preparedness and Response for Chikungunya Virus Introduction in the Americas; Pan American Health Organization/Centers for Diseases Control and Prevention: Washington, DC, USA, 2011. [Google Scholar]
- Matusali, G.; Colavita, F.; Carletti, F.; Lalle, E.; Bordi, L.; Vairo, F.; Ippolito, G.; Capobianchi, M.R.; Castilletti, C. Performance of Rapid Tests in the Management of Dengue Fever Imported Cases in Lazio, Italy 2014–2019. Int. J. Infect. Dis. 2020, 99, 193–198. [Google Scholar] [CrossRef]
- Teoh, B.-T.; Sam, S.-S.; Tan, K.-K.; Johari, J.; Abd-Jamil, J.; Hooi, P.-S.; AbuBakar, S. The Use of NS1 Rapid Diagnostic Test and QRT-PCR to Complement IgM ELISA for Improved Dengue Diagnosis from Single Specimen. Sci. Rep. 2016, 6, 27663. [Google Scholar] [CrossRef] [PubMed]
- Rennie, W.; Phetsouvanh, R.; Lupisan, S.; Vanisaveth, V.; Hongvanthong, B.; Phompida, S.; Alday, P.; Fulache, M.; Lumagui, R.; Jorgensen, P.; et al. Minimising Human Error in Malaria Rapid Diagnosis: Clarity of Written Instructions and Health Worker Performance. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Uzochukwu, B.S.C.; Onwujekwe, E.; Ezuma, N.N.; Ezeoke, O.P.; Ajuba, M.O.; Sibeudu, F.T. Improving Rational Treatment of Malaria: Perceptions and Influence of RDTs on Prescribing Behaviour of Health Workers in Southeast Nigeria. PLoS ONE 2011, 6, e14627. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Healthcare Centres | |||
|---|---|---|---|---|
| AD Lucem Obobogo | CMA Mvog-Betsi | Soa District Hospital | St Joseph Health Centre Dizangué | |
| Region | Centre | Centre | Centre | Littoral |
| Health District | Efoulan | Biyem-assi | Soa | Edea |
| GPS coordinates | 3°52′ N; 3°42′ N 11°25′ E; 11°32′ E | 3°52′ 30′ N; 3°47′ 30′ N 11°27′ E; 11°30′30′ E | 4°8′ N; 3°52′ N 11°31′ E; 11°41′ E | 4°10′ N; 3°10′ N 10°30′ E; 9°40′ E |
| Health facility status | Confessional | Public | Public | Confessional |
| Population covered | 252,501 | 268,428 | 69,084 | 32,627 |
| Number of medical staff | 27 | 43 | 65 | 12 |
| Average number of consultation per week | 87 | 93 | 325 | 40 |
| Environment | Urban | Urban | Urban | Rural |
| Climatic seasons | 4 seasons: 2 rainy and 2 dry | 4 seasons: 2 rainy and 2 dry | 4 seasons: 2 rainy and 2 dry | 2 seasons: 1 rainy and 1 dry |
| Characteristics | All Participants (N = 301) | Dengue | Chikungunya | ||||
|---|---|---|---|---|---|---|---|
| n | Recent Infection (%) | OR (95% CI) | p Value * | Recent Infection (%) | OR (95% CI) | p Value * | |
| Gender | |||||||
| Female | 166 | 49 (29.5) | 1.23 (0.74–2.07) | 0.45 | 39 (23.5) | 1 | _ |
| Male | 135 | 46 (34) | 1 | _ | 37 (27.4) | 1.23 (0.70–2.14) | 0.50 |
| Age | |||||||
| <5 | 65 | 17 (26.1) | 1 | _ | 13 (20) | 1 | _ |
| 5–17 | 99 | 35 (35.3) | 0.65 (0.32–1.29) | 0.21 | 31 (31.3) | 0.55 (0.26–1.15) | 0.11 |
| 18−44 | 119 | 34 (28.6) | 0.88 (0.44–1.75) | 0.72 | 26 (21.8) | 0.89 (0.41–1.88) | 0.77 |
| ≥45 | 18 | 9 (50) | 0.36 (0.12–1.08) | 0.05 | 7 (38.9) | 0.39 (0.13–1.28) | 0.10 |
| YF vaccination | 5 | 2 (40) | 1.31 (0.20–6.90) | 0.71 | 3 (60) | 1 | _ |
| Not vaccinated YF | 296 | 93 (31.4) | 1 | _ | 73 (24.7) | 4.55 (0.51–55.46) | 0.10 |
| Travelled last month | 34 | 7 (20.6) | 0.53 (0.19–1.31) | 0.17 | 7 (20.6) | 0.74 (0.26–1.86) | 0.67 |
| Didn’t travelled | 267 | 88 (33) | 1 | _ | 69 (25.8) | 1 | _ |
| Profession | |||||||
| Civil servant | 15 | 6 (40) | 1 | _ | 3 (20) | 1 | _ |
| Small business | 55 | 20 (36.4) | 0.84 (0.24–2.79) | 0.76 | 15 (27.3) | 0.69 (0.14–2.61) | 0.57 |
| Student | 125 | 43 (34.4) | 1.28 (0.40–3.85) | 0.66 | 36 (28.8) | 0.64 (0.13–2.20) | 0.47 |
| Retired | 3 | 1 (33.3) | 1.25 (0.08–4.54) | 0.82 | 1 (33.3) | 0.50 (0.03–19.46) | 0.61 |
| Jobless | 103 | 35 (34) | 1.30 (0.40–3.98) | 0.64 | 21 (20.4) | 1.01 (0.20–3.60) | 0.97 |
| Site status | |||||||
| Yaoundé (urban) | 198 | 71 (35.8) | 1.83 (1.07–3.19) | 0.02 | 25 (12.6) | 0.92 (0.53–1.61) | 0.78 |
| Dizangué (rural) | 103 | 24 (23.3) | 1 | _ | 27 (26.2) | 1 | _ |
| Characteristics | Tested (N = 301) | Acute * DENV Infection (n = 110) | Negative (n = 191) | OR (95%CI) | p Value # |
|---|---|---|---|---|---|
| Clinical signs | n ** | n ** (%) | n ** (%) | ||
| Headache | 178 | 70 (39.3) | 108 (60.7) | 0.78 (0.45–1.32) | 0.36 |
| Nausea | 42 | 13 (31) | 29 (69) | 0.95 (0.43–2.06) | 0.91 |
| Vomiting | 75 | 19 (25.3) | 56 (74.7) | 0.54 (0.28–0.99) | 0.05 |
| Abdominal pain | 96 | 40 (41.7) | 56 (58.3) | 1.82 (1.05–3.16) | 0.03 |
| Rash | 4 | 0 (0) | 4 (100) | _ | 0.98 |
| Myalgia | 14 | 3 (21.4) | 11 (78.6) | 0.34 (0.07–1.26) | 0.14 |
| Arthralgia | 31 | 11 (35.5) | 20 (64.5) | 0.76 (0.30–1.82) | 0.55 |
| Retro-orbital pain | 9 | 7 (77.8) | 2 (22.2) | 7.14 (1.55–51.63) | 0.02 |
| Diarrhoea | 37 | 10 (27) | 27 (73) | 0.55 (0.22–1.26) | 0.17 |
| Asthenia | 62 | 16 (26.7) | 46 (73.3) | 0.64 (0.33–1.22) | 0.18 |
| Cough | 12 | 7 (58.3) | 5 (41.7) | 3.44 (0.97–13.87) | 0.06 |
| Laboratory results | |||||
| Malaria | 153 | 49 (32) | 104 (68) | 1.55 (0.97–2.52) | 0.06 |
| Typhoid fever | 13 | 7 (53.8) | 6 (46.2) | 0.88 (0.04–10.16) | 0.92 |
| CRP above 6 mg/L | 5 | 4 (80) | 1 (20) | 8.94 (0.81–308.89) | 0.12 |
| Sites | Tested | Serotypes | DENV Co-Infection | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positives (%) | DENV-1 (%) | DENV-2 (%) | DENV-3 (%) | DENV-4 (%) | 1 + 2 (%) | 2 + 3 (%) | 2 + 4 (%) | 3 + 4 (%) | 2 + 3 + 4 (%) | ||
| Yaoundé | 198 | 90 | 7 | 5 | 36 | 18 | 1 | 2 | 1 | 19 | 1 |
| (45.4) | (3.5) | (2.5) | (18.2) | (9.1) | (0.5) | (1) | (0.5) | (9.6) | (0.5) | ||
| Dizangué | 103 | 20 | 3 | 4 | 3 | 10 | _ | _ | _ | _ | _ |
| (19.4) | (2.9) | (3.9) | (2.9) | (9.7) | |||||||
| Total | 301 | 110 | 10 | 9 | 39 | 28 | 1 | 2 | 1 | 19 | 1 |
| (36.5) | (9.1) | (8.2) | (35.5) | (25.4) | (0.9) | (1.8) | (0.9) | (17.3) | (0.9) | ||
| Sites | ||||
|---|---|---|---|---|
| Diagnostic Tools | Virus | Target | Yaoundé n Positives (%) | Dizangué n Positives (%) |
| RDTs | DENV | NS1 | 0 (0) | 0 (0) |
| DENV | IgG | 31 (15.6) | 22 (21.3) | |
| CHIKV | IgG | 67 (33.8) | 19 (18.4) | |
| DENV | IgM | 15 (7.6) | 4 (3.9) | |
| CHIKV | IgM | 9 (4.5) | 13 (12.6) | |
| ELISA | DENV | IgM | 61 (30.8) | 23 (22.3) |
| CHIKV | IgM | 43 (21.7) | 19 (18.4) | |
| rtRT-PCR | DENV | RNA | 90 (45.4) | 20 (19.4) |
| CHIKV | RNA | 1 (0.5) | 0 (0) | |
| ELISA IgM | RDTs IgM |
|---|---|
| Dengue | |
| Positive: 84 | Positive: 8 |
| Negative: 76 | |
| Negative: 217 | Positive: 11 |
| Negative: 206 | |
| Chikungunya | |
| Positive: 62 | Positive: 8 |
| Negative: 54 | |
| Negative: 239 | Positive: 14 |
| Negative: 225 | |
| Tests | % Agreement with ELISA IgM (Kappa) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|
| Dengue | |||||
| RDTs SD Duo (IgM) | 71 (0.09) | 9.52 (4.20–17.91) | 94.93 (91.11–97.44) | 42.11 (23.26–63.57) | 73.05 (71.53–74.52) |
| Chikungunya | |||||
| RDTs Cortez (IgM) | 77 (0.11) | 12.90 (5.74–23.85) | 94.14 (90.37–96.76) | 36.36 (20.07–56.54) | 80.65 (79.02–82.17) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nana-Ndjangwo, S.M.; Djiappi-Tchamen, B.; Mony, R.; Demanou, M.; Keumezeu-Tsafack, J.; Bamou, R.; Awono-Ambene, P.; Bilong Bilong, C.F.; Antonio-Nkondjio, C. Assessment of Dengue and Chikungunya Infections among Febrile Patients Visiting Four Healthcare Centres in Yaoundé and Dizangué, Cameroon. Viruses 2022, 14, 2127. https://doi.org/10.3390/v14102127
Nana-Ndjangwo SM, Djiappi-Tchamen B, Mony R, Demanou M, Keumezeu-Tsafack J, Bamou R, Awono-Ambene P, Bilong Bilong CF, Antonio-Nkondjio C. Assessment of Dengue and Chikungunya Infections among Febrile Patients Visiting Four Healthcare Centres in Yaoundé and Dizangué, Cameroon. Viruses. 2022; 14(10):2127. https://doi.org/10.3390/v14102127
Chicago/Turabian StyleNana-Ndjangwo, Stella Mariette, Borel Djiappi-Tchamen, Ruth Mony, Maurice Demanou, Joyce Keumezeu-Tsafack, Roland Bamou, Parfait Awono-Ambene, Charles Félix Bilong Bilong, and Christophe Antonio-Nkondjio. 2022. "Assessment of Dengue and Chikungunya Infections among Febrile Patients Visiting Four Healthcare Centres in Yaoundé and Dizangué, Cameroon" Viruses 14, no. 10: 2127. https://doi.org/10.3390/v14102127
APA StyleNana-Ndjangwo, S. M., Djiappi-Tchamen, B., Mony, R., Demanou, M., Keumezeu-Tsafack, J., Bamou, R., Awono-Ambene, P., Bilong Bilong, C. F., & Antonio-Nkondjio, C. (2022). Assessment of Dengue and Chikungunya Infections among Febrile Patients Visiting Four Healthcare Centres in Yaoundé and Dizangué, Cameroon. Viruses, 14(10), 2127. https://doi.org/10.3390/v14102127








