Genotype Selection, and Seed Uniformity and Multiplication to Ensure Common Bean (Phaseolus vulgaris L.) var. Liborino
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Adaptation and Yield Field Trials
2.3. Seed Uniformity and Multiplication
3. Results
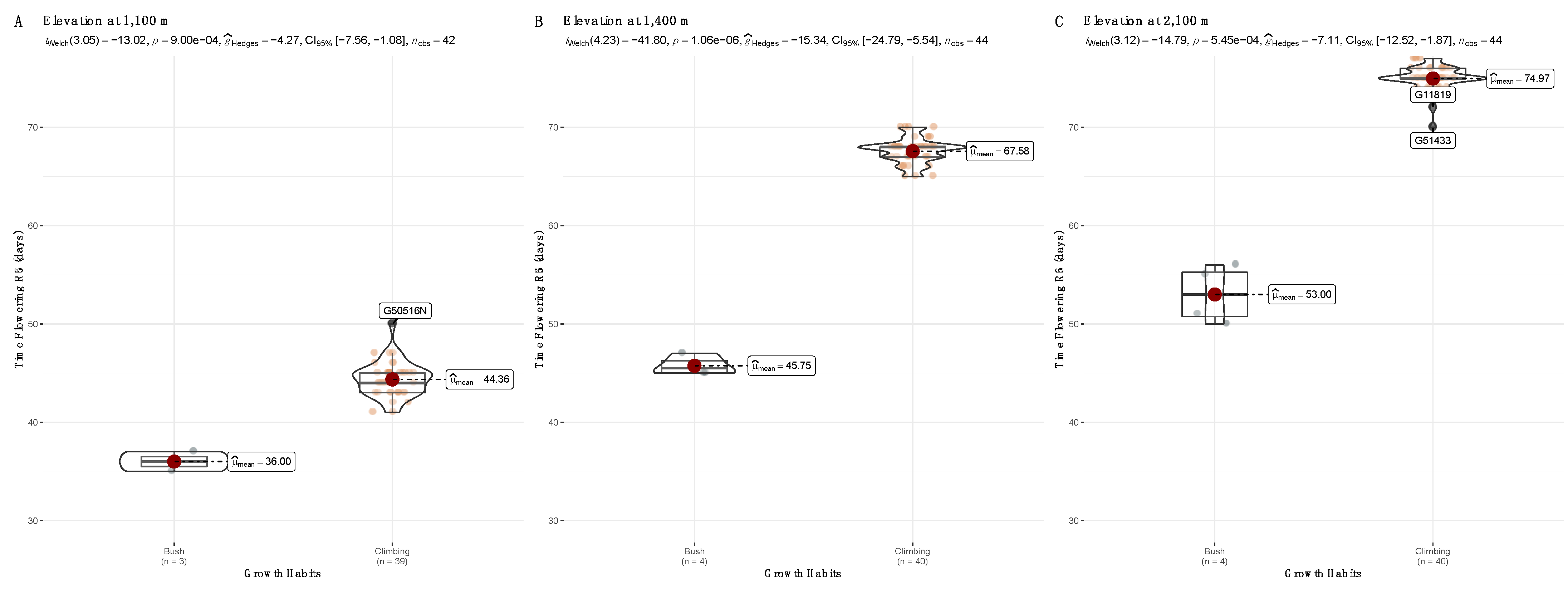
3.1. Adaptation Trials across Three Altitudes for 44 var. Liborino Accessions during the First Season
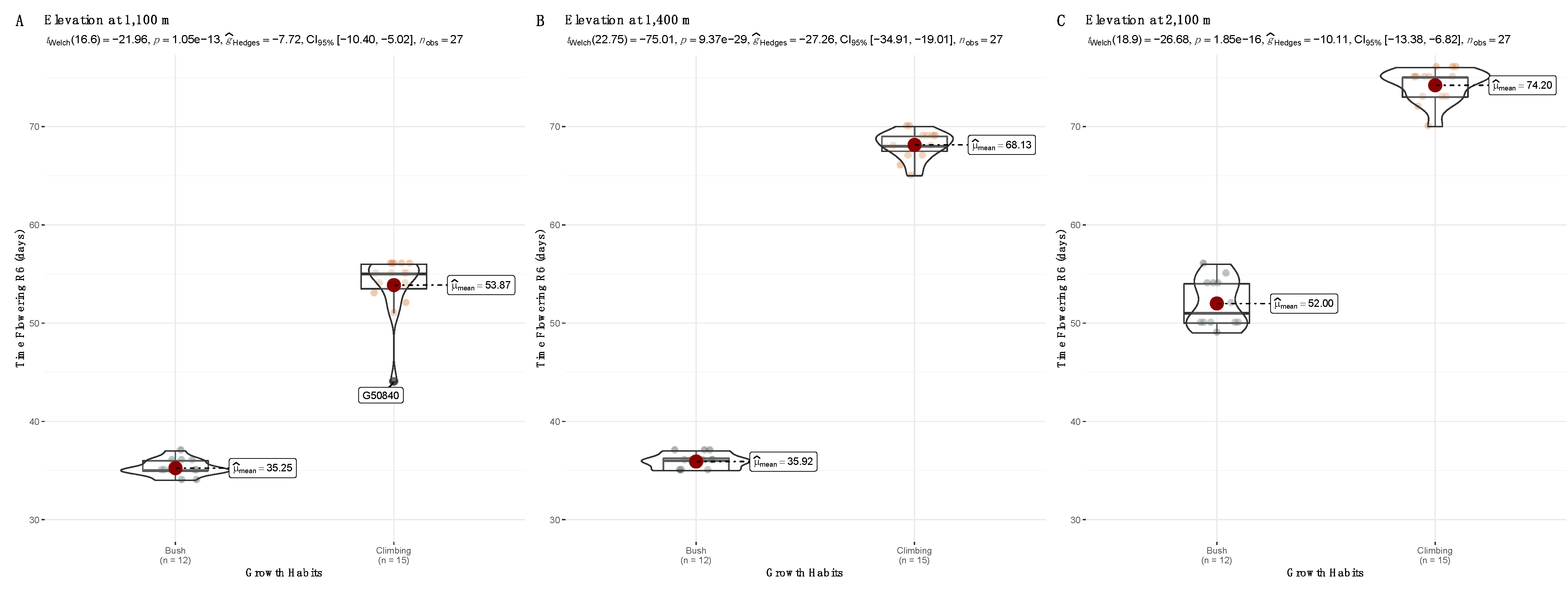
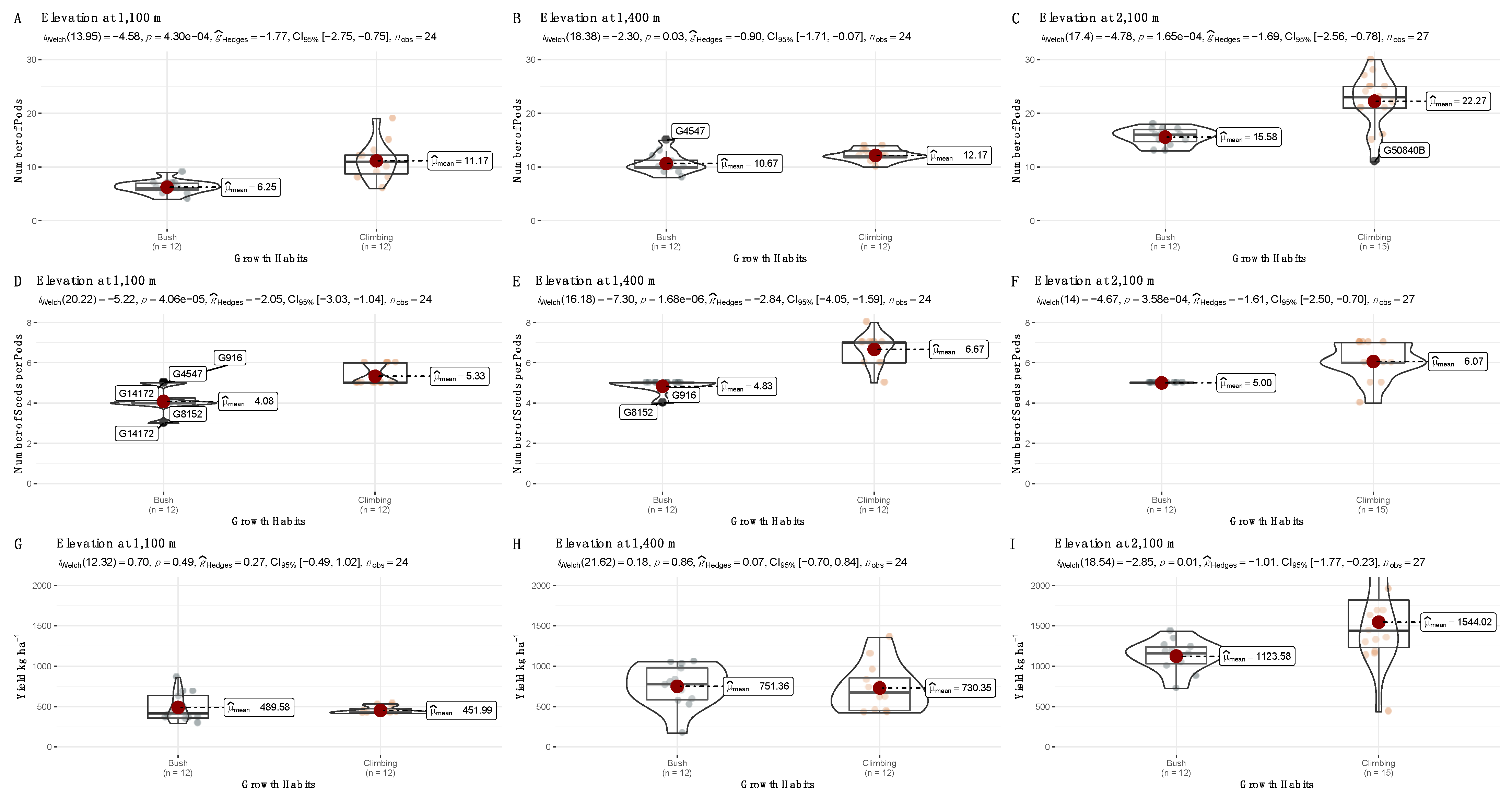
3.2. Yield Trials across Three Altitudes for Nine var. Liborino Accessions during the Second Season
3.3. Participatory Breeding Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welch, R.M.; Graham, R.D. Breeding for micronutrients in staple food crops from a human nutrition perspective. J. Exp. Bot. 2004, 55, 353–364. [Google Scholar] [CrossRef]
- Broughton, W.J.; Hernandez, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)—Model food legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef]
- Pironon, S.; Borrell, J.S.; Ondo, I.; Douglas, R.; Phillips, C.; Khoury, C.K.; Kantar, M.B.; Fumia, N.; Soto Gomez, M.; Viruel, J.; et al. Toward Unifying Global Hotspots of Wild and Domesticated Biodiversity. Plants 2020, 9, 1128. [Google Scholar] [CrossRef]
- Rendon-Anaya, M.; Montero-Vargas, J.M.; Saburido-Alvarez, S.; Vlasova, A.; Capella-Gutierrez, S.; Ordaz-Ortiz, J.J.; Aguilar, O.M.; Vianello-Brondani, R.P.; Santalla, M.; Delaye, L.; et al. Genomic history of the origin and domestication of common bean unveils its closest sister species. Genome Biol. 2017, 18, 60. [Google Scholar]
- Blair, M.W.; Soler, A.; Cortés, A.J. Diversification and Population Structure in Common Beans (Phaseolus vulgaris L.). PLoS ONE 2012, 7, e49488. [Google Scholar] [CrossRef]
- Cortés, A.J. On the Origin of the Common Bean (Phaseolus vulgaris L.). Am. J. Plant Sci. 2013, 4, 1998–2000. [Google Scholar] [CrossRef]
- Kwak, M.; Gepts, P. Structure of genetic diversity in the two major gene pools of common bean (Phaseolus vulgaris L., Fabaceae). Theor. Appl. Genet. 2009, 118, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Bitocchi, E.; Nanni, L.; Bellucci, E.; Rossi, M.; Giardini, A.; Zeuli, P.S.; Logozzo, G.; Stougaard, J.; McClean, P.; Attene, G.; et al. Mesoamerican origin of the common bean (Phaseolus vulgaris L.) is revealed by sequence data. Proc. Natl. Acad. Sci. USA 2012, 109, E788–E796. [Google Scholar] [CrossRef]
- Gepts, P.; Debouck, D. Origin, domestication and evolution of the common bean (Phaseolus vulgaris L.). In Common Beans: Research for Crop Improvement; Van Shoonhoven, A., Voysest, O., Eds.; Wallingford, Commonwealth Agricultural Bureau International: Cali, Colombia, 1991; pp. 7–53. [Google Scholar]
- Schmutz, J.; McClean, P.E.; Mamidi, S.; Wu, G.A.; Cannon, S.B.; Grimwood, J.; Jenkins, J.; Shu, S.; Song, Q.; Chavarro, C.; et al. A reference genome for common bean and genome-wide analysis of dual domestications. Nat. Genet. 2014, 46, 707–713. [Google Scholar] [CrossRef]
- Blair, M.W.; Diaz, J.M.; Hidalgo, R.; Diaz, L.M.; Duque, M.C. Microsatellite characterization of Andean races of common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2007, 116, 29–43. [Google Scholar] [CrossRef]
- Diaz, L.M.; Blair, M.W. Race structure within the Mesoamerican gene pool of common bean (Phaseolus vulgaris L.) as determined by microsatellite markers. Theor. Appl. Genet. 2006, 114, 143–154. [Google Scholar] [CrossRef]
- Sadohara, R.; Izquierdo, P.; Alves, F.C.; Porch, T. The Phaseolus vulgaris L. Yellow Bean Collection: Genetic diversity and characterization for cooking time. Genet. Resour. Crop Evol. 2022, 69, 1627–1648. [Google Scholar] [CrossRef]
- Diaz, A.M.; Caldas, G.V.; Blair, M.W. Concentrations of condensed tannins and anthocyanins in common bean seed coats. Food Res. Int. 2010, 43, 595–601. [Google Scholar] [CrossRef]
- Caldas, G.V.; Blair, M.W. Inheritance of seed condensed tannins and their relationship with seed-coat color and pattern genes in common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2009, 119, 131–142. [Google Scholar] [CrossRef]
- Sadohara, R.; Long, Y.; Izquierdo, P.; Urrea, C.A.; Morris, D.; Cichy, K. Seed coat color genetics and genotype × environment effects in yellow beans via machine-learning and genome-wide association. Plant Genome 2021, 15, e20173. [Google Scholar] [CrossRef]
- Islam, F.M.A.; Rengifo, J.; Redden, R.J.; Basford, K.E.; Beebe, S.E. Association between seed coat polyphenolics (tannins) and disease resistance in common bean. Plant Foods Hum. Nutr. 2003, 58, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.J. Antioqueño Colonization in Western Colombia; University of California Press: Berkeley, CA, USA, 1949. [Google Scholar]
- Myers, J.R.; Formiga, A.K.; Janick, J. Iconography of Beans and Related Legumes Following the Columbian Exchange. Front. Plant Sci. 2022, 13, 851029. [Google Scholar] [CrossRef]
- López-Hernández, F.; Cortés, A.J. Last-Generation Genome–Environment Associations Reveal the Genetic Basis of Heat Tolerance in Common Bean (Phaseolus vulgaris L.). Front. Genet. 2019, 10, 22. [Google Scholar] [CrossRef]
- Diaz, L.M.; Buendia, H.F.; Duque, M.C.; Blair, M.W. Genetic diversity of Colombian landraces of common bean as detected through the use of silver-stained and fluorescently labelled microsatellites. Plant Genet. Resour. Charact. Util. 2011, 9, 86–96. [Google Scholar] [CrossRef]
- Trujillo, H.C.; Contreras, J.L.; Morales, P.A.G.; Gómez, A.B.; Marín, R.G. Desarrollo Económico Local en Ámbitos Territoriales Rurales: El Caso de la Zona Liborina—Sabanalarga, Antioquia, Colombia; Borradores Departamento de Economía: Medellín, Colombia, 2017. [Google Scholar]
- Vásquez, D.L.A.; Balslev, H.; Sklenář, P. Human impact on tropical-alpine plant diversity in the northern Andes. Biodivers. Conserv. 2015, 24, 2673–2683. [Google Scholar] [CrossRef]
- Pérez-Escobar, O.A.; Cámara-Leret, R.; Antonelli, A.; Bateman, R.; Bellot, S.; Chomicki, G.; Cleef, A.; Diazgranados, M.; Dodsworth, S.; Jaramillo, C.; et al. Mining threatens Colombian ecosystems. Science 2018, 359, 1475. [Google Scholar] [CrossRef] [Green Version]
- Guarín-Cifuentes, D.A. Environmental Impact Assessment in Colombia: Review of the Electrical Sector TH Köln Technical; University of Cologne: Cologne, Germany, 2018. [Google Scholar]
- Frischie, S.; Miller, A.L.; Pedrini, S.; Kildisheva, O.A. Ensuring seed quality in ecological restoration: Native seed cleaning and testing. Restor. Ecol. 2020, 28, S239–S248. [Google Scholar] [CrossRef]
- Cortés, A.J.; Blair, M.W. Genotyping by Sequencing and Genome—Environment Associations in Wild Common Bean Predict Widespread Divergent Adaptation to Drought. Front. Plant Sci. 2018, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Cortés, A.J.; Monserrate, F.; Ramírez-Villegas, J.; Madriñán, S.; Blair, M.W. Drought Tolerance in Wild Plant Populations: The Case of Common Beans (Phaseolus vulgaris L.). PLoS ONE 2013, 8, e62898. [Google Scholar] [CrossRef]
- Hussain, A.; Govaerts, B.; Negra, C.; Camacho Villa, T.C.; Chavez Suarez, X.; Espinosa, A.D.; Fonteyne, S.; Gardeazabal, A.; Gonzalez, G.; Gopal Singh, R.; et al. One CGIAR and the Integrated Agri-food Systems Initiative: From short-termism to transformation of the world’s food systems. PLoS ONE 2021, 16, e0252832. [Google Scholar] [CrossRef]
- McCouch, S. Diversifying Selection in Plant Breeding. PLoS Biol. 2004, 2, 1507–1512. [Google Scholar] [CrossRef]
- Cortés, A.J.; López-Hernández, F. Harnessing Crop Wild Diversity for Climate Change Adaptation. Genes 2021, 12, 783. [Google Scholar] [CrossRef]
- Cortés, A.J.; Restrepo-Montoya, M.; Bedoya-Canas, L.E. Modern Strategies to Assess and Breed Forest Tree Adaptation to Changing Climate. Front. Plant Sci. 2020, 11, 583323. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Palacios-Rojas, N.; Hossain, F.; Muthusamy, V.; Menkir, A.; Dhliwayo, T.; Ndhlela, T.; San Vicente, F.; Nair, S.K.; Vivek, B.S.; et al. Molecular Breeding for Nutritionally Enriched Maize: Status and Prospects. Front. Genet. 2020, 10, 1392. [Google Scholar] [CrossRef]
- Palacios-Rojas, N.; McCulley, L.; Kaeppler, M.; Titcomb, T.J.; Gunaratna, N.S.; Lopez-Ridaura, S.; Tanumihardjo, S.A. Mining maize diversity and improving its nutritional aspects within agro-food systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1809–1834. [Google Scholar] [CrossRef]
- Smale, M.; Jamora, N. Valuing genebanks. Food Secur. 2020, 12, 905–918. [Google Scholar]
- Mallor, C.; Barberán, M.; Aibar, J. Recovery of a Common Bean Landrace (Phaseolus vulgaris L.) for Commercial Purposes. Front. Plant Sci. 2018, 9, 1440. [Google Scholar] [CrossRef]
- Keken, Z.; Hanušová, T.; Kulendík, J.; Wimmerová, L.; Zítková, J.; Zdražil, V. Environmental impact assessment—The range of activities covered and the potential of linking to post-project auditing. Environ. Impact Assess. Rev. 2022, 93, 106726. [Google Scholar] [CrossRef]
- Baptiste, B.; Pinedo-Vasquez, M.; Gutierrez-Velez, V.H.; Andrade, G.I.; Vieira, P.; Estupiñán-Suárez, L.M.; Londoño, M.C.; Laurance, W.; Lee, T.M. Greening peace in Colombia. Nat. Ecol. Evol. 2017, 1, 102. [Google Scholar] [CrossRef] [PubMed]
- McCouch, S.; Navabi, K.; Abberton, M.; Anglin, N.L.; Barbieri, R.L.; Baum, M.; Bett, K.; Booker, H.; Brown, G.L.; Bryan, G.J.; et al. Mobilizing Crop Biodiversity. Mol. Plant 2020, 13, 1341–1344. [Google Scholar] [PubMed]
- Crescenzi, R.; De Filippis, F.; Giua, M.; Vaquero-Piñeiro, C. Geographical Indications and local development: The strength of territorial embeddedness. Reg. Stud. 2021, 56, 381–393. [Google Scholar] [CrossRef]
- McCouch, S. Feeding the future. Nature 2013, 499, 23–24. [Google Scholar] [CrossRef]
- Martini, J.W.R.; Molnar, T.L.; Crossa, J.; Hearne, S.J.; Pixley, K.V. Opportunities and Challenges of Predictive Approaches for Harnessing the Potential of Genetic Resources. Front. Plant Sci. 2021, 12, 674036. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S. Decentralized-participatory plant breeding: An example of demand driven research. Euphytica 2006, 155, 349–360. [Google Scholar] [CrossRef]
- Acevedo, M.; Pixley, K.; Zinyengere, N.; Meng, S.; Tufan, H.; Cichy, K.; Bizikova, L.; Isaacs, K.; Ghezzi-Kopel, K.; Porciello, J. A scoping review of adoption of climate-resilient crops by small-scale producers in low- and middle-income countries. Nat. Plants 2020, 6, 1231–1241. [Google Scholar] [CrossRef]
- Wilkus, E.L.; Berny Mier y Teran, J.C.; Mukankusi, C.M.; Gepts, P. Genetic Patterns of Common-Bean Seed Acquisition and Early-Stage Adoption Among Farmer Groups in Western Uganda. Front. Plant Sci. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Letaa, E.; Katungi, E.; Kabungo, C.; Ndunguru, A.A. Impact of improved common bean varieties on household food security on adopters in Tanzania. J. Dev. Effect. 2020, 12, 89–108. [Google Scholar] [CrossRef]
- Huddart, J.E.A.; Crawford, A.J.; Luna-Tapia, A.L.; Restrepo, S.; Di Palma, F. EBP-Colombia and the bioeconomy: Genomics in the service of biodiversity conservation and sustainable development. Proc. Natl. Acad. Sci. USA 2022, 119, e2115641119. [Google Scholar] [CrossRef] [PubMed]
- Cortés, A.J.; Cornille, A.; Yockteng, R. Evolutionary Genetics of Crop-Wild Complexes. Genes 2022, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.W.; Cortés, A.J.; Penmetsa, R.V.; Farmer, A.; Carrasquilla-Garcia, N.; Cook, D.R. A high-throughput SNP marker system for parental polymorphism screening, and diversity analysis in common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2013, 126, 535–548. [Google Scholar] [CrossRef]
- Buitrago-Bitar, M.A.; Cortés, A.J.; López-Hernández, F.; Londoño-Caicedo, J.M.; Muñoz-Florez, J.E.; Muñoz, L.C.; Blair, M.W. Allelic Diversity at Abiotic Stress Responsive Genes in Relationship to Ecological Drought Indices for Cultivated Tepary Bean, Phaseolus acutifolius A. Gray, and Its Wild Relatives. Genes 2021, 12, 556. [Google Scholar] [CrossRef]
- Burbano-Erazo, E.; León-Pacheco, R.; Cordero-Cordero, C.; López-Hernández, F.; Cortés, A.; Tofiño-Rivera, A. Multi-Environment Yield Components in Advanced Common Bean (Phaseolus vulgaris L.) × Tepary Bean (P. acutifolius A. Gray) Interspecific Lines for Heat and Drought Tolerance. Agronomy 2021, 11, 1978. [Google Scholar] [CrossRef]
- Cortés, A.J.; Skeen, P.; Blair, M.W.; Chacón-Sánchez, M.I. Does the genomic landscape of species divergence in Phaseolus beans coerce parallel signatures of adaptation and domestication? Front. Plant. Sci. 2018, 9, 1816. [Google Scholar] [CrossRef]
- Cortés, A.J.; López-Hernández, F.; Blair, M.W. Genome–Environment Associations, an Innovative Tool for Studying Heritable Evolutionary Adaptation in Orphan Crops and Wild Relatives. Front. Genet. 2022, 13, 910386. [Google Scholar] [CrossRef]
- Blair, M.W.; Cortés, A.J.; This, D. Identification of an ERECTA gene and its drought adaptation associations with wild and cultivated common bean. Plant Sci. 2016, 242, 250–259. [Google Scholar] [CrossRef]
- Cortés, A.J.; López-Hernández, F.; Osorio-Rodriguez, D. Predicting thermal adaptation by looking into populations’ genomic past. Front. Genet. 2020, 11, 564515. [Google Scholar] [CrossRef]
- Ulian, T.; Diazgranados, M.; Pironon, S.; Padulosi, S.; Liu, U.; Davies, L.; Howes, M.J.R.; Borrell, J.S.; Ondo, I.; Pérez-Escobar, O.A.; et al. Unlocking plant resources to support food security and promote sustainable agriculture. Plants People Planet 2020, 2, 421–445. [Google Scholar] [CrossRef]

| Accession | Province | Municipality | Growth Habit | Seed Color Tonality (s.l.) | Seed Shape | Seed Brightness | Altitude (m a.s.l.) |
|---|---|---|---|---|---|---|---|
| G11819 | Antioquia | NA | Climbing | Yellow, Red | Rounded | Intermediate | NA |
| G12671 | Antioquia | Medellin | Climbing | Cream, Black | Rounded | Intermediate | NA |
| G12702 | Nariño | Pasto | Climbing | Yellow, Red | Rounded | Intermediate | 2700 |
| G12706 | Nariño | Pasto | Climbing | Yellow, Brown | Rounded | Intermediate | 2800 |
| G12712 | Nariño | Pasto | Climbing | Yellow, Brown | Elongated | Intermediate | 2600 |
| G50516A | Antioquia | Liborina | Climbing | Red | Rounded | Intermediate | 1930 |
| G50516B | Antioquia | Liborina | Climbing | Cream, Red | Rounded | Intermediate | 1930 |
| G50516C | Antioquia | Liborina | Climbing | Red, Cream, Black | Rounded | Intermediate | 1930 |
| G50516D | Antioquia | Liborina | Climbing | Red, Yellow | Rounded | Intermediate | 1930 |
| G50516E | Antioquia | Liborina | Climbing | Yellow, Red | Rounded | Intermediate | 1930 |
| G50516F | Antioquia | Liborina | Climbing | Red | Elongated | Intermediate | 1930 |
| G50516G | Antioquia | Liborina | Climbing | Yellow, Pink | Rounded | Intermediate | 1930 |
| G50516H | Antioquia | Liborina | Climbing | Cream, Other, Black | Rounded | Bright | 1930 |
| G50516I | Antioquia | Liborina | Climbing | Cream, Brown | Rounded | Intermediate | 1930 |
| G50516J | Antioquia | Liborina | Climbing | Black | Rounded | Intermediate | 1930 |
| G50516K | Antioquia | Liborina | Climbing | Brown | Rounded | Bright | 1930 |
| G50516L | Antioquia | Liborina | Climbing | Cream, Brown, Other | Rounded | Intermediate | 1930 |
| G50516N | Antioquia | Liborina | Climbing | Cream, Brown, Black | Rounded | Intermediate | 1930 |
| G50516O | Antioquia | Liborina | Climbing | Pink, Cream, Other | Rounded | Intermediate | 1930 |
| G50516P | Antioquia | Liborina | Climbing | Yellow | Rounded | Intermediate | 1930 |
| G50516Q | Antioquia | Liborina | Climbing | Other, Black | Rounded | Bright | 1930 |
| G50516S | Antioquia | Liborina | Climbing | Pink, Brown | Rounded | Intermediate | 1930 |
| G50516T | Antioquia | Liborina | Climbing | Brown | Rounded | Bright | 1930 |
| G50516U | Antioquia | Liborina | Climbing | Brown | Elongated | Intermediate | 1930 |
| G50834 | Antioquia | Santa Fe de Ant. | Climbing | Yellow, Red | Rounded | Intermediate | 1600 |
| G50840 | Antioquia | Santa Fe de Ant. | Climbing | Yellow, Red | Rounded | Intermediate | 2060 |
| G50840A | Antioquia | Santa Fe de Ant. | Climbing | Cream, Red | Rounded | Intermediate | 2060 |
| G50840B | Antioquia | Santa Fe de Ant. | Climbing | Yellow, Red | Rounded | Intermediate | 2060 |
| G50997 | Antioquia | Andes | Climbing | Yellow, Red | Elongated | Intermediate | 1500 |
| G50997B | Antioquia | Andes | Climbing | Purple | Rounded | Intermediate | 1500 |
| G51013 | Antioquia | Liborina | Bush | Cream, Red | Rounded | Intermediate | 1930 |
| G51013A | Antioquia | Liborina | Climbing | Yellow, Red | Rounded | Bright | 1930 |
| G51018 | Antioquia | Liborina | Climbing | Yellow, Red | Rounded | Intermediate | 1930 |
| G51285 | Antioquia | Liborina | Climbing | Red, Cream | Rounded | Intermediate | 1930 |
| G51285C | Antioquia | Liborina | Climbing | Brown, Other, Purple | Rounded | Intermediate | 1930 |
| G51285E | Antioquia | Liborina | Climbing | Cream, Red | Elongated | Opaque | 1930 |
| G51285F | Antioquia | Liborina | Climbing | Cream, Other, Black | Rounded | Intermediate | 1930 |
| G51285G | Antioquia | Liborina | Climbing | Cream, Other, Purple | Rounded | Intermediate | 1930 |
| G51285H | Antioquia | Liborina | Climbing | Black | Rounded | Intermediate | 1930 |
| G51433 | Antioquia | Santa Fe de Ant. | Climbing | Yellow, Red | Rounded | Intermediate | 1600 |
| G916 | Antioquia | NA | Bush | Yellow, Brown | Elongated | Intermediate | NA |
| G4547 | Antioquia | Medellin | Bush | Yellow, Red | Elongated | Intermediate | NA |
| G8152 | Antioquia | Medellin | Bush | Yellow, Purple | Elongated | Opaque | NA |
| G14172 | Antioquia | Medellin | Bush | Yellow, Purple | Elongated | Intermediate | NA |
| Accession | Growth Habit | FT at 1100 m | FT at 1400 m | FT at 2100 m |
|---|---|---|---|---|
| G50834 | Climbing | 55.3 ± 0.6 a | 67.3 ± 2.1 a | 74.3 ± 1.2 a |
| G50840 | Climbing | 50.3 ± 6.0 a | 67.7 ± 1.6 a | 72.0 ± 1.7 a |
| G50840B | Climbing | 55.3 ± 1.2 a | 68.7 ± 0.6 a | 74.3 ± 2.1 a |
| G51018 | Climbing | 54.3 ± 1.6 a | 68.3 ± 1.6 a | 5.0 ± 1.0 a |
| G51433 | Climbing | 54.0 ± 1.7 a | 68.7 ± 1.6 a | 75.3 ± 0.6 a |
| G916 | Bush | 34.7 ± 0.6 b | 36.0 ± 1.0 b | 51.3 ± 2.3 b |
| G4547 | Bush | 35.7 ± 1.2 b | 35.7 ± 0.6 b | 50.7 ± 1.2 b |
| G8152 | Bush | 35.0 ± 1.0 b | 36.7 ± 0.6 b | 53.3 ± 3.1 b |
| G14172 | Bush | 35.7 ± 0.6 b | 35.3 ± 0.6 b | 52.7 ± 3.2 b |
| Accession | Growth Habit | Yield at 1100 m | Yield at 1400 m | Yield at 2100 m |
|---|---|---|---|---|
| G51433 | Climbing | 521.0 ± 13.3 a | 846.1 ± 275.7 a | 1710.0 ± 471.2 a |
| G50840 | Climbing | 439.9 ± 24.2 a | 670.7 ± 258.6 a | 1429.8 ± 256.5 a |
| G50834 | Climbing | 428.8 ± 4.2 a | 837.9 ± 473.4 a | 1596.2 ± 1032.9 a |
| G51018 | Climbing | NA | NA | 1720.0 ± 588.4 a |
| G50840B | Climbing | 418.3 ± 3.0 a | 566.8 ± 223.9 a | 1264.1 ± 118.5 a |
| G4547 | Bush | 629.3 ± 262.6 a | 874.3 ± 307.0 a | 1093.4 ± 192.2 a |
| G916 | Bush | 555.2 ± 174.2 a | 726.6 ± 257.6 a | 1105.3 ± 333.2 a |
| G8152 | Bush | 411.7 ± 57.7 a | 852.3 ± 103.5 a | 1169.4 ± 228.4 a |
| G14172 | Bush | 362.1 ± 74.9 a | 552.3 ± 337.1 a | 1126.2 ± 192.2 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peláez, D.; Aguilar, P.A.; Mercado, M.; López-Hernández, F.; Guzmán, M.; Burbano-Erazo, E.; Denning-James, K.; Medina, C.I.; Blair, M.W.; De Vega, J.J.; et al. Genotype Selection, and Seed Uniformity and Multiplication to Ensure Common Bean (Phaseolus vulgaris L.) var. Liborino. Agronomy 2022, 12, 2285. https://doi.org/10.3390/agronomy12102285
Peláez D, Aguilar PA, Mercado M, López-Hernández F, Guzmán M, Burbano-Erazo E, Denning-James K, Medina CI, Blair MW, De Vega JJ, et al. Genotype Selection, and Seed Uniformity and Multiplication to Ensure Common Bean (Phaseolus vulgaris L.) var. Liborino. Agronomy. 2022; 12(10):2285. https://doi.org/10.3390/agronomy12102285
Chicago/Turabian StylePeláez, Diana, Paula A. Aguilar, Mariana Mercado, Felipe López-Hernández, Manuel Guzmán, Esteban Burbano-Erazo, Kate Denning-James, Clara I. Medina, Matthew W. Blair, José J. De Vega, and et al. 2022. "Genotype Selection, and Seed Uniformity and Multiplication to Ensure Common Bean (Phaseolus vulgaris L.) var. Liborino" Agronomy 12, no. 10: 2285. https://doi.org/10.3390/agronomy12102285








