Cancer Chemoprevention by Phytochemicals: Nature’s Healing Touch
Abstract
:1. Introduction
2. Preventive and Therapeutic Mechanisms of Phytochemicals
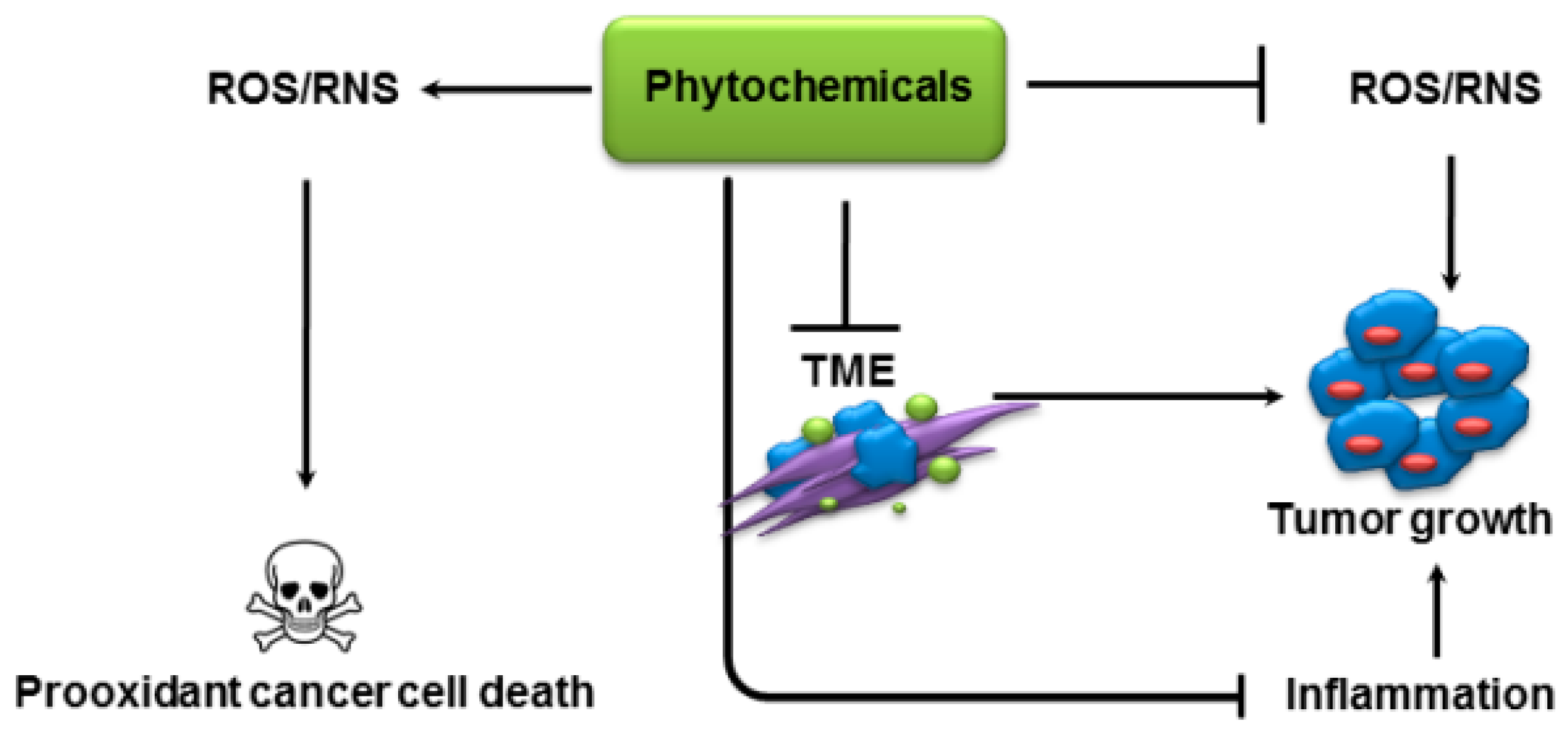
2.1. Modulation of Oxidative Stress
2.2. Inhibition of Inflammation
2.3. Prooxidant Activity
2.4. Modulation of Tumor Metabolism
3. Modulation of Tumor Microenvironment
4. Challenges for Phytochemicals in Cancer Therapy and Emerging Alternatives
4.1. Synthesis of Chemical Analogs
4.2. Novel Formulations
5. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 4-NQO | 4-nitroquinoline-1-oxide |
| 5-FU | 5-FluoroUracil |
| CDF | Curcumin Difluorinated |
| COX2 | CycloOxygenase2 |
| CSC | Cancer Stem Cells |
| CSN5 | COP9 Signalosome 5 |
| CXCR4 | C-X-C chemokine receptor type 4 |
| DEN | DiEthylNitrosamine |
| DMBA | 7,12-Dimethylbenz[a]anthracene |
| EGCG | EpiGalloCatechin Gallate |
| EMT | Epithelial-Mesenchymal Transition |
| FITC | Fluorescein IsoThioCyanate |
| Hh | Hedgehog |
| IL | InterLeukin |
| iNOS | inducible Nitric Oxide Synthase |
| Keap1 | Kelch-like erythroid cell-derived protein with Cap’n’collar homology-associated protein |
| MAPK | Mitogen-Activated Protein Kinases |
| MMP2 | Matrix MetalloProteinase2 |
| MPEG | Mono methoxy Poly Ethylene Glycol |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of activated B cells |
| Nrf2 | NF-E2 p45-related factor 2 |
| NSCLC | Non-Small Cell Lung Cancer |
| PCL | poly (ε-CaproLactone) |
| PD-L1 | Programmed cell Death-Ligand-1 |
| PDLLA-G | Poly(d,l-lactic acid)-glycerol |
| PEG | PolyEthylene Glycol |
| PKC | Protein Kinase C |
| PLA | Poly Lactic Acif |
| PLGA | Poly(Lactic-co-Glycolic Acid) |
| PTEN | Phosphatase and Tensin Homolog |
| RANK | Receptor Activator of Nuclear Factor of κB |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| SMA | Styrene-Maleic Acid copolymer |
| TGF-β | Transforming Growth Factor beta1 |
| TLR | Toll-Like Receptors |
| TME | Tumor MicroEnvironment |
| TNF-α | Tumor Necrosis Factor-Alpha |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| TRAF6 | TNF Receptor Associated Factor 6 |
| UV | UltraViolet |
| VEGF | Vascular Endothelial Growth Factor |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Okimoto, R.A.; Bivona, T.G. Recent advances in personalized lung cancer medicine. Per Med 2014, 11, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Krepler, C.; Xiao, M.; Sproesser, K.; Brafford, P.A.; Shannan, B.; Beqiri, M.; Liu, Q.; Xu, W.; Garman, B.; Nathanson, K.L.; et al. Personalized preclinical trials in braf inhibitor-resistant patient-derived xenograft models identify second-line combination therapies. Clin. Cancer Res. 2016, 22, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.B.; Mohammed, A.; Madka, V.; Kumar, G.; Rao, C.V. Prevention and treatment of cancers by immune modulating nutrients. Mol. Nutr. Food Res. 2016, 60, 1275–1294. [Google Scholar] [CrossRef] [PubMed]
- Chih, H.J.; Lee, A.H.; Colville, L.; Binns, C.W.; Xu, D. A review of dietary prevention of human papillomavirus-related infection of the cervix and cervical intraepithelial neoplasia. Nutr. Cancer 2013, 65, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Terry, P.; Wolk, A. Tea consumption and the risk of colorectal cancer in sweden. Nutr. Cancer 2001, 39, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Van Duyn, M.A.; Pivonka, E. Overview of the health benefits of fruit and vegetable consumption for the dietetics professional: Selected literature. J. Am. Diet. Assoc. 2000, 100, 1511–1521. [Google Scholar] [CrossRef]
- Tseng, M. Diet, cancer and public health nutrition. Public Health Nutr. 2009, 12, 737–738. [Google Scholar] [CrossRef]
- Blokhina, O.; Fagerstedt, K.V. Oxidative metabolism, ros and no under oxygen deprivation. Plant Physiol. Biochem. 2010, 48, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.T.; Smart, R.C.; Wong, C.Q.; Conney, A.H. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-o-tetradecanoylphorbol-13-acetate. Cancer Res. 1988, 48, 5941–5946. [Google Scholar] [PubMed]
- Cho, J.; Rho, O.; Junco, J.; Carbajal, S.; Siegel, D.; Slaga, T.J.; DiGiovanni, J. Effect of combined treatment with ursolic acid and resveratrol on skin tumor promotion by 12-o-tetradecanoylphorbol-13-acetate. Cancer Prev. Res. (Phila) 2015, 8, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, P.M. Protein kinase c as the receptor for the phorbol ester tumor promoters: Sixth rhoads memorial award lecture. Cancer Res. 1988, 48, 1–8. [Google Scholar] [PubMed]
- Shieh, J.M.; Chiang, T.A.; Chang, W.T.; Chao, C.H.; Lee, Y.C.; Huang, G.Y.; Shih, Y.X.; Shih, Y.W. Plumbagin inhibits tpa-induced mmp-2 and u-pa expressions by reducing binding activities of nf-kappab and ap-1 via erk signaling pathway in a549 human lung cancer cells. Mol. Cell Biochem. 2010, 335, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Konoshima, T.; Kozuka, M.; Tokuda, H.; Nishino, H.; Iwashima, A.; Haruna, M.; Ito, K.; Tanabe, M. Studies on inhibitors of skin tumor promotion, ix. Neolignans from magnolia officinalis. J. Nat. Prod. 1991, 54, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Yanaida, Y.; Kohno, H.; Yoshida, K.; Hirose, Y.; Yamada, Y.; Mori, H.; Tanaka, T. Dietary silymarin suppresses 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in male f344 rats. Carcinogenesis 2002, 23, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Nunoshiba, T.; Demple, B. Potent intracellular oxidative stress exerted by the carcinogen 4-nitroquinoline-n-oxide. Cancer Res. 1993, 53, 3250–3252. [Google Scholar] [PubMed]
- Chuang, S.E.; Kuo, M.L.; Hsu, C.H.; Chen, C.R.; Lin, J.K.; Lai, G.M.; Hsieh, C.Y.; Cheng, A.L. Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis. Carcinogenesis 2000, 21, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Komolafe, Y.O.; Bukoye Oloyede, H.O.; Yakubu, M.T.; Adeoye, M.D.; Abdulsalami, I.O.; Oladiji, A.T.; Akanji, M.A. Diethylnitrosamine-induced redox imbalance in rat microsomes: Protective role of polyphenolic-rich extract from sorghum bicolor grains. J. Basic Clin. Physiol. Pharmacol. 2013, 24, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Huang, M.T.; Lou, Y.R.; Xie, J.G.; Reuhl, K.R.; Newmark, H.L.; Ho, C.T.; Yang, C.S.; Conney, A.H. Inhibitory effects of black tea, green tea, decaffeinated black tea, and decaffeinated green tea on ultraviolet b light-induced skin carcinogenesis in 7,12-dimethylbenz[a]anthracene-initiated skh-1 mice. Cancer Res. 1994, 54, 3428–3435. [Google Scholar]
- Wang, Z.Y.; Hong, J.Y.; Huang, M.T.; Reuhl, K.R.; Conney, A.H.; Yang, C.S. Inhibition of n-nitrosodiethylamine- and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced tumorigenesis in a/j mice by green tea and black tea. Cancer Res. 1992, 52, 1943–1947. [Google Scholar] [PubMed]
- Finch, C.E.; Crimmins, E.M. Inflammatory exposure and historical changes in human life-spans. Science 2004, 305, 1736–1739. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Banerjee, S.; Wang, Z.; Kong, D.; Majumdar, A.P.; Sarkar, F.H. Aging and inflammation: Etiological culprits of cancer. Curr. Aging Sci. 2009, 2, 174–186. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [PubMed]
- Ercolini, A.M.; Miller, S.D. The role of infections in autoimmune disease. Clin. Exp. Immunol. 2009, 155, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Michaille, J.J. Promiscuous effects of some phenolic natural products on inflammation at least in part arise from their ability to modulate the expression of global regulators, namely micrornas. Molecules 2016, 21, 1263. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, M.; Schaffer, P.M.; Bar-Sela, G. An update on curcuma as a functional food in the control of cancer and inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Vinayak, M. Long term effect of curcumin in restoration of tumour suppressor p53 and phase-II antioxidant enzymes via activation of Nrf2 signalling and modulation of inflammation in prevention of cancer. PLoS ONE 2015, 10, e0124000. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Maurya, P.; Reddy, S.S.; Singh, V.; Ahmad, H.; Dwivedi, A.K.; Dikshit, M.; Barthwal, M.K. A standardized chemically modified curcuma longa extract modulates irak-mapk signaling in inflammation and potentiates cytotoxicity. Front Pharmacol. 2016, 7, 223. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Farhan Asad, S.; Singh, S.; Hadi, S.M. DNA breakage by resveratrol and cu(II): Reaction mechanism and bacteriophage inactivation. Cancer Lett. 2000, 154, 29–37. [Google Scholar] [CrossRef]
- Ahmad, A.; Syed, F.A.; Singh, S.; Hadi, S.M. Prooxidant activity of resveratrol in the presence of copper ions: Mutagenicity in plasmid DNA. Toxicol. Lett. 2005, 159, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Nakata, R. Resveratrol targets in inflammation. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Latruffe, N.; Lancon, A.; Frazzi, R.; Aires, V.; Delmas, D.; Michaille, J.J.; Djouadi, F.; Bastin, J.; Cherkaoui-Malki, M. Exploring new ways of regulation by resveratrol involving mirnas, with emphasis on inflammation. Ann. N. Y. Acad. Sci. 2015, 1348, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Limagne, E.; Lancon, A.; Delmas, D.; Cherkaoui-Malki, M.; Latruffe, N. Resveratrol interferes with il1-beta-induced pro-inflammatory paracrine interaction between primary chondrocytes and macrophages. Nutrients 2016, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, T.; Selvaraj, M.; Poomalai, S. Epigallocatechin gallate potentially abrogates fluoride induced lung oxidative stress, inflammation via Nrf2/keap1 signaling pathway in rats: An in vivo and in-silico study. Int. Immunopharmacol. 2016, 39, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Kweon, M.H.; Adhami, V.M.; Lee, J.S.; Mukhtar, H. Constitutive overexpression of nrf2-dependent heme oxygenase-1 in a549 cells contributes to resistance to apoptosis induced by epigallocatechin 3-gallate. J. Biol. Chem. 2006, 281, 33761–33772. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.Q.; Qian, S.; Ju, T. Anticancer activity of honokiol against lymphoid malignant cells via activation of ros-jnk and attenuation of nrf2 and nf-kappab. J. BUON 2016, 21, 673–679. [Google Scholar]
- Pan, S.T.; Qin, Y.; Zhou, Z.W.; He, Z.X.; Zhang, X.; Yang, T.; Yang, Y.X.; Wang, D.; Zhou, S.F.; Qiu, J.X. Plumbagin suppresses epithelial to mesenchymal transition and stemness via inhibiting Nrf2-mediated signaling pathway in human tongue squamous cell carcinoma cells. Drug Des. Dev. Ther. 2015, 9, 5511–5551. [Google Scholar]
- Singh, B.; Shoulson, R.; Chatterjee, A.; Ronghe, A.; Bhat, N.K.; Dim, D.C.; Bhat, H.K. Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of nrf2-mediated protective pathways. Carcinogenesis 2014, 35, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Gupta, S.C.; Park, B.; Yadav, V.R.; Aggarwal, B.B. Turmeric (Curcuma longa) inhibits inflammatory nuclear factor (NF)-kappab and nf-kappab-regulated gene products and induces death receptors leading to suppressed proliferation, induced chemosensitization, and suppressed osteoclastogenesis. Mol. Nutr. Food Res. 2012, 56, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Chen, J.; Shen, K.; Wang, X.; Wang, P.; Fu, G.; Meng, H.; Wang, Y.; Jin, B. Mitochondrial modulation by epigallocatechin 3-gallate ameliorates cisplatin induced renal injury through decreasing oxidative/nitrative stress, inflammation and NF-kB in mice. PLoS ONE 2015, 10, e0124775. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Afaq, F.; Kweon, M.H.; Hadi, N.; Bhatia, N.; Spiegelman, V.S.; Mukhtar, H. Green tea polyphenol egcg suppresses cigarette smoke condensate-induced nf-kappab activation in normal human bronchial epithelial cells. Oncogene 2007, 26, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Singh, S.; Piazza, G.A.; Contreras, C.M.; Panyam, J.; Singh, A.P. Honokiol: A novel natural agent for cancer prevention and therapy. Curr. Mol. Med. 2012, 12, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Katiyar, S.K. Honokiol inhibits non-small cell lung cancer cell migration by targeting pge(2)-mediated activation of beta-catenin signaling. PLoS ONE 2013, 8, e60749. [Google Scholar]
- Ahmad, A.; Banerjee, S.; Wang, Z.; Kong, D.; Sarkar, F.H. Plumbagin-induced apoptosis of human breast cancer cells is mediated by inactivation of nf-kappab and bcl-2. J. Cell Biochem. 2008, 105, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Adhami, V.M.; Afaq, F.; Ahmad, N. Suppression of ultraviolet b exposure-mediated activation of nf-kappab in normal human keratinocytes by resveratrol. Neoplasia 2003, 5, 74–82. [Google Scholar] [CrossRef]
- Ben-Neriah, Y.; Karin, M. Inflammation meets cancer, with nf-kappab as the matchmaker. Nat. Immunol. 2011, 12, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Prasad, S.; Yadav, V.R.; Aggarwal, B.B. Cancer cell signaling pathways targeted by spice-derived nutraceuticals. Nutr. Cancer 2012, 64, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ginnebaugh, K.R.; Li, Y.; Padhye, S.B.; Sarkar, F.H. Molecular targets of naturopathy in cancer research: Bridge to modern medicine. Nutrients 2015, 7, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Biersack, B.; Li, Y.; Kong, D.; Bao, B.; Schobert, R.; Padhye, S.B.; Sarkar, F.H. Targeted regulation of pi3k/akt/mtor/nf-kappab signaling by indole compounds and their derivatives: Mechanistic details and biological implications for cancer therapy. Anticancer Agents Med. Chem. 2013, 13, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, I.; Adhami, V.M.; Hafeez, B.B.; Saleem, M.; Mukhtar, H. Fisetin, a natural flavonoid, targets chemoresistant human pancreatic cancer aspc-1 cells through dr3-mediated inhibition of NF-kappaB. Int. J. Cancer 2009, 125, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Hou, D.X. Multiple regulations of keap1/Nrf2 system by dietary phytochemicals. Mol. Nutr. Food Res. 2016, 60, 1731–1755. [Google Scholar] [CrossRef] [PubMed]
- Hadi, S.M.; Ullah, M.F.; Azmi, A.S.; Ahmad, A.; Shamim, U.; Zubair, H.; Khan, H.Y. Resveratrol mobilizes endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: A putative mechanism for chemoprevention of cancer. Pharm. Res. 2010, 27, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.Y.; Zubair, H.; Ullah, M.F.; Ahmad, A.; Hadi, S.M. Oral administration of copper to rats leads to increased lymphocyte cellular DNA degradation by dietary polyphenols: Implications for a cancer preventive mechanism. Biometals 2011, 24, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Zubair, H.; Azim, S.; Khan, H.Y.; Ullah, M.F.; Wu, D.; Singh, A.P.; Hadi, S.M.; Ahmad, A. Mobilization of intracellular copper by gossypol and apogossypolone leads to reactive oxygen species-mediated cell death: Putative anticancer mechanism. Int. J. Mol. Sci. 2016, 17, 973. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Khan, H.Y.; Oves, M.; Al-Harrasi, A.; Rehmani, N.; Arif, H.; Hadi, S.M.; Ahmad, A. Cancer therapy by catechins involves redox cycling of copper ions and generation of reactive oxygen species. Toxins (Basel) 2016, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, M.; Haneda, M.; Naruse, M.; Htay, H.H.; Tsubouchi, R.; Qiao, S.L.; Li, W.H.; Murakami, K.; Yokochi, T. Prooxidant activity of curcumin: Copper-dependent formation of 8-hydroxy-2′-deoxyguanosine in DNA and induction of apoptotic cell death. Toxicol. In Vitro 2004, 18, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Gahlot, S.; Majumdar, S. Oxidative stress induced by curcumin promotes the death of cutaneous t-cell lymphoma (hut-78) by disrupting the function of several molecular targets. Mol. Cancer Ther. 2012, 11, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Nazeem, S.; Azmi, A.S.; Hanif, S.; Ahmad, A.; Mohammad, R.M.; Hadi, S.M.; Kumar, K.S. Plumbagin induces cell death through a copper-redox cycle mechanism in human cancer cells. Mutagenesis 2009, 24, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Foundation, T.B.N. Iron as a pro-oxidant. In Iron: Nutritional and Physiological Significance the Report of the British Nutrition Foundation’s Task Force; Springer: Dordrecht, The Netherlands, 1995; pp. 33–41. [Google Scholar]
- Ninsontia, C.; Phiboonchaiyanan, P.P.; Chanvorachote, P. Zinc induces epithelial to mesenchymal transition in human lung cancer h460 cells via superoxide anion-dependent mechanism. Cancer Cell Int. 2016, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Kim, D.; Kim, B.R.; Jun, J.S.; Yeom, J.S.; Park, J.S.; Seo, J.H.; Park, C.H.; Woo, H.O.; Youn, H.S.; et al. Vitamin C induces apoptosis in ags cells via production of ros of mitochondria. Oncol. Lett. 2016, 12, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Arbault, S.; Ferreira, D.C.M.; Tapsoba, I.; Verchier, Y. Vitamin c stimulates or attenuates reactive oxygen and nitrogen species (ros, rns) production depending on cell state: Quantitative amperometric measurements of oxidative bursts at plb-985 and raw 264.7 cells at the single cell level. J. Electroanal. Chem. 2008, 615, 34–44. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, T.; Ersson, C.; Bergman, J.; Moller, L. Vitamins at physiological levels cause oxidation to the DNA nucleoside deoxyguanosine and to DNA—Alone or in synergism with metals. Mutagenesis 2012, 27, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, S.E.; Chandel, N.S. Targeting mitochondria metabolism for cancer therapy. Nat. Chem. Biol. 2015, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.F. Time and circumstances: Cancer cell metabolism at various stages of disease progression. Front. Oncol. 2016, 6, 257. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J. Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet. Med. 2008, 10, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkanen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Sun, J.; Man, S.; Yang, H.; Ma, L.; Yu, P.; Gao, W. Curcumin attenuates n-nitrosodiethylamine-induced liver injury in mice by utilizing the method of metabonomics. J. Agric. Food Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Chen, X.; Bergmeier, S.C. Novel inhibitors of basal glucose transport as potential anticancer agents. Bioorg. Med. Chem. Lett. 2010, 20, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Bayet-Robert, M.; Morvan, D. Metabolomics reveals metabolic targets and biphasic responses in breast cancer cells treated by curcumin alone and in association with docetaxel. PLoS ONE 2013, 8, e57971. [Google Scholar] [CrossRef] [PubMed]
- Gunnink, L.K.; Alabi, O.D.; Kuiper, B.D.; Gunnink, S.M.; Schuiteman, S.J.; Strohbehn, L.E.; Hamilton, K.E.; Wrobel, K.E.; Louters, L.L. Curcumin directly inhibits the transport activity of glut1. Biochimie 2016, 125, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Wang, Z.; Deng, Z.; Ren, H.; Li, X. Curcumin inhibits lung cancer invasion and metastasis by attenuating glut1/mt1-mmp/mmp2 pathway. Int. J. Clin. Exp. Med. 2015, 8, 8948–8957. [Google Scholar] [PubMed]
- Gwak, H.; Haegeman, G.; Tsang, B.K.; Song, Y.S. Cancer-specific interruption of glucose metabolism by resveratrol is mediated through inhibition of akt/glut1 axis in ovarian cancer cells. Mol. Carcinog. 2015, 54, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Pal, K.; Elkhanany, A.; Dutta, S.; Cao, Y.; Mondal, G.; Iyer, S.; Somasundaram, V.; Couch, F.J.; Shridhar, V.; et al. Plumbagin inhibits tumorigenesis and angiogenesis of ovarian cancer cells in vivo. Int. J. Cancer 2013, 132, 1201–1212. [Google Scholar] [CrossRef]
- Venturelli, L.; Nappini, S.; Bulfoni, M.; Gianfranceschi, G.; Dal Zilio, S.; Coceano, G.; Del Ben, F.; Turetta, M.; Scoles, G.; Vaccari, L.; et al. Glucose is a key driver for glut1-mediated nanoparticles internalization in breast cancer cells. Sci. Rep. 2016, 6, 21629. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Lee, J.H.; Park, J.W.; Moon, S.H.; Cho, Y.S.; Choe, Y.S.; Lee, K.H. Effects of curcumin on cancer cell mitochondrial function and potential monitoring with (1)(8)f-fdg uptake. Oncol. Rep. 2016, 35, 861–868. [Google Scholar] [CrossRef]
- Malhotra, A.; Nair, P.; Dhawan, D.K. Study to evaluate molecular mechanics behind synergistic chemo-preventive effects of curcumin and resveratrol during lung carcinogenesis. PLoS ONE 2014, 9, e93820. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, X.; Li, N.; Liu, H.; Dong, Q.; Zhang, J.; Yang, C.; Liu, Y.; Liang, Q.; Zhang, S.; et al. Resveratrol inhibits hexokinases ii mediated glycolysis in non-small cell lung cancer via targeting akt signaling pathway. Exp. Cell Res. 2016, 349, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.; Araujo, I.; Costa, T.; Correia-Branco, A.; Faria, A.; Martel, F.; Keating, E. Quercetin and epigallocatechin gallate inhibit glucose uptake and metabolism by breast cancer cells by an estrogen receptor-independent mechanism. Exp. Cell Res. 2013, 319, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Spicer, D.; Stanczyk, F.Z.; Tseng, C.C.; Yang, C.S.; Pike, M.C. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormone levels in healthy postmenopausal women. Cancer Prev. Res. (Phila) 2012, 5, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Parimala, R.; Sachdanandam, P. Effect of plumbagin on some glucose metabolising enzymes studied in rats in experimental hepatoma. Mol. Cell Biochem. 1993, 125, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.P.; Zhang, W.; Wu, F.; Zhao, Y.; Cheng, L.F.; Xie, J.J.; Yao, H.P. Honokiol: An effective inhibitor of high-glucose-induced upregulation of inflammatory cytokine production in human renal mesangial cells. Inflamm. Res. 2010, 59, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.R.; Kim, J.; Lisanti, M.P.; Di Vizio, D. A metabolic perturbation by u0126 identifies a role for glutamine in resveratrol-induced cell death. Cancer Biol. Ther. 2011, 12, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Lin, Y.W.; Chiu, H.M.; Chiang, B.H. Curcumin induces apoptosis of colorectal cancer stem cells by coupling with cd44 marker. J. Agric. Food Chem. 2016, 64, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Garcia-Smith, R.; Dorsey, J.; Griffith, J.K.; Bisoffi, M.; Trujillo, K.A. Tumor necrosis factor alpha induces warburg-like metabolism and is reversed by anti-inflammatory curcumin in breast epithelial cells. Int. J. Cancer 2013, 133, 2504–2510. [Google Scholar] [CrossRef] [PubMed]
- Blanquer-Rossello, M.D.; Hernandez-Lopez, R.; Roca, P.; Oliver, J.; Valle, A. Resveratrol induces mitochondrial respiration and apoptosis in sw620 colon cancer cells. Biochim. Biophys. Acta 2017, 1861, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.; Newport, E.; Morten, K.J. The warburg effect: 80 years on. Biochem. Soc. Trans. 2016, 44, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Kim, D. Cancer metabolism: Fueling more than just growth. Mol. Cells 2016, 39, 847–854. [Google Scholar] [PubMed]
- Chang, C.H.; Qiu, J.; O'Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.; et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Giampieri, F.; Battino, M. The use of plant-derived bioactive compounds to target cancer stem cells and modulate tumor microenvironment. Food Chem. Toxicol. 2015, 75, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.H.; Thulasingam, S.; Nagarajan, S. Chemopreventive agents targeting tumor microenvironment. Life Sci. 2016, 145, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Kai, M.; Odate, S.; Iwasaki, H.; Morifuji, Y.; Ogino, T.; Morisaki, T.; Nakashima, Y.; Katano, M. Hypoxia activates the hedgehog signaling pathway in a ligand-independent manner by upregulation of smo transcription in pancreatic cancer. Cancer Sci. 2011, 102, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Spivak-Kroizman, T.R.; Hostetter, G.; Posner, R.; Aziz, M.; Hu, C.; Demeure, M.J.; Von Hoff, D.; Hingorani, S.R.; Palculict, T.B.; Izzo, J.; et al. Hypoxia triggers hedgehog-mediated tumor-stromal interactions in pancreatic cancer. Cancer Res. 2013, 73, 3235–3247. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Xiao, X.; Lei, J.; Duan, W.; Ma, Q.; Li, W. Curcumin inhibits hypoxia-induced epithelialmesenchymal transition in pancreatic cancer cells via suppression of the hedgehog signaling pathway. Oncol. Rep. 2016, 35, 3728–3734. [Google Scholar] [PubMed]
- Li, W.; Cao, L.; Chen, X.; Lei, J.; Ma, Q. Resveratrol inhibits hypoxia-driven ros-induced invasive and migratory ability of pancreatic cancer cells via suppression of the hedgehog signaling pathway. Oncol. Rep. 2016, 35, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Averett, C.; Bhardwaj, A.; Arora, S.; Srivastava, S.K.; Khan, M.A.; Ahmad, A.; Singh, S.; Carter, J.E.; Khushman, M.; Singh, A.P. Honokiol suppresses pancreatic tumor growth, metastasis and desmoplasia by interfering with tumor-stromal cross-talk. Carcinogenesis 2016, 37, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.B.; Hu, C.P.; Xiong, Z.; Yang, H.P.; Li, Y.Y. Treatment with egcg in nsclc leads to decreasing interstitial fluid pressure and hypoxia to improve chemotherapy efficacy through rebalance of ang-1 and ang-2. Chin. J. Nat. Med. 2013, 11, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Ahmad, A.; Li, Y.; Azmi, A.S.; Ali, S.; Banerjee, S.; Kong, D.; Sarkar, F.H. Targeting cscs within the tumor microenvironment for cancer therapy: A potential role of mesenchymal stem cells. Exp. Opin. Ther. Targets 2012, 16, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Kraehe, P.; Lueders, C.; Shayan, P.; Goel, A.; Shakibaei, M. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: Potential role of emt. PLoS ONE 2014, 9, e107514. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Kraehe, P.; Popper, B.; Shayan, P.; Goel, A.; Buhrmann, C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3d alginate tumor microenvironment of colorectal cancer. BMC Cancer 2015, 15, 250. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Kraehe, P.; Popper, B.; Goel, A.; Shakibaei, M. Resveratrol induces chemosensitization to 5-fluorouracil through up-regulation of intercellular junctions, epithelial-to-mesenchymal transition and apoptosis in colorectal cancer. Biochem. Pharmacol. 2015, 98, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Miao, L.; Wang, Y.; Xu, Z.; Zhao, Y.; Shen, Y.; Xiang, G.; Huang, L. Curcumin micelles remodel tumor microenvironment and enhance vaccine activity in an advanced melanoma model. Mol. Ther. 2016, 24, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, S.; Liao, W.; Xiong, Y. Modification of antitumor immunity and tumor microenvironment by resveratrol in mouse renal tumor model. Cell Biochem. Biophys. 2015, 72, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.O.; Li, C.W.; Xia, W.; Cha, J.H.; Chan, L.C.; Wu, Y.; Chang, S.S.; Lin, W.C.; Hsu, J.M.; Hsu, Y.H.; et al. Deubiquitination and stabilization of pd-l1 by csn5. Cancer Cell 2016, 30, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Wang, T.Y.; Fan, Q.M.; Du, L.; Xu, J.K.; Zhai, Z.J.; Li, H.W.; Tang, T.T. Plumbagin attenuates cancer cell growth and osteoclast formation in the bone microenvironment of mice. Acta Pharmacol. Sin. 2014, 35, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiao, J.; Wu, X.; Li, W.; Yang, Z.; Xie, J.; Xu, L.; Cai, X.; Lin, Z.; Guo, W.; et al. Plumbagin inhibits breast tumor bone metastasis and osteolysis by modulating the tumor-bone microenvironment. Curr. Mol. Med. 2012, 12, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Lee, J.K.; Jeon, Y.K.; Kim, C.W. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and m2 polarization. BMC Cancer 2013, 13, 421. [Google Scholar] [CrossRef] [PubMed]
- Salado, C.; Olaso, E.; Gallot, N.; Valcarcel, M.; Egilegor, E.; Mendoza, L.; Vidal-Vanaclocha, F. Resveratrol prevents inflammation-dependent hepatic melanoma metastasis by inhibiting the secretion and effects of interleukin-18. J. Transl. Med. 2011, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Uttarkar, S.; Piontek, T.; Dukare, S.; Schomburg, C.; Schlenke, P.; Berdel, W.E.; Muller-Tidow, C.; Schmidt, T.J.; Klempnauer, K.H. Small-molecule disruption of the myb/p300 cooperation targets acute myeloid leukemia cells. Mol. Cancer Ther. 2016, 15, 2905–2915. [Google Scholar] [CrossRef] [PubMed]
- Azim, S.; Zubair, H.; Srivastava, S.K.; Bhardwaj, A.; Zubair, A.; Ahmad, A.; Singh, S.; Khushman, M.; Singh, A.P. Deep sequencing and in silico analyses identify myb-regulated gene networks and signaling pathways in pancreatic cancer. Sci. Rep. 2016, 6, 28446. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.L.; Stephens, C.A.; Bigelow, R.L.; Coleman, D.T.; Cardelli, J.A. The polyphenols (−)-epigallocatechin-3-gallate and luteolin synergistically inhibit tgf-beta-induced myofibroblast phenotypes through rhoa and erk inhibition. PLoS ONE 2014, 9, e109208. [Google Scholar] [CrossRef] [PubMed]
- Stylos, E.; Chatziathanasiadou, M.V.; Syriopoulou, A.; Tzakos, A.G. Liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) based bioavailability determination of the major classes of phytochemicals. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Maru, G.B.; Hudlikar, R.R.; Kumar, G.; Gandhi, K.; Mahimkar, M.B. Understanding the molecular mechanisms of cancer prevention by dietary phytochemicals: From experimental models to clinical trials. World J. Biol. Chem. 2016, 7, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall'Acqua, S. Natural deep eutectic solvents (nades) as a tool for bioavailability improvement: Pharmacokinetics of rutin dissolved in proline/glycine after oral administration in rats: Possible application in nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Li, Y.; Sarkar, F.H. The bounty of nature for changing the cancer landscape. Mol. Nutr. Food Res. 2016, 60, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Hackler, L., Jr.; Ozsvari, B.; Gyuris, M.; Sipos, P.; Fabian, G.; Molnar, E.; Marton, A.; Farago, N.; Mihaly, J.; Nagy, L.I.; et al. The curcumin analog c-150, influencing nf-kappab, upr and akt/notch pathways has potent anticancer activity in vitro and in vivo. PLoS ONE 2016, 11, e0149832. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Xia, Y.; Chen, Q.; Li, W.; Chen, D.; Ye, H.; Zhao, C.; Du, X.; Shi, D.; Wu, J.; et al. Da0324, an inhibitor of nuclear factor-kappab activation, demonstrates selective antitumor activity on human gastric cancer cells. Drug Des. Dev. Ther. 2016, 10, 979–995. [Google Scholar]
- Liu, G.Y.; Zhai, Q.; Chen, J.Z.; Zhang, Z.Q.; Yang, J. 2,2'-fluorine mono-carbonyl curcumin induce reactive oxygen species-mediated apoptosis in human lung cancer nci-h460 cells. Eur. J. Pharmacol. 2016, 786, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wei, X.; Wang, Z.; Zhang, S.; Ren, J.; Yao, S.; Shi, L.; Yang, L.; Qiu, P.; Wu, J.; et al. A novel double carbonyl analog of curcumin induces the apoptosis of human lung cancer h460 cells via the activation of the endoplasmic reticulum stress signaling pathway. Oncol. Rep. 2016, 36, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Liu, Z.; Cao, Y.; Zhu, C.; Zuo, Y.; Huang, L.; Wen, G.; Shang, N.; Chen, Y.; Yue, X.; et al. Mc37, a new mono-carbonyl curcumin analog, induces g2/m cell cycle arrest and mitochondria-mediated apoptosis in human colorectal cancer cells. Eur. J. Pharmacol. 2016, 796, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhao, C.; Zheng, H.; Lu, K.; Shi, D.; Liu, Z.; Dai, X.; Zhang, Y.; Zhang, X.; Hu, W.; et al. A novel stat3 inhibitor ho-3867 induces cell apoptosis by reactive oxygen species-dependent endoplasmic reticulum stress in human pancreatic cancer cells. Anticancer Drugs 2017. [Google Scholar] [CrossRef] [PubMed]
- Mohankumar, K.; Sridharan, S.; Pajaniradje, S.; Singh, V.K.; Ronsard, L.; Banerjea, A.C.; Somasundaram, D.B.; Coumar, M.S.; Periyasamy, L.; Rajagopalan, R. Bdmc-a, an analog of curcumin, inhibits markers of invasion, angiogenesis, and metastasis in breast cancer cells via nf-kappab pathway--a comparative study with curcumin. Biomed. Pharmacother. 2015, 74, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.; Yoshino, Y.; Kuriyama, S.; Inoue, M.; Komine, K.; Otsuka, K.; Kohyama, A.; Yamakoshi, H.; Ishioka, C.; Tanaka, M.; et al. A curcumin analog, go-y078, effectively inhibits angiogenesis through actin disorganization. Anticancer Agents Med. Chem. 2016, 16, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Faiao-Flores, F.; Quincoces Suarez, J.A.; Fruet, A.C.; Maria-Engler, S.S.; Pardi, P.C.; Maria, D.A. Curcumin analog dm-1 in monotherapy or combinatory treatment with dacarbazine as a strategy to inhibit in vivo melanoma progression. PLoS ONE 2015, 10, e0118702. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Rahman, M.A.; Fuchs, J.R.; Chen, Z.G.; Khuri, F.R.; Shin, D.M.; Amin, A.R. Flll12 induces apoptosis in lung cancer cells through a p53/p73-independent but death receptor 5-dependent pathway. Cancer Lett. 2015, 363, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Jiang, T.; Long, M.; Chen, J.; Ren, D.M.; Wong, P.K.; Chapman, E.; Zhou, B.; Zhang, D.D. A curcumin derivative that inhibits vinyl carbamate-induced lung carcinogenesis via activation of the nrf2 protective response. Antioxid. Redox. Signal 2015, 23, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Gundewar, C.; Ansari, D.; Tang, L.; Wang, Y.; Liang, G.; Rosendahl, A.H.; Saleem, M.A.; Andersson, R. Antiproliferative effects of curcumin analog l49h37 in pancreatic stellate cells: A comparative study. Ann. Gastroenterol. 2015, 28, 391–398. [Google Scholar] [PubMed]
- Ahmad, A.; Sayed, A.; Ginnebaugh, K.R.; Sharma, V.; Suri, A.; Saraph, A.; Padhye, S.; Sarkar, F.H. Molecular docking and inhibition of matrix metalloproteinase-2 by novel difluorinatedbenzylidene curcumin analog. Am. J. Transl. Res. 2015, 7, 298–308. [Google Scholar] [PubMed]
- Roy, S.; Yu, Y.; Padhye, S.B.; Sarkar, F.H.; Majumdar, A.P. Difluorinated-curcumin (cdf) restores pten expression in colon cancer cells by down-regulating mir-21. PLoS ONE 2013, 8, e68543. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Ali, S.; Banerjee, S.; Wang, Z.; Logna, F.; Azmi, A.S.; Kong, D.; Ahmad, A.; Li, Y.; Padhye, S.; et al. Curcumin analogue cdf inhibits pancreatic tumor growth by switching on suppressor micrornas and attenuating ezh2 expression. Cancer Res. 2012, 72, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, S.S.; Yu, Y.; Nautiyal, J.; Patel, B.B.; Padhye, S.; Sarkar, F.H.; Majumdar, A.P. Difluorinated-curcumin (cdf): A novel curcumin analog is a potent inhibitor of colon cancer stem-like cells. Pharm. Res. 2011, 28, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhou, B.; Zhong, P.; Rajamanickam, V.; Dai, X.; Karvannan, K.; Zhou, H.; Zhang, X.; Liang, G. Increased intracellular reactive oxygen species mediates the anti-cancer effects of wz35 via activating mitochondrial apoptosis pathway in prostate cancer cells. Prostate 2016. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Zhang, J.; Xia, Y.; Kanchana, K.; Guo, G.; Chen, W.; Huang, Y.; Wang, Z.; Yang, S.; Liang, G. Ros generation mediates the anti-cancer effects of wz35 via activating jnk and er stress apoptotic pathways in gastric cancer. Oncotarget 2015, 6, 5860–5876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, M.; Zou, P.; Kanchana, K.; Weng, Q.; Chen, W.; Zhong, P.; Ji, J.; Zhou, H.; He, L.; et al. Curcumin analog wz35 induced cell death via ros-dependent er stress and g2/m cell cycle arrest in human prostate cancer cells. BMC Cancer 2015, 15, 866. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Tin, L.; Zhang, Y.; Wu, Y.; Jin, Y.; Jin, X.; Zhang, F.; Li, X. Ef24 suppresses invasion and migration of hepatocellular carcinoma cells in vitro via inhibiting the phosphorylation of src. Biomed. Res. Int. 2016, 2016, 8569684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Bai, H.; Liu, G.; Wang, H.; Chen, F.; Zhang, B.; Zeng, P.; Wu, C.; Peng, C.; Huang, C.; et al. Microrna-33b, upregulated by ef24, a curcumin analog, suppresses the epithelial-to-mesenchymal transition (emt) and migratory potential of melanoma cells by targeting hmga2. Toxicol. Lett. 2015, 234, 151–161. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Feng, C.; Vinothkumar, R.; Chen, W.; Dai, X.; Chen, X.; Ye, Q.; Qiu, C.; Zhou, H.; Wang, Y.; et al. Curcumin analog ef24 induces apoptosis via ros-dependent mitochondrial dysfunction in human colorectal cancer cells. Cancer Chemother. Pharmacol. 2016, 78, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.L.; Liang, Y.J.; Zheng, T.S.; Song, R.P.; Wang, J.B.; Sun, B.S.; Pan, S.H.; Qu, L.D.; Liu, J.R.; Jiang, H.C.; et al. Ef24 inhibits tumor growth and metastasis via suppressing nf-kappab dependent pathways in human cholangiocarcinoma. Sci. Rep. 2016, 6, 32167. [Google Scholar] [CrossRef] [PubMed]
- Yar Saglam, A.S.; Yilmaz, A.; Onen, H.I.; Alp, E.; Kayhan, H.; Ekmekci, A. Hdac inhibitors, ms-275 and salermide, potentiates the anticancer effect of ef24 in human pancreatic cancer cells. Excli J. 2016, 15, 246–255. [Google Scholar] [PubMed]
- Lu, J.; Ho, C.H.; Ghai, G.; Chen, K.Y. Resveratrol analog, 3,4,5,4′-tetrahydroxystilbene, differentially induces pro-apoptotic p53/bax gene expression and inhibits the growth of transformed cells but not their normal counterparts. Carcinogenesis 2001, 22, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.A.; Kim, S.; Heo, Y.H.; Lee, S.K. Resveratrol analog, 3,5,2′,4′-tetramethoxy-trans-stilbene, potentiates the inhibition of cell growth and induces apoptosis in human cancer cells. Arch. Pharm. Res. 2001, 24, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.B.; Kang, H.J.; Kim, H.J.; Rosen, E.M.; Dakshanamurthy, S.; Rondanin, R.; Baruchello, R.; Grisolia, G.; Daniele, S.; Bae, I. Inhibition of cell proliferation by a resveratrol analog in human pancreatic and breast cancer cells. Exp. Mol. Med. 2009, 41, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Saiko, P.; Graser, G.; Giessrigl, B.; Steinmann, M.T.; Schuster, H.; Lackner, A.; Grusch, M.; Krupitza, G.; Jaeger, W.; Somepalli, V.; et al. Digalloylresveratrol, a novel resveratrol analog inhibits the growth of human pancreatic cancer cells. Invest. New Drugs 2013, 31, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Aldawsari, F.S.; Aguayo-Ortiz, R.; Kapilashrami, K.; Yoo, J.; Luo, M.; Medina-Franco, J.L.; Velazquez-Martinez, C.A. Resveratrol-salicylate derivatives as selective dnmt3 inhibitors and anticancer agents. J. Enzyme Inhib. Med. Chem. 2016, 31, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Aldawsari, F.S.; Aguiar, R.P.; Wiirzler, L.A.; Aguayo-Ortiz, R.; Aljuhani, N.; Cuman, R.K.; Medina-Franco, J.L.; Siraki, A.G.; Velazquez-Martinez, C.A. Anti-inflammatory and antioxidant properties of a novel resveratrol-salicylate hybrid analog. Bioorg. Med. Chem. Lett. 2016, 26, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Gopalakrishnan, V.; Hegde, M.; Kumar, S.; Karki, S.S.; Raghavan, S.C.; Choudhary, B. A novel resveratrol based tubulin inhibitor induces mitotic arrest and activates apoptosis in cancer cells. Sci. Rep. 2016, 6, 34653. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Molavi, O.; Haddadi, A.; Lai, R.; Gossage, R.A.; Lavasanifar, A. Resveratrol analog trans 3,4,5,4′-tetramethoxystilbene (dmu-212) mediates anti-tumor effects via mechanism different from that of resveratrol. Cancer Chemother. Pharmacol. 2008, 63, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, M.; Baer-Dubowska, W.; Wierzchowski, M.; Murias, M.; Jodynis-Liebert, J. 3,4,5,4′-trans-tetramethoxystilbene (dmu-212) modulates the activation of nf-kappab, ap-1, and stat3 transcription factors in rat liver carcinogenesis induced by initiation-promotion regimen. Mol. Cell Biochem. 2014, 391, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Penthala, N.R.; Thakkar, S.; Crooks, P.A. Heteroaromatic analogs of the resveratrol analog dmu-212 as potent anti-cancer agents. Bioorg. Med. Chem. Lett. 2015, 25, 2763–2767. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.Y.; Yoon, Y.G.; Rho, J.H.; Lee, J.S.; Lee, S.Y.; Yoo, K.S.; Song, S.; Suh, H.; Choi, Y.H.; Yoo, Y.H. The novel resveratrol analog hs-1793-induced polyploid lncap prostate cancer cells are vulnerable to downregulation of bcl-xl. Int. J. Oncol. 2011, 38, 1597–1604. [Google Scholar] [PubMed]
- Jeong, M.H.; Yang, K.M.; Choi, Y.J.; Kim, S.D.; Yoo, Y.H.; Seo, S.Y.; Lee, S.H.; Ryu, S.R.; Lee, C.M.; Suh, H.; et al. Resveratrol analog, HS-1793 enhance anti-tumor immunity by reducing the CD4+CD25+ regulatory T cells in FM3A tumor bearing mice. Int. Immunopharmacol. 2012, 14, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Song, I.S.; Kim, H.K.; Lee, S.R.; Song, S.; Suh, H.; Yoon, Y.G.; Yoo, Y.H.; Kim, N.; Rhee, B.D.; et al. An analogue of resveratrol hs-1793 exhibits anticancer activity against mcf-7 cells via inhibition of mitochondrial biogenesis gene expression. Mol. Cells 2012, 34, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.K.; Yang, K.; Park, Y.S.; Choi, Y.J.; Oh, S.J.; Lee, C.W.; Lee, K.Y.; Jeong, M.H.; Jo, W.S. Interferon gamma induced by resveratrol analog, hs-1793, reverses the properties of tumor associated macrophages. Int. Immunopharmacol. 2014, 22, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yang, K.M.; Park, Y.S.; Choi, Y.J.; Yun, J.H.; Son, C.H.; Suh, H.S.; Jeong, M.H.; Jo, W.S. The novel resveratrol analogue hs-1793 induces apoptosis via the mitochondrial pathway in murine breast cancer cells. Int. J. Oncol. 2012, 41, 1628–1634. [Google Scholar] [PubMed]
- Choi, Y.J.; Heo, K.; Park, H.S.; Yang, K.M.; Jeong, M.H. The resveratrol analog hs-1793 enhances radiosensitivity of mouse-derived breast cancer cells under hypoxic conditions. Int. J. Oncol. 2016, 49, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Waleh, N.S.; Chao, W.R.; Bensari, A.; Zaveri, N.T. Novel d-ring analog of epigallocatechin-3-gallate inhibits tumor growth and vegf expression in breast carcinoma cells. Anticancer Res. 2005, 25, 397–402. [Google Scholar] [PubMed]
- Hashimoto, O.; Nakamura, A.; Nakamura, T.; Iwamoto, H.; Hiroshi, M.; Inoue, K.; Torimura, T.; Ueno, T.; Sata, M. Methylated-(3″)-epigallocatechin gallate analog suppresses tumor growth in huh7 hepatoma cells via inhibition of angiogenesis. Nutr. Cancer 2014, 66, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Qin, X.L.; Gu, Y.Y.; Chen, D.; Cui, Q.C.; Jiang, T.; Wan, S.B.; Dou, Q.P. Prodrugs of fluoro-substituted benzoates of egc as tumor cellular proteasome inhibitors and apoptosis inducers. Int. J. Mol. Sci. 2008, 9, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sun, D.K.; Chen, D.; Cui, Q.C.; Gu, Y.Y.; Jiang, T.; Chen, W.; Wan, S.B.; Dou, Q.P. Antitumor activity of novel fluoro-substituted (−)-epigallocatechin-3-gallate analogs. Cancer Lett. 2010, 292, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.; Yang, H.; Cui, Q.C.; Dou, Q.P.; Chan, T.H. Proteasome inhibition in human breast cancer cells with high catechol-o-methyltransferase activity by green tea polyphenol egcg analogs. Bioorg. Med. Chem. 2010, 18, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, P.; Vemuri, K.; Venkateswara Swamy, K.; Khan, E.M.; Sritharan, M.; Padhye, S. Synthesis, characterization, molecular docking and anti-tubercular activity of plumbagin-isoniazid analog and its beta-cyclodextrin conjugate. Bioorg. Med. Chem. Lett. 2014, 24, 5070–5075. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.R.; Karlsson, A.I.; Bonner, M.Y.; Arbiser, J.L.; Singh, S.V. Honokiol inhibits androgen receptor activity in prostate cancer cells. Prostate 2014, 74, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Bonner, M.Y.; Karlsson, I.; Rodolfo, M.; Arnold, R.S.; Vergani, E.; Arbiser, J.L. Honokiol bis-dichloroacetate (honokiol dca) demonstrates activity in vemurafenib-resistant melanoma in vivo. Oncotarget 2016, 7, 12857–12868. [Google Scholar] [PubMed]
- Arora, S.; Tyagi, N.; Bhardwaj, A.; Rusu, L.; Palanki, R.; Vig, K.; Singh, S.R.; Singh, A.P.; Palanki, S.; Miller, M.E.; et al. Silver nanoparticles protect human keratinocytes against uvb radiation-induced DNA damage and apoptosis: Potential for prevention of skin carcinogenesis. Nanomed.: Nanotechnol. Biol. Med. 2015, 11, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Aras, A.; Khokhar, A.R.; Qureshi, M.Z.; Silva, M.F.; Sobczak-Kupiec, A.; Pineda, E.A.; Hechenleitner, A.A.; Farooqi, A.A. Targeting cancer with nano-bullets: Curcumin, egcg, resveratrol and quercetin on flying carpets. Asian Pac. J. Cancer Prev. 2014, 15, 3865–3871. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.P.; Mills, T.; Norton, I. Lipid based nanosystems for curcumin: Past, present and future. Curr. Pharm. Des. 2016, 22, 4247–4256. [Google Scholar] [PubMed]
- Rahimi, H.R.; Nedaeinia, R.; Sepehri Shamloo, A.; Nikdoust, S.; Kazemi Oskuee, R. Novel delivery system for natural products: Nano-curcumin formulations. Avicenna J. Phytomed. 2016, 6, 383–398. [Google Scholar] [PubMed]
- Strojny, B.; Grodzik, M.; Sawosz, E.; Winnicka, A.; Kurantowicz, N.; Jaworski, S.; Kutwin, M.; Urbanska, K.; Hotowy, A.; Wierzbicki, M.; et al. Diamond nanoparticles modify curcumin activity: In vitro studies on cancer and normal cells and in ovo studies on chicken embryo model. PLoS ONE 2016, 11, e0164637. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.S.; Park, J.H.; Kang, H.J.; Choe, J.H.; Goh, M.S.; Kim, D.D.; Cho, H.J. Poly(d,l-lactic acid)-glycerol-based nanoparticles for curcumin delivery. Int. J. Pharm. 2015, 488, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.S.; Chauhan, N.; Yallapu, M.M.; Gara, R.K.; Maher, D.M.; Kumari, S.; Sikander, M.; Khan, S.; Zafar, N.; Jaggi, M.; et al. Curcumin nanoformulation for cervical cancer treatment. Sci. Rep. 2016, 6, 20051. [Google Scholar] [CrossRef] [PubMed]
- Kheiri Manjili, H.; Ghasemi, P.; Malvandi, H.; Mousavi, M.S.; Attari, E.; Danafar, H. Pharmacokinetics and in vivo delivery of curcumin by copolymeric mpeg-pcl micelles. Eur. J. Pharm. Biopharm. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Lian, S.; Sun, J.; Liu, Z.; Zhao, F.; Jiang, Y.; Gao, M.; Sun, K.; Liu, W.; Fu, F. Design of novel multifunctional targeting nano-carrier drug delivery system based on cd44 receptor and tumor microenvironment ph condition. Drug Deliv. 2016, 23, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Ndong Ntoutoume, G.M.; Granet, R.; Mbakidi, J.P.; Bregier, F.; Leger, D.Y.; Fidanzi-Dugas, C.; Lequart, V.; Joly, N.; Liagre, B.; Chaleix, V.; et al. Development of curcumin-cyclodextrin/cellulose nanocrystals complexes: New anticancer drug delivery systems. Bioorg. Med. Chem. Lett. 2016, 26, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Khan, S.; Maher, D.M.; Ebeling, M.C.; Sundram, V.; Chauhan, N.; Ganju, A.; Balakrishna, S.; Gupta, B.K.; Zafar, N.; et al. Anti-cancer activity of curcumin loaded nanoparticles in prostate cancer. Biomaterials 2014, 35, 8635–8648. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.T.; Chanchal, A.; Yavvari, P.S.; Bhagat, S.D.; Gujrati, M.; Mishra, R.K.; Srivastava, A. Cell permeating nano-complexes of amphiphilic polyelectrolytes enhance solubility, stability, and anti-cancer efficacy of curcumin. Biomacromolecules 2016, 17, 2375–2383. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, Y.; Zhang, X.; Liu, S.; Tian, C.; Wang, H. Targeted nanomedicine for prostate cancer therapy: Docetaxel and curcumin co-encapsulated lipid-polymer hybrid nanoparticles for the enhanced anti-tumor activity in vitro and in vivo. Drug Deliv. 2016, 23, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.B.; Zhang, J.M.; Xie, X.; Liu, D.; He, C.W.; Wan, J.B.; Chen, M.W. Ph-sensitive micelles based on acid-labile pluronic f68-curcumin conjugates for improved tumor intracellular drug delivery. Int. J. Pharm. 2016, 502, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, W.; Tu, P. Synergistically improved anti-tumor efficacy by co-delivery doxorubicin and curcumin polymeric micelles. Macromol. Biosci. 2015, 15, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Banerjee, S.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Parenterally administrable nano-micelles of 3,4-difluorobenzylidene curcumin for treating pancreatic cancer. Colloids Surf. B Biointerfaces 2015, 132, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.K.; Zinabadi, A.; Wu, A.W.; Venkatesan, N.; Duarte, V.M.; Kang, J.J.; Dalgard, C.L.; Srivastava, M.; Sarkar, F.H.; Wang, M.B.; et al. Liposome encapsulated curcumin-difluorinated (cdf) inhibits the growth of cisplatin resistant head and neck cancer stem cells. Oncotarget 2015, 6, 18504–18517. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Banerjee, S.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Hyaluronic acid engineered nanomicelles loaded with 3,4-difluorobenzylidene curcumin for targeted killing of cd44+ stem-like pancreatic cancer cells. Biomacromolecules 2015, 16, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Xie, L.; Banerjee, S.; Mao, G.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3,4-difluorobenzylidene curcumin to cd44 overexpressing pancreatic cancer cells. Colloids Surf. B Biointerfaces 2015, 136, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Schlesinger, M.; Rupp, A.; Schubert, R.; Nolting, J.; Wenzel, J.; Holdenrieder, S.; Brossart, P.; Bendas, G.; Feldmann, G. A liposomal formulation of the synthetic curcumin analog ef24 (lipo-ef24) inhibits pancreatic cancer progression: Towards future combination therapies. J. Nanobiotechnol. 2016, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; An, F.F.; Liu, J.; Jin, S.; Zhang, J.C.; Wang, P.C.; Zhang, X.; Lee, C.S.; Liang, X.J. Self-carried curcumin nanoparticles for in vitro and in vivo cancer therapy with real-time monitoring of drug release. Nanoscale 2015, 7, 13503–13510. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Bansal, S.S.; Aqil, F.; Jeyabalan, J.; Cao, P.; Kausar, H.; Russell, G.K.; Munagala, R.; Ravoori, S.; Vadhanam, M.V. Controlled-release systemic delivery—A new concept in cancer chemoprevention. Carcinogenesis 2012, 33, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Siddiqui, I.A.; Sechi, M.; Mukhtar, H. Resveratrol-loaded nanoparticles based on poly(epsilon-caprolactone) and poly(d,l-lactic-co-glycolic acid)-poly(ethylene glycol) blend for prostate cancer treatment. Mol. Pharm. 2013, 10, 3871–3881. [Google Scholar] [CrossRef] [PubMed]
- Pulliero, A.; Wu, Y.; Fenoglio, D.; Parodi, A.; Romani, M.; Soares, C.P.; Filaci, G.; Lee, J.L.; Sinkam, P.N.; Izzotti, A. Nanoparticles increase the efficacy of cancer chemopreventive agents in cells exposed to cigarette smoke condensate. Carcinogenesis 2015, 36, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Pujara, N.; Jambhrunkar, S.; Wong, K.Y.; McGuckin, M.; Popat, A. Enhanced colloidal stability, solubility and rapid dissolution of resveratrol by nanocomplexation with soy protein isolate. J. Colloid Interface Sci. 2017, 488, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Chae, S.Y.; Park, J.O.; Lee, K.J.; Park, G. Gold-conjugated resveratrol nanoparticles attenuate the invasion and mmp-9 and cox-2 expression in breast cancer cells. Oncol. Rep. 2016, 35, 3248–3256. [Google Scholar] [CrossRef] [PubMed]
- Han, D.W.; Matsumura, K.; Kim, B.; Hyon, S.H. Time-dependent intracellular trafficking of fitc-conjugated epigallocatechin-3-o-gallate in l-929 cells. Bioorg. Med. Chem. 2008, 16, 9652–9659. [Google Scholar] [CrossRef] [PubMed]
- Shutava, T.G.; Balkundi, S.S.; Vangala, P.; Steffan, J.J.; Bigelow, R.L.; Cardelli, J.A.; O'Neal, D.P.; Lvov, Y.M. Layer-by-layer-coated gelatin nanoparticles as a vehicle for delivery of natural polyphenols. ACS Nano 2009, 3, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Mukhtar, H. Nanochemoprevention by bioactive food components: A perspective. Pharm. Res. 2010, 27, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Taylor, E.W.; Wang, Y.; Wan, X.; Zhang, J. Encapsulated nanoepigallocatechin-3-gallate and elemental selenium nanoparticles as paradigms for nanochemoprevention. Int. J. Nanomed. 2012, 7, 1711–1721. [Google Scholar]
- Siddiqui, I.A.; Adhami, V.M.; Ahmad, N.; Mukhtar, H. Nanochemoprevention: Sustained release of bioactive food components for cancer prevention. Nutr. Cancer 2010, 62, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, S.S.; Solati-Hashjin, M.; Samadikuchaksaraei, A.; Kalantarinejad, R.; Asadi-Eydivand, M.; Abu Osman, N.A. Epigallocatechin gallate/layered double hydroxide nanohybrids: Preparation, characterization, and in vitro anti-tumor study. PLoS ONE 2015, 10, e0136530. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Mony, U.; Vijaykumar, D.K.; Koyakutty, M.; Paul-Prasanth, B.; Menon, D. Sequential release of epigallocatechin gallate and paclitaxel from plga-casein core/shell nanoparticles sensitizes drug-resistant breast cancer cells. Nanomed.: Nanotechnol. Biol. Med. 2015, 11, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Duraipandy, N.; Lakra, R.; Kunnavakkam Vinjimur, S.; Samanta, D.; K, P.S.; Kiran, M.S. Caging of plumbagin on silver nanoparticles imparts selectivity and sensitivity to plumbagin for targeted cancer cell apoptosis. Metallomics 2014, 6, 2025–2033. [Google Scholar] [CrossRef] [PubMed]
- Appadurai, P.; Rathinasamy, K. Plumbagin-silver nanoparticle formulations enhance the cellular uptake of plumbagin and its antiproliferative activities. IET Nanobiotechnol. 2015, 9, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.A.; Snima, K.S.; Kamath, R.C.; Nair, S.V.; Lakshmanan, V.K. Plumbagin nanoparticles induce dose and ph dependent toxicity on prostate cancer cells. Curr. Drug Deliv. 2015, 12, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Zheng, L.; Peng, X.; Men, K.; Zheng, X.; Zeng, S.; Guo, G.; Luo, F.; Zhao, X.; Chen, L.; et al. Poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) (pcl-peg-pcl) nanoparticles for honokiol delivery in vitro. Int. J. Pharm. 2009, 375, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gou, M.; Zheng, X.; Wei, X.; Gong, C.; Wang, X.; Zhao, Y.; Luo, F.; Chen, L.; Qian, Z.; et al. Co-delivery honokiol and doxorubicin in mpeg-pla nanoparticles. J. Nanosci. Nanotechnol. 2010, 10, 4166–4172. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Kan, B.; Gou, M.; Fu, S.; Zhang, J.; Men, K.; Chen, L.; Luo, F.; Zhao, Y.; Zhao, X.; et al. Preparation of mpeg-pla nanoparticle for honokiol delivery in vitro. Int. J. Pharm. 2010, 386, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Qiu, N.; Cai, L.L.; Xie, D.; Wang, G.; Wu, W.; Zhang, Y.; Song, H.; Yin, H.; Chen, L. Synthesis, structural and in vitro studies of well-dispersed monomethoxy-poly(ethylene glycol)-honokiol conjugate micelles. Biomed. Mater. 2010, 5, 065006. [Google Scholar] [CrossRef] [PubMed]
- Alaarg, A.; Jordan, N.Y.; Verhoef, J.J.; Metselaar, J.M.; Storm, G.; Kok, R.J. Docosahexaenoic acid liposomes for targeting chronic inflammatory diseases and cancer: An in vitro assessment. Int. J. Nanomed. 2016, 11, 5027–5040. [Google Scholar] [CrossRef] [PubMed]
- Molfino, A.; Amabile, M.I.; Monti, M.; Arcieri, S.; Rossi Fanelli, F.; Muscaritoli, M. The role of docosahexaenoic acid (dha) in the control of obesity and metabolic derangements in breast cancer. Int. J. Mol. Sci. 2016, 17, 505. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.H.; Fu, X.; Ababaikeli, G. Docosahexaenoic acid induces growth suppression on epithelial ovarian cancer cells more effectively than eicosapentaenoic acid. Nutr. Cancer 2016, 68, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.A.; Xu, Z.; Saaddatzadeh, M.R.; Wang, H.; Pollok, K.; Cohen-Gadol, A.A.; Siddiqui, R.A. Enhanced anticancer properties of lomustine in conjunction with docosahexaenoic acid in glioblastoma cell lines. J. Neurosurg. 2015, 122, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Oliveira, L.T.; Oger, C.; Galano, J.M.; Bultel-Ponce, V.; Richard, S.; Guimaraes, A.G.; Vilela, J.M.; Andrade, M.S.; Durand, T.; et al. Polymeric nanocapsules prevent oxidation of core-loaded molecules: Evidence based on the effects of docosahexaenoic acid and neuroprostane on breast cancer cells proliferation. J. Exp. Clin. Cancer Res. 2015, 34, 155. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Enhancing the bioaccessibility of hydrophobic bioactive agents using mixed colloidal dispersions: Curcumin-loaded zein nanoparticles plus digestible lipid nanoparticles. Food Res. Int. 2016, 81, 74–82. [Google Scholar] [CrossRef]
- Neves, A.R.; Lucio, M.; Martins, S.; Lima, J.L.; Reis, S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar]
- Niu, Y.; Bai, J.; Kamm, R.D.; Wang, Y.; Wang, C. Validating antimetastatic effects of natural products in an engineered microfluidic platform mimicking tumor microenvironment. Mol. Pharm. 2014, 11, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Adhami, V.M.; Bharali, D.J.; Hafeez, B.B.; Asim, M.; Khwaja, S.I.; Ahmad, N.; Cui, H.; Mousa, S.A.; Mukhtar, H. Introducing nanochemoprevention as a novel approach for cancer control: Proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009, 69, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Bharali, D.J.; Nihal, M.; Adhami, V.M.; Khan, N.; Chamcheu, J.C.; Khan, M.I.; Shabana, S.; Mousa, S.A.; Mukhtar, H. Excellent anti-proliferative and pro-apoptotic effects of (−)-epigallocatechin-3-gallate encapsulated in chitosan nanoparticles on human melanoma cell growth both in vitro and in vivo. Nanomed.: Nanotechnol. Biol. Med. 2014, 10, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; Srivastava, S.K.; Arora, S.; Omar, Y.; Ijaz, Z.M.; Al-Ghadhban, A.; Deshmukh, S.K.; Carter, J.E.; Singh, A.P.; Singh, S. Comparative analysis of the relative potential of silver, zinc-oxide and titanium-dioxide nanoparticles against uvb-induced DNA damage for the prevention of skin carcinogenesis. Cancer Lett. 2016, 383, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Annu; Chaudhry, S.A.; Ikram, S. A review on biogenic synthesis of zno nanoparticles using plant extracts and microbes: A prospect towards green chemistry. J. Photochem. Photobiol. B 2017, 166, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.L.; Wong, W.Y.; Bian, Z.; Chui, C.H.; Gambari, R. Recent advances in green nanoparticulate systems for drug delivery: Efficient delivery and safety concern. Nanomedicine (Lond.) 2017. [Google Scholar] [CrossRef] [PubMed]
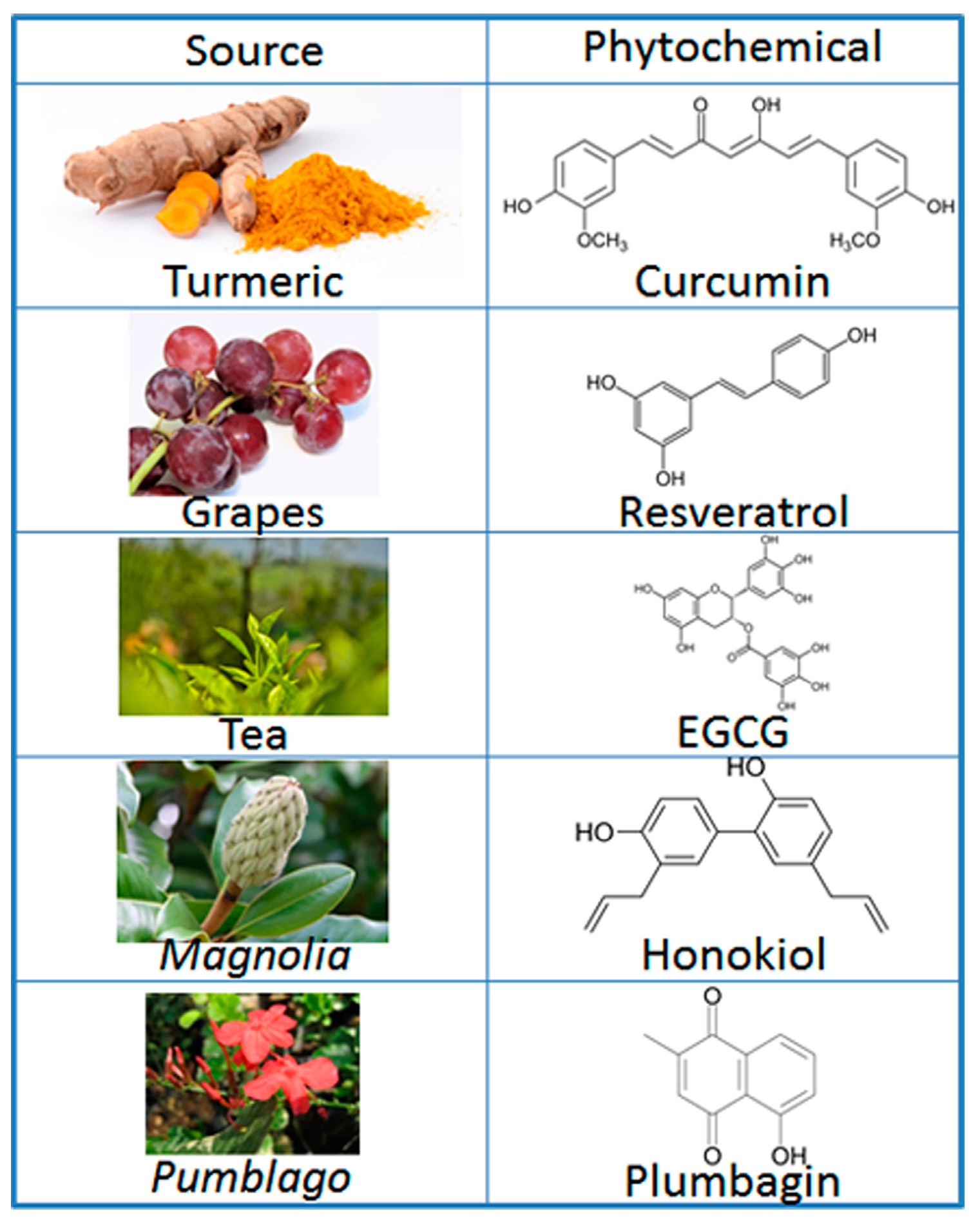
| Signaling Factor/Pathway | Phytochemical | Reference |
|---|---|---|
| COX2 | Curcumin | [30] |
| IL-1β | Resveratrol | [36] |
| IL-6 | Resveratrol | [35] |
| IL-8 | Resveratrol | [35] |
| iNOS | Curcumin | [30] |
| TLR/IL-1R | Curcumin | [31] |
| Keap1/Nrf2 | Curcumin | [30] |
| EGCG | [37,38] | |
| Honokiol | [39] | |
| Plumbagin | [40] | |
| Resveratrol | [41] | |
| NF-κB | Curcumin | [42] |
| EGCG | [43,44] | |
| Honokiol | [45,46] | |
| Plumbagin | [47] | |
| Resveratrol | [48] |
| TME Component | Phytochemical | Cancer Model | Reference |
|---|---|---|---|
| CSCs | Curcumin | Pancreatic | [105] |
| Colorectal | [106] | ||
| Hh signaling | Curcumin | Pancreatic | [101] |
| Honokiol | Pancreatic | [103] | |
| Resveratrol | Pancreatic | [102] | |
| CXCR4 | Honokiol | Pancreatic | [103] |
| IL-6 | Curcumin | Melanoma | [109] |
| Resveratrol | Renal | [110] | |
| EGCG | Breast | [114] | |
| IL-18 | Resveratrol | Melanoma | [115] |
| Microvasculature | EGCG | NSCLC | [104] |
| Myofibroblast Differentiation | EGCG | Prostate | [118] |
| RANK | Plumbagin | Breast | [113] |
| Regulatory T-cells | Curcumin | Melanoma | [109] |
| Resveratrol | Renal | [110] |
| Phytochemical | Analogue | Reported Activity | Reference |
|---|---|---|---|
| Curcumin | C-150 | Inhibits NF-κB | [123] |
| Da0324 | Inhibits NF-κB | [124] | |
| 2-2′-fluorine mono-carbonyl analog | Modulates ROS | [125] | |
| A17 | Induces ER stress | [126] | |
| MC37 | Induces cell cycle arrest | [127] | |
| HO-3867 | Inhibits STAT3 | [128] | |
| BDMC-A | Inhibits NF-κB | [129] | |
| GO-Y078 | Inhibits invasion of endothelial cells | [130] | |
| DM-1 | Induces apoptosis | [131] | |
| FLLL12 | Induces apoptosis | [132] | |
| BHBA | Activates nrf2 | [133] | |
| L49H37 | Induces apoptosis in pancreatic stellate cells | [134] | |
| CDF | Multiple pathways affected | [135,136,137,138] | |
| WZ35 | Modulates ROS | [139,140,141] | |
| EF24 | Multiple pathways affected | [142,143,144,145,146] | |
| EGCG | D-ring analog | Targets VEGF | [163] |
| Methylated analog | Targets VEGF | [164] | |
| Fluoro-substituted | Inhibits proteasomal activity | [165,166] | |
| Honokiol | Dichloroacetate ester | Inhibits AR, chemosensitizes | [169,170] |
| Plumbagin | Isoniazid analog | Multiple pathways affected | [168] |
| Resveratrol | DMU-212 | Inhibits NF-κB | [155] |
| HS-1793 | Multiple pathways affected | [157,158,159,160,161,162] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubair, H.; Azim, S.; Ahmad, A.; Khan, M.A.; Patel, G.K.; Singh, S.; Singh, A.P. Cancer Chemoprevention by Phytochemicals: Nature’s Healing Touch. Molecules 2017, 22, 395. https://doi.org/10.3390/molecules22030395
Zubair H, Azim S, Ahmad A, Khan MA, Patel GK, Singh S, Singh AP. Cancer Chemoprevention by Phytochemicals: Nature’s Healing Touch. Molecules. 2017; 22(3):395. https://doi.org/10.3390/molecules22030395
Chicago/Turabian StyleZubair, Haseeb, Shafquat Azim, Aamir Ahmad, Mohammad Aslam Khan, Girijesh Kumar Patel, Seema Singh, and Ajay Pratap Singh. 2017. "Cancer Chemoprevention by Phytochemicals: Nature’s Healing Touch" Molecules 22, no. 3: 395. https://doi.org/10.3390/molecules22030395
APA StyleZubair, H., Azim, S., Ahmad, A., Khan, M. A., Patel, G. K., Singh, S., & Singh, A. P. (2017). Cancer Chemoprevention by Phytochemicals: Nature’s Healing Touch. Molecules, 22(3), 395. https://doi.org/10.3390/molecules22030395








